Abstract
The pattern of shell shape variation in populations of the mussel, Mytilus chilensis (Hupe 1854) from Southern Chile was analyzed as a function of sample origin (cultivated vs. wild) and latitude, using standard tools of geometric morphometrics for landmark data. Additionally, posterior adductor muscle index (PAMI), Freeman condition index and shell thickness were measured in each sample. Highly significant differences in shell shape components were found among mussel populations. These differences are related to the origin of samples (expansion of the posterior adductor muscle scar, elongation of the lateral ligament and of the ventral umbo position in non-cultivated samples) and to latitude (more elongated shells and more extended posterior adductor muscle scar in most southern samples when compared with the northernmost ones). PAMI and shell thickness were statistically higher in wild population, and Freeman condition index was higher in cultivated shells. It is suggested that in wild populations of M. chilensis, the mussels may face higher predator pressures and other environmental stress factors. Consequently, individuals may be using higher energy fraction to reinforce shells and to promote adductor muscle growth at the expense of somatic growth. In contrast, individuals found in calm aquaculture environments are relatively protected from predators and use most of their assimilated energy in somatic growth. In turn, this growth depends on changes that covariate with shell morphology.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Morphological variation in bivalve molluscs has been addressed in several studies dealing with changes in shell morphology. However, few studies have addressed the factors that impact on morphological patterns in Mytilus chilensis. Krapivka et al. (2007) studied the latitudinal variation of shell shape in wild populations of M. chilensis using geometric morphometric techniques for outline data (Fourier elliptical analysis). These authors found significant differences in the convexity of the shell ventral margin, umbo shape and shell elongation. These differences may be explained by a latitudinal gradient. Similar patterns were observed in Brachidontes sp., another important mussel species of South America (Aguirre et al. 2006).
The coexistence of aquaculture sites and natural populations of M. chilensis and their distribution along with a wide latitudinal gradient provide a unique scenario to address comparative morphological studies of sessile marine species. The bivalve Mytilus chilensis (Hupe 1854) is an important ecological and economic resource available along the southern coast of the Pacific Ocean. In Chile, this intertidal species is distributed from Arica (18° SL) to Cape of Horn (56° SL) (Lancelloti and Vásquez 2000). Exploitation of natural populations and their depletion north of Concepción (35° SL) (Toro et al. 2006) have promoted extensive farming of this species (Clasing et al. 1998) in the region of Los Lagos and the island of Chiloé (41°30′–43° SL), using long-line techniques (Lovatelli et al. 2007). Favorable environmental conditions have facilitated a rapid growth of the industry with 187,540 tons total landings in year 2008 (Sernapesca Annual Report 2008).
Although bivalve morphological changes have been traditionally studied using interlandmark distances and angles (Bertness and Grosholz 1985; Innes and Bates 1999; Alunno-Bruscia et al. 2001; Steffani and Branch 2003; Ubukata 2003; Lajtner et al. 2004; Briones and Guiñez 2005; Funk and Reckendorfer 2008), geometric morphometric techniques are turning into a standard approach to dilucidate the pattern of bivalve shell shape variation. Examples of this approach are Palmer et al. (2004) (Chamelea gallina), Aguirre et al. (2006) (Brachidontes sp.), Rufino et al. (2006) (Chamelea gallina and Chamelea striatula), Krapivka et al. (2007) (Mytilus chilensis), Sousa et al. (2007) (Corbicula fluminea) and Costa et al. (2008) (Tapes decussatus and T. philippinarum). Geometric morphometric techniques are based on the acquisition of 2D and 3D discrete (landmarks) or continue (outlines) matrix data. This procedure allows the partitioning of shape and size components of morphological change. Such partitioning is not possible to obtain using interlandmark distances because of their one dimensionality. Moreover, geometric morphometric techniques also preserve the main geometric properties of the biological specimens generating a visual output of the morphometric change after applying thin-plate splines, a mapping function for coordinate transformation (Bookstein 1991).
