Abstract
The phytoplankton of the Bahía Blanca Estuary, Argentina, has been surveyed since 1978. Chlorophyll a, phytoplankton abundance, species composition and physico-chemical variables have been fortnightly recorded. From 1978 to 2002, a single winter–early spring diatom bloom has dominated the main pattern of phytoplankton interannual variability. Such pattern showed noticeable changes since 2006: the absence of the typical winter bloom and changes in phenology, together with the replacement of the dominant blooming species, i.e. Thalassiosira curviseriata, and the appearance of different blooming species, i.e. Cyclotella sp. and Thalassiosira minima. The new pattern showed relatively short-lived diatom blooms that spread throughout the year. In addition, shifts in the phytoplankton size structure toward small-sized diatoms, including the replacement of relatively large Thalassiosira spp. by small Cyclotella species and Chaetoceros species have been noticed. The changes in the phenology and composition of the phytoplankton are mainly attributed to warmer winters and the extremely dry weather conditions evidenced in recent years in the Bahía Blanca area. Changing climate has modified the hydrological features in the inner part of the estuary (i.e. higher temperatures and salinities) and potentially triggered the reorganization of the phytoplankton community. This long-term study provides evidence on species-specific and structural changes at the bottom of the pelagic food web likely related to the recent hydroclimatic conditions in a temperature estuary of the southwestern Atlantic.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Estuaries are among the most dynamic ecosystems where hydrological conditions vary markedly at several spatio-temporal scales. In recent years, evidence has been growing on the influence of anthropogenic and climatic forces on the interannual variations of phytoplankton communities (Cloern et al. 2007; Nixon et al. 2009). Phytoplankton provides food supply to upper tropic levels, and any modification in its structure and/or dynamics (i.e. phenology, size structure, species composition) may trigger changes in the ecosystem functioning. Phytoplankton blooms are ubiquitous in coastal ecosystems, and noticeable changes in phenology and species composition have been linked to global changes (Edwards and Richardson 2004; Wiltshire and Manly 2004). Most of phytoplankton monitoring programs, however, are based on bulk measurements of chlorophyll or total biomass, and structural changes related to modifications at species level have been generally overlooked. Phytoplankton blooms have a characteristic species-specific dimension that respond to changing environmental dynamics (Legendre 1990). Complex patterns of competition exist among phytoplankton species due to their sensitivity to variations in light and nutrients concentrations (e.g. Tilman 1982; Huisman and Weissing 1999; Roelke et al. 2003). Changes in external forcing (i.e. light, precipitation, temperature) might obscure the role of intrinsic species interactions in complex food webs and promote species coexistence in plankton communities (Benincà et al. 2008). In fact, although phytoplankton species share the same potentially limiting resources, they differ in regard to their physiological requirements, i.e. ratios of resources required are species specific (Litchman et al. 2007). Given the concerns about global warming (Hays et al. 2005), long-term phytoplankton records are essential to understand how pelagic ecosystems respond to variations in climate and anthropogenic perturbations or their synergies.
The Bahía Blanca Estuary, located on the southwestern Atlantic in Argentina, is a mesotidal estuary, highly turbid and characterized by a eutrophic inner zone (Freije and Marcovecchio 2004). In this area, the phytoplankton bloom has been investigated throughout the period 1978–2002 and showed a relatively regular pattern characterized by a recurrent winter–early spring diatom bloom in the inner zone of the estuary (Popovich et al. 2008b and references therein). The bloom has been characterized by interannual variations in duration and magnitude; however, a consistent recurrence of the same species assemblage (Thalassiosira spp. and Chaetoceros spp., with T. curviseriata as the most abundant species) has been observed throughout the years. In this study, we extended such investigations and compared the long-term pattern observed during 1978–2002 with the years 2006–2008. Our goals were twofold: (1) to examine the long-term variations of the phytoplankton bloom in regards to the rising temperatures in the Southern Hemisphere and (2) to quantify changes in species composition, timing and magnitude of the bloom and identify possible underlying processes.
Materials and methods
Study area
The Bahía Blanca Estuary (38°42′–39°25′S, 61°50′–62°22′W) is located in a temperate region in the south of Buenos Aires Province, Argentina, on the southwestern Atlantic coast. The estuary has a semidiurnal tidal cycle and high turbidity and nutrient concentrations (Popovich and Marcovecchio 2008). The inner zone is characterized by a restricted circulation where tidal velocities range between 0.69 and 0.77 ms−1, with low advection (Perillo et al. 2001) and a residence time ca. 28 days (G. Perillo unpublished). The water column is generally well mixed all year-round (Gayoso 1998). Two freshwater tributaries enter the estuary from the northern shore, the Sauce Chico River and the Napostá Grande Creek, with a mean annual runoff of 1.9 and 0.8 m3 s−1, respectively (Melo and Limbozzi 2008). The Ingeniero White port, one of the most important ports in South America, and Bahía Blanca City (300,000 inhab.) are located in this area.
Sampling and analysis
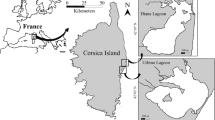
Samples were collected at a fixed station (Puerto Cuatreros, 38°50′S; 62°20′W), a shallow harbor (mean depth of 6 m) at the head of the Bahía Blanca Estuary (Fig. 1). The sampling protocol (i.e. frequency, collection and processing of samples) was consistent from 1978 to 2008. Samples were collected fortnightly (weekly or twice by week during the phytoplankton bloom) at high tide and around noon time. Surface water samples were taken using a Van Dorn bottle (2.5 l) and used for phytoplankton cells enumeration (the samples were preserved in acidified Lugol’s solution), biomass quantification and dissolved nutrient analysis. For species identification, samples were collected using a Nansen 30-μm net and preserved with formaldehyde (final concentration 0.4%).
