Abstract
Globally, many commercial bivalve populations have declined in recent decades. In addition to overharvesting and habitat loss, the increasing frequency and intensity of harmful algal blooms (HABs) are likely to contribute to bivalve losses, particularly in cases where blooms negatively impact larval stages. This paper reports on the lethal effects of clonal cultures and blooms of Cochlodinium polykrikoides from the US Atlantic coast on the larvae of three species of commercially and ecologically valuable bivalves: the Eastern oyster (Crassostrea virginica), the bay scallop (Argopecten irradians), and the Northern quahog (hard clam; Mercenaria mercenaria). Both cultures and blooms of C. polykrikoides were highly toxic to all three species of bivalve larvae causing 80–100% mortality during 24- to 72-h exposures at concentrations of 1–2 × 103 cells ml−1. Toxicity was dependent on cell densities, growth stage of C. polykrikoides (i.e. cultures in exponential stage growth were more toxic than later stages), exposure time of larvae to cells (i.e. longer exposure caused higher mortality), the age of larvae (i.e. younger larvae were more sensitive), and the relative abundance of C. polykrikoides (i.e. the presence of other microalgae decreased toxicity). Free radical-scavenging enzymes (peroxidase and catalase) and the removal of C. polykrikoides cells (i.e. culture filtrate) significantly increased larval survival suggesting toxicity is maximized by contact with live cells and may involve labile toxins bound by these compounds including e.g. reactive oxygen species. The toxicity of C. polykrikoides to bivalve larvae was generally more severe than other HAB species (e.g. Karenia brevis, Karlodinium veneficum, Alexandrium tamarense, Prorocentrum minimum). Since the bivalves in this study spawn in the months when C. polykrikoides blooms on the east coast of North America, these results suggest that these blooms may have detrimental effects on efforts to restore these already diminished populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Benthic suspension feeders, such as bivalves, are important in coastal zones because they provide a variety of ecosystem services through their filtration. Many species are considered “ecosystem engineers” and have considerable commercial value (Reise 2002; Bruno et al. 2003). The filtration provided by bivalves has the potential to control eutrophication (Officer et al. 1982), increase pelagic light penetration (Newell and Koch 2004), and enrich the nutrient content of sediment (Smaal and Prins 1993) with the latter two processes providing benefit to submerged aquatic vegetation (Carroll et al. 2008; Wall et al. 2008). During the past century, many estuarine bivalve populations have suffered tremendous declines due to overharvesting, diseases, and the loss of key habitats (Jackson et al. 2001; Kemp et al. 2005; Lotze et al. 2006). Furthermore, there is growing evidence that harmful algal blooms (HABs) have negative impacts on their populations in ecosystems around the world (Jin et al. 2008) due to their synthesis of toxins and/or high levels of algal biomass (Sunda et al. 2006).
HABs afflict most temperate and tropical coastal nations, and the frequency of HAB events and their negative impacts on fisheries have increased markedly in recent decades (Anderson et al. 2002; Hoagland et al. 2002; Heisler et al. 2008; Jin et al. 2008). HABs can be toxic to bivalves (Shumway 1990; Briceji and Shumway 1998) and more recently, the lethal effects of HABs on bivalve larvae have been documented (e.g. Matsuyama et al. 2001; Yan et al. 2001, 2003; Leverone et al. 2006; Shumway et al. 2006; Stoecker et al. 2008). Larvae represent a critical life stage for bivalve populations as reductions in the metamorphosis and survival of larvae can translate into substantial declines in adult populations (Gosselin and Qian 1997; Schneider et al. 2003; Arnold 2008). Elevated densities of Pfiesteria piscicida can cause rapid and complete mortality in the larvae of Eastern oysters (Crassostrea virginica) and bay scallops (Argopecten irradians; Springer et al. 2002), and Alexandrium tamarense can decrease the survival of the larvae of Japanese scallops (Chlamys farreri; Yan et al. 2001) and bay scallops (Argopecten irradians concentricus; Yan et al. 2003). The brown tide-forming pelagophyte Aureococcus anophagefferens causes reduced survival, growth, and lipid content of Northern quahog larvae (Mercenaria mercenaria; Padilla et al. 2006; Bricelj and MacQuarrie 2007) and can slow the growth of bay scallop larvae (Gallager et al. 1989). Karlodinium veneficum, an unarmoured dinoflagellate, can cause significant declines in survival of early life history stages of the Eastern oyster (Stoecker et al. 2008) and Karenia brevis, another unarmoured dinoflagellate, decreases survival and lengthens the development time of the larvae of Northern quahog, Eastern oyster, and bay scallop (Leverone et al. 2006).