Other environmental and ecological factors that impact on shell morphology in some species of bivalves have been observed, such as sediment type (Funk and Reckendorfer 2008), tidal and wave exposure (Steffani and Branch 2003; Funk and Reckendorfer 2008), matrix stratification (Briones and Guiñez 2005), population density (Bertness and Grosholz 1985; Alunno-Bruscia et al. 2001; Briones and Guiñez 2005) and predation (Reimer and Tedengren 1996, Reimer and Harms-Ringdahl 2001; Beadman et al. 2003; Caro and Castilla 2004).
The hypothesis of this study is that shell shape variation can be explained by site origin (wild vs. cultivated) and/or latitude (northern vs. southern). Furthermore, we consider shape variation as a phenotype correlated with observed physiological changes (indexes).
Materials and methods
Samples and sampling sites
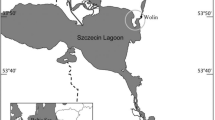
Samples of Mytilus chilensis were obtained by certified samplers of the National Shellfish Sanitation Program of the Servicio Nacional de Pesca (Sernapesca), following standard procedures for sample gathering and shipment as stated in norm LAB-NT1 (Sernapesca 2009). Sixty adult individuals (>6 cm) were obtained from each site (Fig. 1), except in Conchas Blancas (N = 28) (Table 1). Mussel populations were classified according to sample origin (wild or cultivated) and latitude (most northern Los Lagos and most southern Magallanes geographical regions).
Geographical distribution of samples used in this study. Samples were obtained from six areas from southern Chile. Los Lagos region: Caicaén Channel and Conchas Blancas (cultivated), Yaldad and Quilalar (wild population). Magallanes and Chilean Antarctic region: Almirante Montt and Punta Paulo (both wild populations)
Sample preparation and calculation of physiological indexes
Each pair of shell valves was labeled with a single key number. Shell area was estimated in the intact animal from measurements of shell length, height and width with a hand caliper (±0.05 mm), following Beadman et al. (2003). Thereafter, posterior adductor muscle and other tissues were dissected and kept separately for dry weight measurements. Dry weights were obtained after 70 h in an oven at 60°C. Estimations of allocated energy and its distribution within individuals were obtained. A Freeman condition index (Filgueira et al. 2008) was used to estimate the relative contribution of somatic tissues. Posterior adductor muscle index (PAMI; muscle dry weight/total soft tissue dry weight) was employed to assess the relative contribution of the specific muscle and shell thickness (Beadman et al. 2003) to assess the relative contribution of the shells.
Image analysis
The internal aspect (face) of the left valve from each individual was scanned using a desktop scanner (CanoScan D646U, 300 dpi) and stored in *.jpg format. Images were later processed for balance, brightness and contrast with Adobe Photoshop Image, version 7.0.
Geometric morphometric analyses
Geometric morphometric analyses (GMA) were carried out using free access programs from the Rohlf’s Tps series (http://life.bio.sunysb.edu/morph). For acquisition of two-dimensional primary data, a homologous map of nine discrete landmarks was constructed according to Bookstein’s criteria. The level of information contained by each landmark was defined after Bookstein (1991) (Fig. 2). Map digitization of scanned images and subsequent scaling was performed with the program tpsDig v.2.05 (Rohlf 2005). Differences due to rotation, translation and scale were filtered out by Procrustes analysis, following the algorithm from the tpsRelwarp v.1.36 program (Rohlf 2003a). The resulting matrices were projected as unique points to a biplot with axes tangent to Kendall shape space (relative warps analysis). These axes (relative warps), analogous to the principal components of multivariate analysis, correspond to the shape components of the Procrustes analysis (partial warps) and represent the total variance of shape change in decreasing order. The consensus configuration is defined as the point localized at x = 0, y = 0 corresponding to the reference thereafter used to visualize the direction and magnitude of the morphometric change in the morphometric space. The visualization of the pattern of shell shape variation was obtained after applying the thin-plate spline function in the positive and negative extreme values of each axis.
Confirmatory analysis
The statistical hypothesis about differences between groups was tested through the shell shape components’ regression into vectors of variable categories (−1, 0, 1), representing sample origin and latitude. The shells were evaluated by a generalized F Goodall’s test using sample origin (cultivated (1)/wild (−1)) and latitude (Los Lagos (1)/Magallanes (−1)) (program TpsReg v. 1.28, Rohlf 2003b) as categorical variables as follows:
-
(a)
Morphometric variation due to sample origin. Samples from cultivated populations (Caicaén Channel and Conchas Blancas) vs. wild samples (Yaldad and Quilalar) were analyzed.