Physical data
Environmental data correspond to surface water temperature and conductivity measured with a digital multi-sensor Horiba U-10. Thereafter, the conductivity was transformed into salinity and expressed as practical salinity units. Precipitation data (January 2006–February 2008) were taken from a meteorological station located near the sampling station, at the pier in Puerto Cuatreros. For the long-term scale, regional monthly records of precipitation over the period 1980–2005 at Bahía Blanca City (about 20 km of Puerto Cuatreros) were obtained from the Argentine National Meteorological Service. In addition, field patterns of atmospheric variability (i.e. precipitation and temperature) over the Bahía Blanca region during the period 1970–2008 were assessed. The dataset used is the National Center for Environmental Prediction–National Center for Atmospheric Research (NCEP–NCAR) gridded reanalysis (Kalnay et al. 1996).
Dissolved inorganic nutrients
Water samples were filtered through Watman GF/C filters and frozen in plastic bottles until analysis. Dissolved nitrate \( \left( {{\text{NO}}_{3}^{ - } } \right) \), nitrite \( \left( {{\text{NO}}_{2}^{ - } } \right) \), ammonium \( \left( {{\text{NH}}_{4}^{ + } } \right) \), phosphate \( \left( {{\text{PO}}_{4}^{3 - } } \right) \) and silicate (SiO2) concentrations were measured according to APHA (1998) using a Technicon AA-II Autoanalyzer expanded to five channels. Total dissolved inorganic nitrogen concentration (DIN) was calculated as the sum of \( {{\text{NO}}_{3}^{ - } } \), \( {{\text{NO}}_{2}^{ - } } \) and \( {{\text{NH}}_{4}^{ + } } \).
Chlorophyll determinations
Chlorophyll concentration (in μg l−1) was measured with spectrophotometer using the method described in APHA (1998). Water samples (250 ml) were filtered through Whatman GF/C filters, which were immediately frozen and stored at −20°C. Pigment extraction was done in 90% acetone for 20 min at ambient temperature.
Phytoplankton enumeration
Phytoplankton was identified at species level using a Zeiss Standard R microscope and a Nikon Eclipse microscope, with magnification of 1,000× and phase contrast. For the phytoplankton identification, we largely followed Ross et al. 1979, Gayoso 1981, 1988, 1989 and Thomas 1997, among other specific references on diatom taxonomy. The phytoplankton abundance (in cells l−1) was determined using a Sedgwick-Rafter chamber (1 ml) which was considered as a suitable size due to the amount of suspended particles. The entire chamber was examined at 200×, and each cell was counted as a unit.
Historical data compilation
All published phytoplankton data (Gayoso 1989, 1998, 1999; Pettigrosso and Popovich 2009; Popovich 1997, 2004; Popovich and Gayoso 1999; Popovich and Marcovecchio 2008; Popovich et al. 2008a) were compiled to compare the phytoplankton annual pattern observed in the period 2006–2008 with the one previously described in the estuary.
Statistical analysis
From the thirteen available years in the literature (1978–1982, 1988–1994 and 2002) in which chlorophyll concentrations and phytoplankton densities were recorded weekly or twice a week, we calculated the monthly means and their respective 95% confidence intervals. Afterward, three main periods were formed for comparison: (1) 1978–1982, (2) 1988–1994 (consecutive years), and (3) 2002–2007 (3 years, 2002, 2006 and 2007). These periods were compared by applying non-parametric Mann–Whitney tests on monthly chlorophyll concentration and phytoplankton abundance during the blooming winter episode (from May to September). In addition, coefficients of variation (CV) were estimated to evaluate the annual variability of chlorophyll and phytoplankton abundance (after log-transformation) within each period. In addition, in those years where the phytoplankton enumeration was done up to species level, we compared species-specific maximal abundances reached during the winter bloom.
The interannual variability of phytoplankton abundance and chlorophyll concentration was analyzed in their standardized and non-dimensional form as standard deviations (SDs) from the mean of the reference period 1978–2002. This allowed assessing the magnitude of changes, expressed as SD from the global mean and to identify patterns in their interannual variability.
Changes in the phytoplankton bloom during the three specified periods were evaluated by computing the probability density distribution of phytoplankton during the months May to September. Density distribution was estimated using non-parametric Kernel smoothing. This allows assessing changes in the probability distribution of the phytoplankton bloom that have occurred in the period investigated (Jones 1990). Also, to evaluate potential shifts in the timing of the winter bloom, the onset and the decline were defined according to Gayoso (1998) in Julian days and then analyzed by regression analysis. According to Gayoso (1998), the bloom usually started in late June when cell densities rapidly increased by three orders of magnitude and chlorophyll a augmented fourfold above the annual average within a few days. The decline of the bloom was characterized by a dramatic reduction in cell densities, generally in September.
Test for detecting departures from normality (Shapiro–Wilk W-statistic), linear regressions and Mann–Whitney Wilcoxon tests for sample comparisons were performed using a software package developed at the Mathematical Department of the Universidad Nacional del Sur, Argentina. The probability density distribution and kernel smoothing were done with MatLab software V.7 (Mathworks inc., 2005).
Results
Water temperature, salinity, precipitation and dissolved nutrients
Water temperature changes registered at Puerto Cuatreros for the periods 1974–1982, 1983–1992, 1993–2002 and 2006–2007 are shown in Table 1. Increases in both maximum and minimum values were apparent with a highest record of mean and minimum values in 2006. The fortnightly variability of temperature from January 2006 to February 2008 showed similar patterns in both years, with maximal values during summer (Fig. 2a). The mean salinity over the period 1974–2002 (the only period available in the literature) was 32.8 ± 3.7 (minimum 17.3, maximum 41.9). During the period 2006–2008, the salinity was higher and, like temperature, it presented maximal values during summer (Fig. 2a). Conversely, in winter, the salinity showed different patterns between 2006 and 2007, probably in response to the differences in the precipitation regime, as we will discuss later. In winter 2006, hydrographic conditions were anomalously warm (minimum of 8.2°C) and salty (maximum 34.8 ± 1.4). During 2006–2008, precipitation was low (434 mm in 2006 and 392 mm in 2007) compared to the period 1980–2005 where mean precipitation values registered in the estuary reached 614 mm (Fig. 2b). Dissolved nutrients concentrations in 2006–2008 were similar to the historical records with a marked annual depletion in early spring (Fig. 2c). The mean values (in μmol l−1) during the periods 1974–2002 (the only period available in the literature, Freije and Marcovecchio 2004) and 2006–2008 with their respective standard deviations were 1.77 ± 1.86 and 2.26 ± 1.97 for nitrite, 8.02 ± 13.64 and 10.06 ± 6.50 for nitrate, 32.31 ± 25.78 and 28.52 ± 14.41 for ammonium, 1.83 ± 2.25 and 2.92 ± 1.49 for phosphate and 87.30 ± 31.46 and 82.11 ± 27.50 for silicate, respectively. Regional precipitation showed a trend to decrease, while regional atmospheric temperature showed a significant growing trend (Fig. 3).