Another dinoflagellate that may negatively impact bivalve larvae is Cochlodinium polykrikoides, which is notorious for causing ichthyotoxic blooms around the world. Blooms of C. polykrikoides reported from Japan, South Korea, the Philippines, Malaysia, Indonesia, and China (Iwataki et al. 2008) have caused hundreds of millions of dollars in fisheries losses (Kim 1998; Kim et al. 1999). In the United States, blooms of Cochlodinium species have become annual events on both the Atlantic and Pacific coasts (Curtiss et al. 2008; Gobler et al. 2008). While the effects of these blooms on fish are well established (Whyte et al. 2001; Gobler et al. 2008; Tang and Gobler 2009), their impacts on bivalve larvae are less clear. A field study using seawater with high abundances of C. polykrikoides (>104 cells ml−1) from the York River, Virginia, USA demonstrated that this water could rapidly kill Eastern oyster larvae (Crassostrea virginica; Ho and Zubkoff 1979). However, the study did not report the relative abundance of C. polykrikoides and other plankton in York River water and did not include experiments with clonal isolates of C. polykrikoides (Ho and Zubkoff 1979), making interpretation of these results difficult. In contrast, Matsuyama et al. (2001) reported that a clonal culture of C. polykrikoides from Japan, which was concentrated by centrifugation (up to 3 × 104 cells ml−1), delayed the metamorphosis of Pacific oyster larvae (Crassostrea gigas), but did not significantly alter their survival. Finally, Jeong et al. (2004) found that among six red tide dinoflagellates, an Asian strain of C. polykrikoides was an optimal prey for the larvae of the mussel Mytilus galloprovincialis. In addition, the difficulty in culturing C. polykrikoides, the variable physiological activity of C. polykrikoides within different growth stages (Tang and Gobler 2009), and the limited availability of robust bivalve larvae may have restricted progress to date in understanding the impacts of C. polykrikoides on bivalve larvae. Clearly, the impacts of C. polykrikoides on bivalve larvae are uncertain and to date, the impacts of North American isolates of C. polykrikoides on larvae have not been investigated.
The goal of this study was to assess the effects of clonal cultures of C. polykrikoides isolated from the US Atlantic coast and estuarine water on the survival of three species of commercially valuable and ecologically important bivalve larvae including the Eastern oyster, Crassostrea virginica; the bay scallop, Argopecten irradians; and the Northern quahog, Mercenaria mercenaria. We also examined the effects of co-occurring phytoplankton, of cell-removal, and of free radical-scavenging enzymes on the impact of C. polykrikoides on larval survival.