-
(b)
Morphometric variation due to latitudinal distribution. Samples from wild populations Los Lagos (Yaldad and Quilalar) vs. Magallanes (Almirante Montt and Punta Paulo) were analyzed.
-
(c)
Analysis of morphometric variation among all populations (Caicaén Channel, Conchas Blancas, Yaldad, Quilalar, Almirante Montt and Punta Paulo).
Furthermore, a distance analysis by UPGMA of the Procrustes distances obtained from the consensus configurations from each group was performed using standard algorithms of NTSYS-pc v.2.10a program (Applied Biostatistics, Inc.). The centroid size was utilized as an estimation of the geometric size for each specimen and corresponds to the square root of the sum of the Euclidean distances between each landmark and the centroid of each specimen (Bookstein 1991).
-
(d)
In order to assess the level of concordance between the observed shell shape changes and the effect of the independent variables controlled in this study, a discriminant analysis was carried out (Statistica; www.statsoft.com). Only the shape components representing more than 90% of overall variance were considered. In this context, it is necessary to consider that uniform components (UNI) are those that do not require bending energy. Thus, parallel lines in a Cartesian grid remain parallel after the transformation has been carried out. In contrast, non-uniform shape components (PW) produce highly localized effects on the grid (Zelditch et al. 2004). Biologically, the effect of a uniform transformation can be seen along major anatomical axes, i.e. anteroposterior. In this study, the contribution of uniform shape components to overall shape variation is negligible (cf. P values in Table 2).
Table 2 Effect of origin and latitude sampling on M. chilensis shell shape components
Results
Shell morphometric variability due to origin (cultivated vs. wild)
Exploratory analysis of shell morphometric variability, as shown by relative warps distributions, showed clear differences among wild and cultivated mussel samples. The axis of higher variability (relative warp RW1) accounted for near 62% of the total variance. These differences were statistically significant (cf. Table 2), and they could be visualized as a clear extension of the posterior adductor muscle scar, an elongation of the lateral ligament in natural populations as opposed to cultivated ones (Fig. 3, X axis). In addition, the umbo changed its orientation toward the ventral shell border in wild populations as opposed to a shift toward the anterior shell border in cultivated samples. The ventral shell border was clearly concave in wild populations as opposed to a convex shape in cultivated samples.
Shell morphometric variability due to latitudinal distribution (Los Lagos vs. Magallanes)
Shell morphometric variability was clearly observed across different locations, and statistically significant differences were found among mussel populations obtained along a longitudinal axis of ca. 1,350 km encompassing 42° to 53° SL. The first shape component (RW1) explained 35.21% of the total variance. These differences were statistically significant (cf. Table 2), and they are visualized as more elongated and concave shells in Magallanes samples characterized by an extension of the posterior adductor muscle scar, compared with the most northern Los Lagos samples (Fig. 3, Y axis).
Morphometric analysis of all samples
Exploratory analysis of shell morphometric variability considering all samples corroborated the previously described pattern of shell shape variation considering origin and latitude. Main morphometric changes (RW1, 50.83% of total variation) were associated with shell shape differences among wild and cultivated samples (origin).
All previous observations could be a consequence of shell size and shell shape variation. To reject the possible correlations between the geometric size of the shell (centroid size) and shape, shells were analyzed in each sample separately (one-way Anova). Both attributes were found to be statistically independent (P > 0.05) with the exception of a very weak correlation in Conchas Blancas (cultivated) (r = 0.40; P = 0.032) and Quilalar (wild) (r = 0.32; P = 0.013), both from Los Lagos region. When the analysis of size variation was carried out excluding the cultivated populations, the centroid size of southern wild populations was bigger than the centroid size observed in the northern wild populations. These differences were statistically significant (mean centroid size Los Lagos = 7.8 cm, mean centroid size Magallanes = 9.3 cm; ttest, P < < 0.01).