Temporal variation in a water surface temperature, b precipitation and c dissolved nutrient concentration (phosphate, silicate and DIN = ammonium + nitrite + nitrate) in Puerto Cuatreros from January 2006 to February 2008. In (b), the monthly average precipitation during the period 1980–2005 is represented as a line and repeated twice
Seasonal variability of phytoplankton community: 1978–2002
The data compiled for the years 1978–2002 showed a recurrent unimodal annual pattern of phytoplankton (dashed lines in Fig. 4) characterized by a winter–early spring diatom bloom. Although interannual variations were noticed, as shown in Fig. 4 by the 95% confident intervals of each monthly mean, the blooming episode took place every winter. The recurrent pattern was registered not only in the magnitude of the bloom in terms of chlorophyll concentration and phytoplankton abundance but also in the dominance of blooming species (Table 2). The winter–early spring bloom lasted for 3 months, the onset was typically in June and the decline in early September. Maximal annual values of chlorophyll concentration were 54 μg l−1 in July 1980 and 44 μg l−1 in July 2002, and the maximal phytoplankton abundance registered reached 12,720 × 103 cells l−1 in July 1991. The genus Thalassiosira was the most conspicuous component of the phytoplankton winter bloom in abundance and species richness (T. curviseriata, T. anguste-lineata, T. rotula, T. pacifica, T. eccentrica and T. hibernalis) followed by the genus Chaetoceros (4 species) (see Table 2 for maximal winter abundances). Thalassiosira curviseriata was by far the most abundant species in the phytoplankton annual cycle; it was observed all year-round with a strong peak in winter. The highest densities showed by this species varied between 2.8 × 106 and 12.7 × 106 cells l−1. Some non-blooming species (i.e. other diatoms, dinoflagellates and the Xantophyceae Ophiocytium sp.; sensu Popovich et al. 2008a) also appeared during the blooming season (Table 2), although they were less recurrent and showed important interannual variations. In 1981, a noticeable peak (4.3 × 105 cells l−1) of Ditylum brightwellii was registered during the phytoplankton bloom whose overall magnitude was particularly low (up 8 × 105 cells l−1). The mean water temperature in winter 1981 was 11°C, which corresponded to the maximal value registered between 1978 and 1992 in the sampling station (Gayoso 1998).
Temporal variation in a chlorophyll a concentrations and b abundances of phytoplankton. The period 2006–2008 was sampled fortnightly from January 2006 to February 2008; the period 1978–2002 is based on monthly means (vertical bars are the 95% confidence intervals) of eleven non-consecutive years sampled fortnightly or weekly. The long-term mean annual cycle (1978–2002) is repeated twice
Seasonal variability of phytoplankton community: 2006–2008
Chlorophyll
The chlorophyll concentration was highly variable in the years 2006–2008 (coefficient of variation = 86%) and showed numerous peaks. The values ranged between 0.23 and 24.55 μg l−1 (Fig. 4a). The 2 years had similar annual mean chlorophyll concentration: 5.8 μg l−1 in 2006 and 5.6 μgl−1 in 2007, with maximal values during winter, 19.97 μg l−1 in 2006 and 24.55 μg l−1 in 2007.
Bloom dynamics
The phytoplankton variability showed several peaks during the sampling period (Fig. 4b). The first peak in 2006 was observed ca. mid-summer (February–March), and it was characterized by phytoplankton densities ca. 2,271 × 103 cells l−1 and 12.06 μg l−1 of chlorophyll concentration. The second peak occurred in late autumn–early winter period (June–July), when the density reached up to 2,267 × 103 cells l−1 and chlorophyll concentration reached a maximum of 19.97 μg l−1. A third peak in 2006 appeared late winter (August–September) with phytoplankton densities that reached up to 1,792 × 103 cells l−1 and chlorophyll concentration of 14.94 μg l−1. In 2007, the first peak of abundance occurred in mid summer (February), and it was characterized by phytoplankton densities of 1,343 × 103 cells l−1 and 7.38 μg l−1 of chlorophyll concentration. The second peak persisted almost during the entire winter season (June to August). The phytoplankton density reached 8,032 × 103 cells l−1, and the chlorophyll concentration was 24.55 μg l−1. During summer 2008, a peak was found in January, and it was characterized by densities of 4,960 × 103 cells l−1 and chlorophyll concentration of 11.48 μg l−1.