Materials and methods
Algal cultures
Culture isolates of Cochlodinium polykrikoides, strains CP1 and CPPB17, were obtained by pipetting single cells into polystyrene cell culture plates containing sterile GSe culture medium under an inverted microscope. The bloom water samples from which the cultures were established were collected on August 31, 2006 from the most western basin of the Peconic Estuary, Flanders Bay (40.923°N, 72.587°W) and September 4, 2008 from Great Peconic Bay (40.936°N, 72.512°W), New York, USA. Molecular and microscopic identification of strain CP1 has been reported previously (Gobler et al. 2008). The C. polykrikoides strain CPCB-10 was isolated from Cotuit Bay, Massachusetts, USA. Identification of both isolates as C. polykrikoides was confirmed with large subunit (LSU) rDNA sequencing (Gobler et al. 2008; Iwataki et al. 2008). Cultured Isochrysis galbana (Tahitian strain; T-Iso) was used as a control since it fosters maximal growth and survival in bivalve larvae (e.g. Padilla et al. 2006). All cultures were maintained in sterile GSe medium with a salinity of 31–32, made with autoclaved and 0.2 μm-filtered seawater (Doblin et al. 1999), at 21° C in an incubator with a 12 h light: 12- h dark photoperiod, illuminated by a bank of fluorescent lights providing a light intensity of ~100 μmol quanta m−2 s−1. For all cultures, an antibiotic–antimycotic solution (a mixture of 10,000 I.U. penicillin, 10,000 μg ml−1 streptomycin, and 25 μg ml−1 amphotericin B; Mediatech. Inc., Hemdon, Virginia) was added into the medium immediately before inoculation, with a final concentration of 1% to minimize contamination by bacteria and fungus. This antibiotic mixture has no negative effects on the growth and survivorship of bivalve larvae (Padilla et al. 2006). Periodic DAPI-staining of cultures during the study indicated the general absence of bacteria in the cultures most of the time.
Bivalve larvae
Eastern oyster larvae (Crassostrea virginica) were provided by Dave Veilleux and Dr. Gary Wikfors of Milford laboratory, NOAA Fisheries Northeast Fisheries Science Center (Connecticut, USA); bay scallop larvae (Argopecten irradians) were obtained from the East Hampton Shellfish Hatchery (New York, USA), and Northern quahog larvae (Mercenaria mercenaria) were obtained from the Cornell Cooperative Extension shellfish hatchery facility in Southold (New York, USA) as well as from the East Hampton Town Shellfish Hatchery (New York, USA). Before experiments, all larvae were maintained in filtered seawater with daily feeding of T-Iso cultures at ~5 × 104 cells ml−1 and mild aeration at ~22°C.
Experiments
All experiments were conducted during June through September of 2008 with sterile, 6-well polystyrene culture plates (n = 6 for each treatment). For each treatment of each experiment, 10-ml experimental cultures of (CP1, CPPB17, or CPCB-10 or bloom water) of Cochlodinium polykrikoides were added to each well. Unless otherwise indicated, all cultures were within exponential stage growth. Approximately, 20 larvae (i.e. ~2 larvae ml−1) were gently transferred into each well with a modified 1.0-ml pipette, yielding a final density consistent with those used for prior experiments investigating the toxicity of harmful algae to bivalve larvae (Leverone et al. 2006; Bricelj and MacQuarrie 2007; Stoecker et al. 2008). At 24 and 72 h (except for the first scallop larva bioassay, which was 10 h), the number of dead larvae in each well was enumerated under an inverted light microscope (total magnification × 40, occasionally × 100) by scanning the entire well. The total number of larvae was determined the same way after all samples were fixed with Lugol’s solution (final concentration 1%) at the end of each experiment (immediately after the final enumeration of dead larvae). A larva was considered dead when swimming and movements of the velum and, when visible, internal organs, ceased. Experiments were conducted using 24-h-old veligers (D-stage) and with older larvae (8–11 days), which were also veligers. The single exception was an experiment conducted with 10-day-old bay scallop larvae, which were a mix of veligers and a small portion of pediveligers. All plates (with covers) were maintained with a salinity of 31–32 at light intensity of 10 μE m−2 s−1 and ~24°C, a temperature yielding maximal growth rates for Crassostrea virginica, Argopecten irradians, and Mercenaria mercenaria larvae (Carriker 2001; Matthiessen 2001; Cragg 2006; Padilla et al. 2006).
For all experiments, a treatment of T-Iso at 1–4 × 104 cells ml−1 was used as the control, densities that yield maximal growth rates for shellfish larvae (Padilla et al. 2006). Dilution series of C. polykrikoides cultures (CP1, CPPB17, and CPCB-10) or bloom water were prepared by diluting treatment water with GSe medium with a salinity similar to culture and bloom water. For several experiments, a parallel series of dilutions was prepared with 1.0 to 4.0 × 104 cells ml−1 of T-Iso (identical density to the control) added into each well with the CP1 and CPCB-10 cultures. For one experiment (24-h Northern quahog larvae), 0.22 μm-filtered CP1 cultures, with and without the addition of T-Iso, were used as treatments to assess the importance of intracellular and extracellular toxicity of C. polykrikoides.