A discriminant analysis among shape components (dependent variable) (Viðarsdóttir et al. 2002) using origin and latitude as discriminant variables was performed, considering the first four shape components explaining over 90% of overall shape variation. For origin, the percentage of correctly classified individuals was 92% in both natural and cultivated populations (Wilks’ Lambda: 0.35440; approx. F (4.203) = 92.451; P < 0.001; Tolerance > 97%). For latitude, the percentage of correctly classified individuals was 93% in Los Lagos and 92% in Magallanes (Wilks’ Lambda: 0.32254; approx. F (4.235) = 123.40; P < 0.001; Tolerance > 97%). This analysis confirms the consistency of the results obtained in this study after applying the standard tools kit of geometric morphometric approach.
Analysis of Procrustes distances
Dendrograms were constructed using matrices of morphometric distances (Procrustes distances) and UPGMA as explained above (see “Materials and methods”). Dendrograms confirmed results obtained with relative warps analysis and displayed separate branches for wild and cultivated populations (Fig. 4). In addition, these two main branches displayed further subdivisions associated with geographical origin (Los Lagos vs. Magallanes). Morphometric distances between cultivated populations (Caicaén Channel vs. Conchas Blancas) were minor but statistically significant, while differences between wild populations were very distinct and highly significant (i.e. Yaldad vs. Quilalar in Chiloé, Los Lagos region).
Morphological variation and physiological indexes
In the analysis, it was observed that the posterior adductor muscle scar was larger in wild populations than in cultivated ones, a finding that could be a consequence of a larger and more powerful posterior adductor muscle in wild populations. This led us to examine whether the size of the posterior adductor muscle scar (centroid size, cm) correlated with the muscle dry weight (g). Statistical analysis revealed a high degree of correlation (r = 0.91; P < 0.001). In addition, the ratio (muscle dry weight/dry total soft tissue weight) (PAMI: posterior adductor muscle index) was significantly higher in wild vs. cultivated populations (Fig. 5a). These correlations suggest that variation of morphological patterns could be associated with classical physiological variables. To examine this hypothesis, Freeman condition index and shell thickness were measured for each individual in all populations. It was found that Freeman condition index was significantly higher in cultivated populations (Fig. 5b), while shell thickness was clearly higher in wild populations (Fig. 5c).
Discussion
In this study, morphometric differences were strongly associated with origin (cultivated vs. wild) and latitude (Los Lagos vs. Magallanes) (Fig. 3). As we hypothesized, morphometric characters were strongly distinguishable between cultivated and wild populations of Mytilus chilensis. This is the first quantitative analysis reporting these significant differences for this commercially relevant species. Wild individuals displayed a concave ventral border, displacement of the umbo toward the ventral border, bigger posterior adductor muscles and thicker shells. These results are consistent with findings by Kirk et al. (2007), who reported that shell morphology in Mytilus edulis differed between cultivated and wild populations. As observed by Krapivka et al. (2007), this study confirms that shell shape did not display any significant correlation with geometric size. This precludes a biological explanation of morphometric differences between wild and cultivated populations based solely on size components.
This study shows that southernmost specimens display shells more concave and elongated when compared to northernmost specimens. This pattern of shell shape variation agrees with the results reported by Krapivka et al. (2007) that used a standard geometric morphometric tool kit for outline data. According to these authors, this pattern could be associated with a salinity latitudinal cline, with more elongated specimens belonging to lower salinity environments. In Chile, Mytilus chilensis can be found in ecosystems with widely varying salinities (Reid and Osorio 2002) and has been regarded as the most euryhaline species found in the southern fjords system.
Variations in salinity have widespread effects on aquatic organisms and can influence the geographical distribution of mussels (Mytilus californianus) (Young 1941) and its genetic structure. Koehn et al. (1980) demonstrated that salinity was a significant selective forcing that could change the genetic composition of bivalve populations. These authors demonstrated that the expression of osmotic pressure of Mytilus edulis individuals subjected to a salinity gradient was an object of natural selection, and this was reflected in the distribution of gen frequencies for the Lap locus that code for that phenotype. As shown by Shurova (2001), variations in salinity can modify size, age, sex and phenotypic structures of mussel populations, a fact that can be considered as an adaptive strategy. Thus, the latitudinal variation patterns of physiological and morphometric factors found in our study for Mytilus chilensis could be associated with the latitudinal salinity gradient described in southern Chile by Dávila et al. (2002). This factor could additionally affect specific attributes of the genetic structure of Mytilus chilensis populations.