Species composition
The phytoplankton community was dominated by diatoms during the whole sampling period (Fig. 5). The Xantophyceae Ophiocytium sp. was relatively abundant (244 × 103 cells l−1) during summer 2008. Small flagellates (2–20 μm) and some dinoflagellates were also present but with relatively low abundances (e.g. the maximal concentration of Scrippsiella trochoidea reached 30 × 103 cells l−1 during early spring). A few diatoms species were responsible for the maximal abundance peaks previously described (Figs. 4b, 5). These species were Thalassiosira minima (summer peaks), Cyclotella sp. (late autumn–early winter peak 2006), Guinardia delicatula (late winter peak 2006) (Fig. 5a), Chaetoceros sp. 1, C. ceratosporus and C. debilis (winter peak 2007) (Fig. 5b). Thalassiosira minima represented more than 75% of the total phytoplankton abundance in the summer peaks. It reached a maximum on January 2008 with 4,267 × 103 cells l−1 (86% of the total abundance). In the summer peak 2006, T. curviseriata appeared as the second most frequent species (Fig. 5c), with abundances over the 10% of the samples (320 × 103 cells l−1). In the late autumn–early winter peak 2006, Cyclotella sp. (diameter 5–12 μm) was present at a maximal concentration of 1,283 × 103 cells l−1 (56% of the total abundance) followed by Thalassiosira sp. (diameter 15–45 μm) with an abundance of 370 × 103 cells l−1. In the late winter 2006 (August–September), a short-term succession of phytoplankton peaks was observed. This was characterized by a dominance of Chaetoceros diadema, which accounted for the 77% of the total phytoplankton abundance on August 16. Afterward, on August 31, Thalassiosira pacifica, Guinardia delicatula and T. eccentrica were dominant and accounted for the 34, 32 and 20% of the total abundance, respectively. Finally, on September 15, the species G. delicatula (74% of the total abundance) with a population density of 1,333 × 103 cells l−1 and Ditylum brightwellii (21% of the total abundance) were by far the most abundant ones. In 2007, Thalassiosira curviseriata (467 × 103 cells l−1) and Leptocylindrus minimus (243 × 103 cells l−1) co-dominated in the samples on June 8 with 39 and 20% of the total abundance, respectively. On June 29, a small unidentified Chaetoceros (diameter of 3–8 μm and delicate, short setae), here called Chaetoceros sp. 1, reached maximal densities of 5,367 × 103 cells l−1. This species represented 67% of the total abundance. From June 22 to July 10, other species were also found with relatively high abundances as Chaetoceros ceratosporus, Cyclotella sp., Leptocylindrus minimus, Thalassiosira sp. and T. pacifica. On July 17, 65% of the phytoplankton abundance was due to Chaetoceros debilis that reached high densities (2,653 × 103 cells l−1).
Long-term changes of the winter bloom
The mean chlorophyll concentration observed during the months May–September in the three analyzed periods (1978–1982, 1988–1994 and 2002–2007) was 15.9, 15.7 and 10.1 μg l−1, and their CV were 15.1, 11.7 and 21.6%, respectively (Fig. 6a). Statistical evidence was found that the magnitude of the peak in terms of chlorophyll concentration was significantly different between the periods 1978–1982 and 2002–2007 (Mann–Whitney test; P < 0.05), with a decline of about 65% in the latter period. A significant reduction (about 69%) in the maximum of phytoplankton abundance was also found in 2006–2007 compared with the former years 1978–2002 (Fig. 6b), although these variations were not significant when comparing the mean values of phytoplankton abundances during the three periods 1978–1982 (CV of 16.5%), 1988–1994 (CV of 5.1%) and 2002–2007 (CV of 2.7%) (Mann–Whitney test; P > 0.05).
a Mean annual values of chlorophyll a concentration (points) during the blooming months (May–September) with their standard deviations (vertical lines). The horizontal dashed lines represent the mean values of the three groups of years (1978–1982, 1988–1994 and 2002–2007). b Monthly means (columns) of the phytoplankton abundance during the blooming period (May–September) and the mean values of the three groups of years (horizontal dashed lines)
The analysis of chronological changes in the winter bloom showed a decreasing pattern in both chlorophyll (Fig. 7a) and phytoplankton abundance (Fig. 7b) that was accentuated in the last 2 years, as shown by the SD from the mean of the period 1978–2002. These changes were also observed in the interannual dispersion of the phytoplankton abundance between May and September (Fig. 8a). Higher variability at the annual scale was found during the first years related to the pronounced magnitude in the winter bloom that substantially decreased in recent years, which explain the observed lower variability of phytoplankton abundance. This was illustrated by the density distribution of the phytoplankton abundance in the winter bloom, which showed marked differences among the three periods (Fig. 8b). The pattern in the periods 1978–1982 and 1988–1994 displayed a narrow peak driving by the dominance of few species. Conversely, we found that in the last years such pattern has dramatically changed toward a wider dome-shaped typology, related to the decrease in the few dominant species and the occurrence of new ones. Regarding the timing of the phytoplankton winter bloom (Fig. 8c), in 2006 and 2007, it started significantly earlier (R 2 = 0.502 P < 0.01) in May (Julian days 124–130) than in previous years, around June–July (Julian days 154–184). On the other hand, the decline of the bloom moved forward slightly (R 2 = 0.269 P < 0.05).
a Box-plots describing the distributions of the phytoplankton abundance during the blooming period. The horizontal lines across the boxes correspond to the lower quartile, median and upper quartile values. The whiskers are lines extending from each end of the box to show the extent of the rest of the data. b Density distribution of the phytoplankton abundance (dimensionless) during the winter bloom in the periods 1978–1981, 1988–1994 and 2002–2007. c Timing of the phytoplankton annual bloom indicated by the onset and decline of the bloom expressed in Julian days
Discussion
Changes in phytoplankton community structure
Species-specific changes
Despite the high variability in the estuarine habitat, the phytoplankton in the Bahía Blanca Estuary showed a quasi-regular annual pattern, characterized by a winter bloom during the period 1978–2002. Such annual pattern showed few interannual changes in the relative abundances of some species (e.g. Skeletonema costatum, Ditylum brightwellii, Guinardia delicatula), duration and magnitude (Popovich et al. 2008b and references therein). From 1978 to 2002, the bloom was dominated by the same assemblage of species: Thalassiosira curviseriata, T. hibernalis, T. anguste-lineata, T. rotula, T. pacifica, Chaetoceros similis, C. ceratosporus, C. debilis and C. diadema. From this group, Thalassiosira curviseriata has been the dominant species accounting for 60–90% of the total number of cells (Popovich and Gayoso 1999). However, in the last few years, the Bahía Blanca Estuary showed significant changes in the dynamics and composition of the phytoplankton bloom. The most noticeable changes between the years 1978–2002 and 2006–2008 were the shifts of the unimodal annual pattern by numerous episodes of phytoplankton growth throughout the latter period. These bloom events (sensu Smayda 1997) were generally short (~15 days) and characterized by unknown blooming species so far in the Bahía Blanca Estuary. These results illustrate the modification undergone by the phytoplankton assemblage (Table 3): shifts in species predominance (i.e. replacement of T. curviseriata), trends of increasing abundance of specific species (i.e. T. minima and Cyclotella sp.) and disappearance (T. rotula, T. anguste-lineata, T. hibernalis), as well as the occurrence of irregular growth events (Chaetoceros sp. 1).