An experiment with Northern quahog larvae was conducted using Cochlodinium polykrikoides bloom water collected from Old Fort Pond, (40.868°N, 72.446°W), a tidal tributary in Eastern Shinnecock Bay, and Great Peconic Bay (40.936°N, 72.512°W), on September 5, 2008, both dominated by C. polykrikoides. Dilutions of bloom water were made with GSe medium as described earlier. Initial concentrations of C. polykrikoides and other microalgae >10 μm diameter were determined by counting cells in 1.0 ml of triplicate bloom water samples added to a Sedgewick-Rafter counting chamber after fixation with Lugol’s solution. Cochlodinium polykrikoides abundances in the bloom water were 1.8 × 103 and 0.8 × 103 cells ml−1 for Shinnecock Bay (Old Fort Pond) and the Peconic Estuary (Great Peconic Bay), respectively, while the abundances of other phytoplankton >10 μm were 1.6 × 103 and 0.2 × 103 cells ml−1, respectively.
Since prior research has demonstrated that reactive oxygen species (ROS)-scavenging enzymes can mitigate or reduce the toxicity of C. polykrikoides to fish (Kim et al. 1999; Tang and Gobler 2009), addition of peroxidase and catalase to CP1 cultures was used as treatment during experiments with bay scallop and Northern quahog larvae. The stock solutions of horseradish peroxidase (MP Biomedicals, LLC., Aurora, Ohio) and catalase (MP Biomedicals, LLC., Solon, Ohio) were added to the cultures of CP1 in a final concentration of 1.25 μg ml−1 for peroxidase and 0.5 U ml−1 for catalase. In the stock solutions of enzymes, horseradish peroxidase was dissolved in 10 mM phosphate buffer, 0.15 M NaCl, pH 7.4, containing 1.0% BSA and 0.1% proclin as a preservative, with a concentration of 2.5 mg ml−1, while catalase was dissolved in an aqueous solution with a concentration of 1,108 U ml−1 (one unit decomposes 1.0 μmole of hydrogen peroxide per minute at pH 7.0, 25°C). We have previously demonstrated that these compounds at the concentrations used here do not negatively affect the growth or viability of C. polykrikoides (Tang and Gobler 2009).
Since the ichthyotoxicity of C. polykrikoides can also be affected by the growth stages of cultures (Tang and Gobler 2009), three batches of CP1 culture in exponential, stationary, and late-stationary growth phases at equal cell densities were used as treatments for a Northern quahog larvae bioassay. The cell densities of CP1, CPCB-10, and C. polykrikoides in bloom water, cell densities of T-Iso, species and ages of larvae, average numbers of larvae in each well, and final concentrations of peroxidase and catalase are summarized in Table 1.
Statistics
All percentage data (percent mortality) were arcsine square root-transformed before ANOVAs were performed. One-, two-, or three-way ANOVAs were performed to assess the effects of the source of C. polykrikoides cells (strain CP1, strain CBCP-10, or bloom water), density of C. polykrikoides cells, the duration of larval exposure to C. polykrikoides cells (24 or 72 h), the addition of other microalgae (T-Iso), the addition of enzymes, and/or culture filtrate on larval survival. Only subsets of these factors were part of each experiment. Differences among treatments were generally assessed with post hoc Holm-Sidak methods for multiple pairwise comparisons with SigmaStat 3.1. When transformations of non-normally distributed data sets were unsuccessful, a Kruskal–Wallis ANOVA on ranks was employed. In all cases, significance levels were set at P < 0.05.