Our results show that physiological indexes are closely related to morphological attributes such as shell shape variation as well as population origin (cultivated vs. natural). A significant increase in the size of the posterior adductor muscle scar in natural populations was found, and this was related to a bigger muscle size. Values of PAMI (Posterior Adductor Muscle Index) and shell thickness indexes were found to be significantly higher in natural populations. This indicates that in these populations energy could be preferentially allocated toward higher muscle growth and thicker shells, attributes directly related to individual survival.
Wild populations of Mytilus chilensis displayed thicker shells, a characteristic already registered in other bivalves related to higher predator pressure in wild environments, as shown for Placopecten magellanicus (Lafrance et al. 2003) and Pecten maximus (Grefsrud and Strand 2006).
Predation is an important factor associated with morphological plasticity in bivalves (Reimer and Tedengren 1996; Reimer and Harms-Ringdahl 2001; Beadman et al. 2003; Caro and Castilla 2004). Mussels are predated by a variety of predators such as gastropods, crabs, starfishes and marine birds and mammals (Reimer and Tedengren 1996; Reimer and Harms-Ringdahl 2001; Beadman et al. 2003; Soto et al. 2004; Kirk et al. 2007). This results in the formation and/or strengthening of body structures (Sälgeback 2006; Kirk et al. 2007) such as bigger adductor muscles and thicker shells, which are related to the increasing of individual fitness. In contrast, in cultivated populations, those predators are routinely eliminated by cultivators (Castro 2007). Reimer and Harms-Ringdahl (2001) showed that predator presence caused a significant increase in shell thickness and adductor muscle size in Mytilus edulis, when compared to individuals growing in areas free of predators. Coastal areas in Chile have highly diversified populations of the main predator groups of mussels, such as the gastropod Concholepas concholepas (loco), the asteroid Heliaster helianthus and decapods Acanthocyclus gayi y A. hassleri (Soto 1996). For the gastropod Acanthina monodon, mussels constitute ca. 95% of its diet (Soto et al. 2004).
Consistent with previous studies on morphological variation in other mussel species, a simple ecophysiological explanation of the observed morphological changes can be stated. According to this explanation, in wild populations of M. chilensis facing higher predator pressure and other environmental stress factors, individuals usually allocate a higher energy fraction to reinforce shells and to promote adductor muscle growth at the expense of somatic growth. In contrast, individuals placed in calm aquaculture environments are relatively protected from predators and use most of their assimilated energy in somatic growth. This could be the expression of a balance where individuals in natural environments reinforce their defensive structures at the expense of lower somatic growth. In turn, this growth depends on somatic changes that covariate with shell morphology change.