In pelagic communities, the appearance of new species followed by the persistence, perennial predominance or even establishment as keystone species has been increasingly documented (Smayda 1998; Hays et al. 2005), and some changes have been related to long-term environmental variations (Lange et al. 1992, Thackeray et al. 2008). In particular, this is the case of the Cyclotella taxa that has increased its abundance in lakes since the nineteenth century apparently in response to global warming (Rühland et al. 2008, Winder et al. 2008). The rising temperatures enhance water stratification in lakes and nutrient depletion in surface layers. Such conditions favor the dominance of small-sized diatoms like Cyclotella spp. as they present higher surface to volume ratios and lower sinking velocities and small diffusion boundary layers (i.e. more efficient nutrient uptake and superior ability to harvest light). This mechanism, however, does not seem to explain the dominance of smaller species (e.g. Cyclotella sp., Chaetoceros sp.1) in recent years in the Bahía Blanca Estuary, where the water column is nutrient-rich and completely mixed all year-round. Alternative explanations for the increase of these species seem to be related to the hydroclimatic modifications experienced in the estuary in the last years. These changes in the environment could have directly affected the ecological niches and the species-specific interactions in the phytoplankton leading to shifts in the community composition. However, indirect effects trough changes in the community structure of zooplankton could also be related to the new occurrence of smaller diatom species, as we will discuss later.
Modifications in magnitude, size structure and phenology of phytoplankton
In the years 2006 and 2007, except for one phytoplankton growth event in winter 2007 (8 × 106 cells l−1), winter bloom lasted for no longer than 1 month and was significantly lower in magnitude (65–69%) compared to the winter bloom reported in earlier studies (Popovich et al. 2008b and references therein). Together with the unchanged phytoplankton densities, the significantly lower mean chlorophyll concentration in the years 2006 and 2007 suggests a shift within the diatom assemblage, from a dominance of large cells (i.e. Thalassiosira curviseriata, T. hibernalis, T. rotula, T. anguste-lineata, T. pacifica) toward smaller ones (Chaetoceros sp. 1, Cyclotella sp.). In the last decades, increasing evidence for changes in plankton size structure has been reported worldwide in relation to global warming (Daufresne et al. 2009; Morán et al. 2009). Temperature effects on the size structure (i.e. a reduction in the mean size) have been detected in microzooplankton (Molinero et al. 2006), stream fish communities (Daufresne and Boët 2007; Genner et al. 2010) and suggested in pelagic marine copepods (Beaugrand et al. 2003). Other field studies (Gómez and Souissi 2007; Wiltshire et al. 2008; Winder et al. 2008) and empirical investigations (Sommer and Lengfellner 2008) have reported a decrease in the mean cell size of the blooming species with increasing water temperature. In agreement to this, the replacement of large cells during the winter bloom by smaller ones could be related to the enhanced water temperatures trough shifts of the species environmental optimum for growth (e.g. Gebühr et al. 2009) or changes in grazing rate or selectivity of zooplankton (Sommer and Sommer 2006; Sommer and Lengfellner 2008).
Regarding the phytoplankton dynamics during the bloom, our results showed that in the periods 1978–1981 and 1988–1994, the bloom was dominated by a single major bloom between June and August, while in the period 2002–2007, it was composed by several bloom events from May to September. This is consistent with modifications in the periodicity of the main peak of chlorophyll around 1990 that shifted from a 12-month period to a 6-month period (Winder and Cloern 2010). The former pattern was characterized by the phytoplankton biomass accumulation in winter–early spring while the latter showed both phytoplankton peaks, in winter–early spring and in summer. This might be related with the decrease or disappearance of the dominant blooming species and the successive peaks of different species observed in recent years. Particularly noticeable was the marked decrease in Thalassiosira curviseriata, which in the past dominated the winter assemblages forming almost monospecific blooms but in the last years has been almost absent. In addition, as suggested by our results, the winter diatom bloom in the Bahía Blanca Estuary has moved forward ca. 1 month, likely in relation to the observed increase in salinity and temperature. In agreement to this, rising temperatures in both marine and freshwater systems have been directly related to the advancement of phenological events in phyto- and zooplankton (Parmesan and Yohe 2003, Edwards and Richardson 2004).
Potential underlying mechanisms
Environmental changes
The effect of the climate warming on water temperature and salinity has been found more pronounced in shallow and semi-enclosed areas like the head of the Bahía Blanca Estuary (mean depth: 10 m and residence time ca. 28 days), where evaporation is high and river runoff is low (Freije and Marcovecchio 2004). The reduction in precipitation in the last years has reduced the flow of freshwater and consequently has led to an increase in salinity. In particular, the precipitation rate in 2009 has been the third lowest in the last century in the Bahía Blanca region. Temperature is a key parameter affecting physiological rates at the individual level (e.g. enzymatic reactions, respiration, rates of feeding) impacting growth rate, body size and generation time (Peters 1983; Mauchline 1998). Salinity has also important implications in plankton physiology, like on copepod hatching success (Berasategui et al. 2009), germination of resting stages, growth rates and development of phytoplankton blooms in coastal waters (McQuoid 2005; Shikata et al. 2008; Gebühr et al. 2009). As phytoplankton species have different tolerances to variations in salinity and temperature (e.g. Gebühr et al. 2009), changes in these parameters have probably triggered a reorganization of diatom community structure in the estuary. These changes have likely favored the development of the fast-growing opportunistic species, which are able to exploit open niches and may establish as dominant in the system (Cloern and Dufford 2005). A particular example of a significant increase in the population abundance of a diatom due to shifts in its ecological niche is the case of Paralia sulcata at Helgoland Roads in the North Sea (Gebühr et al. 2009). The increased occurrence of this species seems to be related to changes in temperature, light and nutrient conditions, which lead a shift from a specialized to a more generalized niche of P. sulcata. Accordingly, we suggest that the hydrometeorological changes observed in the last decades in the Bahía Blanca Estuary could be in part responsible for the occurrence of the diatoms Cyclotella sp., Chaetoceros sp.1 and Thalassiosira minima. Future experimental analysis of the response of these species to environmental parameters in mono- and multicultures will be performed to determine their ecological niches. A detailed study of the species-specific interactions has to be considered to allow deeper insights into the intrinsic properties of the community.