References
Aguirre M, Pérez S, Sirch Y (2006) Morphological variability of Brachidontes Swainson (Bivalvia, Mytilidae) in the marine Quaternary of Argentina (S.W. Atlantic). Palaeogeogr Palaeoclimatol Palaeoecol 239:100–125. doi:10.1016/j.palaeo.2006.01.019
Alunno-Bruscia M, Bourget E, Fréchette M (2001) Shell allometry and length-mass-density relationship for Mytilus edulis in an experimental food-regulated situation. Mar Ecol Prog Ser 219:177–188
Beadman H, Caldow R, Kaiser M, Willows R (2003) How to toughen up your mussels: using mussel shell morphological plasticity to reduce predation losses. Mar Biol 142:487–494. doi:10.1007/s00227-002-0977-4
Bertness M, Grosholz E (1985) Population dynamics of the ribbed mussel, Geukensia demissa: the costs and benefits of an aggregated distribution. Oecologia 67:192–204
Bookstein F (1991) Morphometrics tools for landmark data: geometry for biology. Cambridge University Press, Cambridge
Briones C, Guiñez R (2005) Asimetría bilateral de la forma de las valvas y posición espacial en matrices del chorito Perumytilus purpuratus (Lamarck, 1819) (Bivalvia: Mytilidae). Rev Chil Hist Nat 78:3–14
Caro A, Castilla J (2004) Predator-inducible defences and local intra-population variability of the intertidal mussel Semimytilus algosus in central Chile. Mar Ecol Prog Ser 276:115–123
Castro H (2007) Biología marina. McGraw-Hill, Madrid
Clasing E, Oñate A, Arriagada H (1998) Cultivo de Choritos en Chile. Universidad Austral de Chile, Imprenta Universitaria S.A., Valdivia
Costa C, Menesatti P, Aguzzi J, D’Andrea S, Antonucci F, Rimatori V, Pallottino F, Mattoccia M (2008) External shape differences between sympatric populations of commercial clams Tapes decussatus and T. philippinarum. Food Bioprocess Technol. doi:10.1007/s11947-008-0068-8
Dávila PM, Figueroa D, Müller E (2002) Freshwater input into the coastal ocean and its relation with the salinity distribution off austral Chile (35–55°S). Continental Shelf Res 22:521–534
Filgueira R, Labarta U, Fernández-Reiriz M (2008) Effect of condition index on allometric relationship of clearance rate in Mytilus galloprovincialis Lamarck, 1819. Revista de Biología Marina y Oceanografía 43:391–398
Funk A, Reckendorfer W (2008) Environmental heterogeneity and morphological variability in Pisidium subtruncatum (Sphaeriidae, Bivalvia). Internat Rev Hydrobiol 93:188–199. doi:10.1002/iroh.200710969
Grefsrud E, Strand Ø (2006) Comparison of shell strength in wild and cultured scallops (Pecten maximus). Aquaculture 251:306–313. doi:10.1016/j.aquaculture.2005.06.004
Innes D, Bates J (1999) Morphological variation of Mytilus edulis and Mytilus trossulus in eastern Newfoundland. Mar Biol 133:691–699
Kirk M, Esler D, Boyd W (2007) Morphology and density of mussels on natural and aquaculture structure habitats: implications for sea duck predators. Mar Ecol Prog Ser 346:179–187. doi:10.3354/meps07046
Koehn R, Newell R, Immermann F (1980) Maintenance of an aminopeptidase allele frequency cline by natural selection. Proc Natl Acad Sci USA 77:5385–5389
Krapivka S, Toro J, Alcapán A, Astorga M, Presa P, Pérez M, Guiñez R (2007) Shell-shape variation along the latitudinal range of the Chilean blue mussel Mytilus chilensis (Hupe 1984). Aquacult Res 38:1770–1777. doi:10.1111/j.1365-2109.2007.01839.x
Lafrance M, Cliche G, Haugum G, Guderley H (2003) Comparison of cultured and wild sea scallops Placopecten magellanicus, using behavioral responses and morphometric and biochemical indices. Mar Ecol Prog Ser 250:183–195
Lajtner J, Marušić Z, Klobučar G, Maguire I, Erben R (2004) Comparative shell morphology of the zebra mussel, Dreissena polymorpha in the Drava river (Croatia). Biologia Bratislava 59:595–600
Lancelloti D, Vásquez J (2000) Zoografía de macroinvertebrados bentónicos de la costa de Chile: contribución para la conservación marina. Rev Chil Hist Nat 73:99–129. doi:10.4067/S0716-078X2000000100011
Lovatelli A, Farías A, Uriarte I (eds) Estado actual del cultivo y manejo de moluscos bivalvos y su proyección futura: factores que afectan su sustentabilidad en América Latina. Taller Técnico Regional de la FAO. 20–24 de agosto de 2007, Puerto Montt, Chile. FAO Actas de Pesca y Acuicultura, No. 12. Roma, FAO, 359 p