Dissolved nutrient concentrations
The assessment of the dynamics of dissolved nutrient concentrations in the Bahía Blanca Estuary did not reveal any change in the general annual pattern (Freije and Marcovecchio 2004, Popovich and Marcovecchio 2008, Popovich et al. 2008a). This suggests that the long-term changes in the phytoplankton community structure were not related to modifications in nutrients supply. Support to this was also given by the absence of any shift in taxonomic groups (diatoms toward non-diatoms species), as it was observed in other systems where significant changes in nutrient ratios have been reported (Cloern 2001). Instead, in the Bahía Blanca Estuary, a dominance of diatoms was observed throughout the whole period (1978–2008). On the other hand, taxonomic group species have different nutrient concentration/ratios requirements (Sommer 1993) which may explain modifications in species dominance.
Zooplankton pressure
Concurrent with the hydrological changes and the potential shift in nutrient ratio, trophodynamics interactions could also been affected in the estuary leading to a reorganization of the phytoplankton community composition. Growth and grazing of consumers are strongly influenced by thermal conditions. Aberle et al. (2007) showed that an increase in the winter temperature produces accelerated growth and large ciliate biomass, altering the specific composition and creating an asynchrony between the components of the plankton. Previous studies on trophic groups in the Bahía Blanca Estuary have shown a close correlation between the largest aloricate ciliates and the size fractions of the phytoplankton (Pettigrosso and Popovich 2009). During the winter diatom bloom, long chains of diatom have been observed inside large ciliates, although the magnitude of ciliates predation on phytoplankton has not been quantified (Barría de Cao et al. 2005). The lack of information in regards to the zooplankton pressure on the phytoplankton community of the Bahía Blanca Estuary precludes quantifying the real effect of predation. The mesozooplankton is dominated by the copepod Acartia tonsa most of the year, which peaks in summer (January–March) and shows minimum abundances during the phytoplankton bloom (June–August) (Hoffmeyer 2004). Also, the copepod Eurytemora americana dominates during late winter–spring and feeds almost exclusively on blooming diatoms (Diodato and Hoffmeyer 2008). Studies on the environmental regulation of the key copepods in the estuary, A. tonsa and E. Americana, have revealed a close link between their population dynamics (abundance, eggs production and hatching success) and water temperature and salinity (Berasategui et al. 2009; Hoffmeyer et al. 2009). In addition, modifications in the seasonal pattern of ciliates (Pettigrosso and Barria de Cao 2007) and copepods (Hoffmeyer 2004; Hoffmeyer et al. 2009) in recent years have been documented in the Bahía Blanca Estuary. In a mesocosms study, Sommer and Lengfellner (2008) found higher grazer activities in the warmer mesocosms due to enhanced metabolic demand of copepods at higher temperatures, which could explain both the decreased phytoplankton biomass during the spring bloom and the shift toward smaller phytoplankton at higher temperatures. This was also consistent with the finding that copepods feed preferentially on phytoplankton >500–1,000 μm3 cell volume (Sommer and Stibor 2002; Sommer and Sommer 2006), while exerting less grazing pressure on smaller ones. Based on these observations and our results, we suggest two indirect mechanisms to explain the potential temperature-dependent shifts in phytoplankton size structure in the Bahía Blanca Estuary. On the one hand, the new dominance of small cells has likely affected the capture efficiency of copepods leading to a larger magnitude in the bloom of such species. On the other hand, potential shift in grazing selectivity toward predation of large cells could have eventually triggered the dominance of the smaller ones. Further studies on zooplankton dynamics should be considered in order to disentangle potential match–mismatch situations or a top–down control of the phytoplankton community structure.
Underwater light conditions
Changes in the underwater light conditions due to possible modifications in turbidity could also be responsible for the shifts observed in the phytoplankton dynamics in recent years. Experimental studies of blooming species (e.g. Thalassiosira curviseriata) isolated from the Bahía Blanca Estuary showed that the phytoplankton community in the inner zone of the estuary was adapted to growth at relatively low light intensities i.e. growth became inhibited at ~150 μEm−2 s−1 under laboratory conditions (Popovich and Gayoso 1999). In addition, seasonal variability in water turbidity was not noticed in previous years (Popovich and Marcovecchio 2008) whereas in 2007, the winter phytoplankton bloom (dominated by Chaetoceros sp.1) occurred when water transparency increased as a consequence of significant shifts in wind effect (Guinder et al. 2009). Similarly, in the Narragansett Bay (USA), changes in the phytoplankton annual pattern over the last 50 years (i.e. decrease in the winter–spring bloom and occurrence of relatively short diatom blooms in spring, summer and fall) have been related to warming water especially in winter, cloudiness and a significant decline in the wind speed (Nixon et al. 2009).
Concluding remarks
We stress the importance of investigating the specific composition of phytoplankton assemblages to identify species-specific changes that shape the long-term variations of the community. The changes in phenology and structure of phytoplankton in the Bahía Blanca Estuary are likely related to the noticeable warmer and drier weather conditions during the last decade. However, a thorough assessment of the drivers of the observed long-term changes can only be achieved by investigating dynamics of nutrient ratios, species-specific interactions and grazing pressure. The results presented here are likely indicators of more complex modifications in the pelagic food web of the Bahía Blanca Estuary and may be considered as a baseline for further investigations. They also stress on the importance of maintaining long-term ecological surveys in order to track the ecosystem state of estuaries.
References
Aberle N, Lengfellner K, Sommer U (2007) Spring bloom succession, grazing impact and herbivore selectivity of ciliate communities in response to winter warming. Oecologia 150:668–681
American Public Health Association (APHA) (1998) In: Clesceri LS, Greenberg AE, Easton AD (eds) Standard methods for examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC, USA
Barría de Cao MS, Beigt D, Piccolo C (2005) Temporal variability of diversity and biomass of tintinnids (Ciliophora) in a southwestern Atlantic temperate estuary. J Plankton Res 27(11):1103–1111
Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC (2003) Plankton effect on cod recruitment in the North Sea. Nature (Lond) 426:661–664
Benincà E, Huisman J, Heerkloss R et al (2008) Chaos in a long-term experiment with a plankton community. Nature 451:822–825
Berasategui AA, Hoffmeyer MS, Biancalana F, Fernandez Severini M, Menendez MC (2009) Temporal variation in abundance and fecundity of the invading copepod Eurytemora americana in Bahía Blanca Estuary during an unusual year. Estuar Coast Shelf Sci 85:82–88
Cloern JE (2001) Our evolving conceptual model of the coastal eutrophication problem. Mar Ecol Prog Ser 210:223–253
Cloern JE, Dufford R (2005) Phytoplankton community ecology: principles applied in San Francisco Bay. Mar Ecol Prog Ser 285:11–28
Cloern JE, Jassby AD, Thomson JK, Hieb KA (2007) A cold phase of the East Pacific triggers new phytoplankton blooms in San Francisco Bay. PNAS 104(47):18561–18565
Daufresne M, Böet P (2007) Climate change impacts on structure and diversity of fish communities in rivers. Glob Change Biol 13:2467–2478
Daufresne M, Lengfellner K, Sommer U (2009) Global warming benefits the small in aquatic ecosystems. PNAS 106(31):12788–12793
Diodato SL, Hoffmeyer MS (2008) Contribution of planktonic and detritic fractions to the natural diet of mesozooplankton in Bahía Blanca Estuary. Hydrobiol 614:83–90
Edwards M, Richardson A (2004) Impact of climate change on marine pelagic phenology and trophic mismatch. Nature 430:881–884
Freije RH, Marcovecchio J (2004) Oceanografía química. In: Piccolo MC, Hoffmeyer M (eds) El ecosistema del estuario de Bahía Blanca. Instituto Argentino de Oceanografía, Bahía Blanca, Argentina, pp 69–78
Gayoso AM (1981) Estudio de las diatomeas del estuario de Bahía Blanca. Doctoral Dissertation, Universidad nacional de La Plata, Argentina, p 100
Gayoso AM (1988) Seasonal variations of the phytoplankton from the most inner part of Bahía Blanca Estuary (Buenos Aires province, Argentine). Gayana Bot 45:241–247
Gayoso AM (1989) Species of the Diatom Genus Thalassiosira from the coastal Zone of the South Atlantic (Argentina). Bot Mar 32:331–337
Gayoso AM (1998) Long-term phytoplankton studies in the Bahía Blanca Estuary, Argentina. ICES J Mar Sci 55:655–660
Gayoso AM (1999) Seasonal succession patterns of phytoplankton in the Bahía Blanca Estuary (Argentina). Bot Mar 42:367–375
Gebühr C, Wiltshire KH, Aberle N, van Beusekom JEE, Gerdts G (2009) Influence of nutrients, temperature, light and salinity on the occurrence of Paralia sulcata at Helgoland Roads, North Sea. Aquat Biol 7:185–197
Genner MJ, Sims DW, Southward AJ et al (2010) Body size-dependent responses of a marine fish assemblage to climate change and fishing over a century-long scale. Glob Change Biol 16:517–527
Gomez F, Souissi S (2007) Unusual diatoms linked to climatic events in the northeastern English channel. J Sea Res 58:283–290
Guinder VA, Popovich CA, Perillo GME (2009) Particulate suspended matter concentrations in the Bahía Blanca Estuary, Argentina: implication for the development of phytoplankton blooms. Estuar Coast Shelf Sci 85:157–165
Hays GC, Richardson AJ, Robinson C (2005) Climate change and marine plankton. Trends Ecol Evol 20:337–344
Hoffmeyer MS (2004) Decadal change in zooplankton seasonal succession in the Bahía Blanca Estuary, Argentina, following introduction of two zooplankton species. J Plankton Res 26:181–189
Hoffmeyer MS, Berasategui AA, Beigt D, Piccolo MC (2009) Environmental regulation of the estuarine copepods Acartia tonsa and Eurytemora americana during coexistence period. J Mar Biol Assoc UK 89(2):355–361
Huisman J, Weissing FJ (1999) Biodiversity of plankton by species oscillations and chaos. Nature 402:407–410
Jones MC (1990) The performance of kernel density functions in kernel distribution function estimation. Stat Probabil Lett 9:129–132
Kalnay E, Kanamitsu M, Kistler R, Collins W et al (1996) The NCEP/NCAR 40-year reanalysis project. Bull Am Meteorol Soc 77:437–471
Lange CB, Hasle GR, Syversten EE (1992) Seasonal cycle of diatoms in the Skagerrak, North Atlantic, with emphasis on the period 1980–1990. Sarsia 77:173–187
Legendre L (1990) The significance of microalgae blooms for fisheries and for the export of particulate organic carbon in oceans. J Plankton Res 12(4):681–699
Litchman E, Klausmeier CA, Schofield OM, Falkowski PG (2007) The role of functional traits and trade-offs in structuring phytoplankton communities: scaling from cellular to ecosystem level. Ecol Lett 10:1170–1181
Mauchline J (1998) The biology of calanoid copepods. Academic Press, San Diego
McQuoid M (2005) Influence of salinity on seasonal germination of resting stages and composition of microplankton on the Swedish west coast. Mar Ecol Prog Ser 289:151–163
Melo WD, Limbozzi F (2008) Geomorphology, hidrological systems and land use of Bahía Blanca Estuary reigon. In: Neves R, Baretta J, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Scientific Publishers, Lisboa, Portugal, pp 317–331
Molinero JC, Anneville O, Souissi S, Balvay G, Gerdeaux D (2006) Anthropogenic and climate forcing on the long-term changes of planktonic rotifers in Lake Geneva, Europe. J Plankton Res 28:287–296
Morán XAG, López-Urrutia Á, Calvo-Díaz A, Li WKW (2009) Increasing importance of small phytoplankton in a warmer ocean. Global Change Biol 16:1137–1144