Palmer M, Pons G, Linde M (2004) Discriminating between geographical groups of a Mediterranean commercial clam (Chamelea gallina (L): Veneridae) by shape analysis. Fish Res 67:93–98. doi:10.1016/j.fishres.2003.07.006
Reid D, Osorio C (2002) The shallow-water marine Mollusca of the Estero Elefantes and Laguna San Rafael, southern Chile. Bull Nat Hist Mus, London (Zool) 66:109–146
Reimer O, Harms-Ringdahl S (2001) Predator-inducible changes in blue mussels from the predator-free Baltic Sea. Mar Biol 139:959–965
Reimer O, Tedengren M (1996) Phenotypical improvement of morphological defences in the mussel Mytilus edulis induced by exposure to the predator Asterias rubens. Oikos 75:383–390
Rohlf FJ (2003a) tpsRelw, relative warps analysis, version 1.36. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohlf FJ (2003b) tpsRegr, shape regression, version 1.28. Department of Ecology and Evolution, State University of New York, Stony Brook
Rohlf FJ (2005) tpsDig, digitize landmarks and outlines, version 2.05. Department of Ecology and Evolution, State University of New York, Stony Brook
Rufino M, Gaspar M, Pereira A, Vasconcelos P (2006) Use of shape to distinguish Chamelea gallina and Chamelea striatus (Bivalvia: Veneridae): linear and geometric morphometric methods. J Morphol 267:1433–14440. doi:10.1002/jmor
Sälgeback J (2006) Functional morphology of gastropods and bivalves. Digital Comprehensive Summaries of Uppsala Dissertations from the Faculty of Science and Technology 257, Universitatis Upsaliensis, 31 pp
Sernapesca (2009) Norm LAB NT1 http://www.sernapesca.cl/index.php?option=com_remository&Itemid=246&func=fileinfo&id=198
Sernapesca Annual Report (2008) http://www.sernapesca.cl/index.php?option=com_remository&Itemid=54&func=fileinfo&id=3302
Shurova N (2001) Influence of salinity on the structure and the state of bivalve Mytilus galloprovincialis populations. Russ J Mar Biol 27:151–155
Soto R (1996) Estructura gremial de un ensamble de depredadores de la zona intermareal rocosa en Chile Central. Invest Mar 24:97–105
Soto R, Castilla J, Bozinovic F (2004) Conducta de forrajeo del gastrópodo Acanthina monodon Pallas, 1774 (Gastropoda: Muricidae) en el intermareal rocoso de Chile central. Rev Chil Hist Nat 77:157–175. doi:10.4067/S0716-078X2004000100012
Sousa R, Freire R, Rufino M, Mendez J, Gaspar M, Antunes C, Guilhermino L (2007) Genetic and shell morphological variability of the invasive bivalve Corbicula fluminea (Müller, 1774) in two Portuguese estuaries. Estuar Coast Shelf Sci 74:166–174. doi:10.1016/j.ecss.2007.04.011
Steffani C, Branch G (2003) Growth rate, condition, and shell shape of Mytilus galloprovincialis: responses to wave exposure. Mar Ecol Prog Ser 246:197–209
Toro J, Castro G, Ojeda J, Vergara A (2006) Allozymic variation and differentiation in the Chilean blue mussel, Mytilus chilensis, along its natural distribution. Genet Mol Biol 29:174–179
Ubukata T (2003) A morphometrical study on morphological plasticity of shell form in crevice-dwelling Pterioida (Bivalvia). Biol J Linn Soc 79:285–297
Viðarsdóttir U, O′Higgins P, Stringer C (2002) A geometric morphometric study of regional differences in the ontogeny of the modern human facial skeleton. J Anat 201:211–229
Young R (1941) The distribution of the mussel (Mytilus californianus) in relation to the salinity of its environment. Ecology 22:379–386
Zelditch M, Swiderski D, Sheets H, Fink W (2004) Geometric morphometrics for biologists. Elsevier Academic Press, New York and London
Acknowledgments
Partial support was obtained from International Atomic Energy Agency Technical Cooperation Grant CHI 07/011 to B.A.S.I. and Fondecyt 1050279 to G.M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Valladares, A., Manríquez, G. & Suárez-Isla, B.A. Shell shape variation in populations of Mytilus chilensis (Hupe 1854) from southern Chile: a geometric morphometric approach. Mar Biol 157, 2731–2738 (2010). https://doi.org/10.1007/s00227-010-1532-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1532-3