Nixon SW, Fulweiler RW, Buckley BA, Granger SL, Nowicki BL, Henry KM (2009) The impact of changing climate on phenology, productivity and benthic-pelagic coupling in Narragansett Bay. Estuar Coast Shelf Sci 82:1–18
Parmesan C, Yohe G (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature 421:37–42
Perillo GME, Pierini JO, Pérez DE, Gómez EA (2001) Suspended sediment circulation in semienclosed docks Puerto Galván, Argentina. Terra et Aqua 83:1–8
Peters RH (1983) The ecological implications of body size. Cambridge University Press, Cambridge
Pettigrosso RE, Barría de Cao MS (2007) Ciliados planctónicos. In: Piccolo MC, Hoffmeyer M (eds) El ecosistema del estuario de Bahía Blanca. Instituto Argentino de Oceanografía, Bahía Blanca, Argentina, pp 1221–1231
Pettigrosso RE, Popovich CA (2009) Phytoplankton-aloricate ciliate community in the Bahía Blanca Estuary (Argentina): seasonal patterns and trophic groups. Braz J Oceanogr 57(3):215–227
Popovich CA (1997) Autoecología de Thalassiosira curviseriata Takano (Bacillariophyceae) y su importancia en el entendimiento de la floración anual de diatomeas en el estuario de Bahía Blanca (Pcia. Bs. As., Argentina). PhD dissertation, Universidad Nacional del Sur, Bahía Blanca, Argentina
Popovich CA (2004) Fitoplancton. In: Piccolo MC, Hoffmeyer M (eds) El ecosistema del estuario de Bahía Blanca. Instituto Argentino de Oceanografía, Bahía Blanca, Argentina, pp 91–100
Popovich CA, Gayoso AM (1999) Effect of irradiance and temperature on the growth rate of Thalassiosira curviseriata Takano (Bacillariophyceae), a bloom diatom in Bahía Blanca estuary (Argentina). J Plankton Res 21(6):1101–1110
Popovich CA, Marcovecchio JE (2008) Spatial and temporal variability of phytoplankton and environmental factors in a temperate estuary of South America (Atlantic coast, Argentina). Cont Shelf Res 28:236–244
Popovich CA, Spetter CV, Marcovecchio JE, Freije RH (2008a) Dissolved nutrient availability during winter diatom bloom in a turbid and shallow estuary (Bahía Blanca, Argentina). J Coast Res 24:95–102
Popovich CA, Guinder VA, Pettigrosso RE (2008b) Composition and dynamics of phytoplankton and aloricate ciliate communities in the Bahía Blanca Estuary. In: Neves R, Baretta J, Mateus M (eds) Perspectives on integrated coastal zone management in South America. IST Press, Scientific Publishers, Lisboa, Portugal, pp 257–272
Roelke D, Augustine S, Buyukates Y (2003) Fundamental predictability in multispecies competition: the influence of large disturbance. Am Nat 162:615–623
Ross R, Cox EJ, Karayeva NI et al (1979) An amended terminology for the siliceous components of the diatom cell. Nova Hedwigia Beih 64:513–533
Rühland K, Paterson AM, Smol JP (2008) Hemispheric-scale patterns of climate-related shifts in planktonic diatoms from North American and European lakes. Glob Change Biol 14:2740–2754
Shikata T, Nagasoe S, Matsubara T et al (2008) Factors influencing the initiation of blooms of the raphidophyte Heterosigma akashiwo and the diatom Skeletonema costatum in a port in Japan. Limnol Oceanogr 53(6):2503–2518
Smayda TJ (1997) What is a bloom? A commentary. Limnol Oceanogr 42 (5 part 2):1132–1136
Smayda TJ (1998) Patterns of variability characterizing marine phytoplankton, with examples from Narragansett Bay. Ices J Mar Sci 55:562–573
Sommer U (1993) Phytoplankton competition in Pluβsee: a field test of the resource-ratio hypothesis. Limnol Oceanogr 38(4):838–845
Sommer U, Lengfellner K (2008) Climate change and the timing, magnitude, and composition of the phytoplankton spring bloom. Glob Change Biol 14:1199–1208
Sommer U, Sommer F (2006) Cladocerans versus copepods: the cause of contrasting top-down controls on freshwater and marine phytoplankton. Oecologia 147:183–194
Sommer U, Stibor H (2002) Copepoda Tunicata: the role of three major mesozooplankton groups in pelagic food webs. Ecol Res 17:161–174
Thackeray SJ, Jones ID, Maberly SC (2008) Long-term change in the phenology of spring phytoplankton: species–specific responses to nutrient enrichment and climate change. J Ecol 96(3):523–535
Thomas CR (1997) Identifying marine phytoplankton. Academic Press, USA
Tilman D (1982) Resource competition and community structure. Princeton University Press, Princeton, NJ
Wiltshire KH, Manly BFJ (2004) The warming trend at Helgoland Roads, North Sea: phytoplankton response. Helgol Mar Res 58:269–273
Wiltshire KH, Malzahn AM, Wirtz K et al (2008) Resilience of North Sea phytoplankton spring bloom dynamics: An ANALYSIS o long-term data at Helgoland Roads. Limnol Oceanogr 53:1294–1302
Winder M, Cloern JE (2010) The annual cycles of phytoplankton biomass. Philos Trans R Soc B. doi:10.1098/rstb.2010.0125
Winder M, Reuter JE, Schladow SG (2008) Lake warming favours small-sized planktonic diatom species. Proc R Soc B 276:427–443
Acknowledgments
We are grateful to U. Sommer, V. N. de Jonge, G. Plumley and S. Hawkins for their enriching comments and suggestions to improve this manuscript. V.A.G. acknowledges the hospitality and support of the research group of Prof. U. Sommer during her stay at the IfM-GEOMAR funded by the Ministerio de Ciencia, Técnica e Innovación Productiva (MINCYT) and the German Academic Exchange Service (DAAD). We also thank W. Melo for preparing the map of the estuary, and R. Astesuain, A. Astesuain and J. Arlengui for their participation in the field work and the analytical determination of chlorophyll. We thank M. Winder and two anonymous reviewers for providing helpful comments to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Guinder, V.A., Popovich, C.A., Molinero, J.C. et al. Long-term changes in phytoplankton phenology and community structure in the Bahía Blanca Estuary, Argentina. Mar Biol 157, 2703–2716 (2010). https://doi.org/10.1007/s00227-010-1530-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1530-5