Results
Toxicity of C. polykrikoides to larvae of the Eastern oyster (Crassostrea virginica)
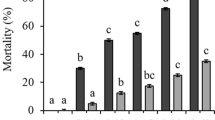
Both strains of C. polykrikoides, CP1 and CPCB-10, caused dramatic mortality of 24-h C. virginica larvae (Fig. 1). In a 24-h exposure, the culture of CP1 (1.56 × 103 cells ml−1) and CPCB-10 (1.64 × 103 cells ml−1) caused 94 and 64% mortality compared to complete survival in control treatments, while lower cell densities also caused elevated (i.e. significantly higher than in the control) mortalities in oyster larvae (Fig. 1). After 72 h of exposure, CPCB-10 caused higher mortality than did the 24-h exposure for all cell densities, as did CP1 at lower cell densities only (0.39–0.78 × 103 cells ml−1; Fig. 1). However, although cell density of C. polykrikoides had a statistically significant treatment effect on oyster larvae survival (Three-way ANOVA, P < 0.001), strains and exposure time did not (Three-way ANOVA, P > 0.05). Even the lowest C. polykrikoides densities (0.16 × 103 cells ml−1) yielded mortality in oyster larvae significantly higher than that in the control (Holm-Sidak post hoc pairwise comparison (termed post hoc comparison onward); P < 0.001; Fig. 1).
Eastern oyster (Crassostrea virginica) larval bioassay for C. polykrikoides strains CP1 and CPCB-10, showing relationship between mortality of larvae and cell density of CP1 and CPCB-10. Larvae were 4-day-old, and the final concentration of Isochrysis galbana (T-Iso) in negative control and treatments was 2.2 × 104 cells ml−1. Error bars indicate SD of 6 replicates
Toxicity of C. polykrikoides cultures to larvae of bay scallops (Argopecten irradians)
Cochlodinium polykrikoides (CP1, 2.58 × 103 cells ml−1) caused 99% mortality in 24-h scallop larvae after a 10-h exposure compared to 8.5% in the control (Fig. 2a). Lower densities of CP1 yielded lower larval mortalities, which were still significantly higher than that of the control (post hoc comparison; P < 0.001; Fig 2a). Eight-day-old larvae exposed to CP1 for 24 h experienced mortalities of 60 to 100% at cell densities from 0.88 to 2.19 × 103 cells ml−1, all of which were significantly higher than mortalities in the control (post hoc comparison; Fig. 2b). The addition of T-Iso to CP1 significantly lessened its toxic effect on 8-days larvae at cell densities >0.88 × 103 cells ml−1 (P < 0.001, two-way ANOVA; Fig. 2b).
Bay scallop (Argopecten irradians) larval bioassays for C. polykrikoides CP1, showing relationship between mortality of larvae and cell density of CP1 and mitigation effect of Isochrysis galbana (T-Iso) on toxicity. a Experiment using larvae of 24 h old. Exposure time of larvae to CP1 culture was 10 h, and cell density of T-Iso control was 4.0 × 104 cells ml−1; b experiment using larvae of 8-day-old and using CP1 culture with and without addition of T-Iso. Exposure time was 24 h. Cell density of T-Iso in control and CP1 culture was 2.2 × 104 cells ml−1. Error bars indicate SD of 6 replicates
Ten-day larvae were more resistant to C. polykrikoides cultures than the younger larvae (Fig. 3). For example, 10-days larvae exposed to 1.7 × 103 CP1 cells ml−1 displayed only 9.6% mortality in 24 h (Fig. 3), whereas 24-h larvae exposed to 1.5 × 103 CP1 cells ml−1 for 10 h experienced 83% mortality (Fig. 1a). In general, the mortality of 10-days larvae was dependent on C. polykrikoides cell densities (P < 0.001; Three-way ANOVA) but did not differ between strains CP1 and CB-CP10 (P > 0.05; Three-way ANOVA). For example, strain CPCB-10 caused larval mortalities of 54–80% at cell densities of 1.07 to 1.78 × 103 cells ml−1 in 72 h, while CP1 caused similar mortalities (50 and 89% at cell densities of 0.87 and 1.74 × 103 cells ml−1; Fig. 3). Finally, exposure time had a significant treatment effect, as 72-h exposure yielded three- to ten-times greater mortalities than those observed at 24 h at densities ≥8.7 × 102 cells ml−1 (P < 0.05; Three-way ANOVA; post hoc comparison).
Toxicity of C. polykrikoides to the larvae of Northern quahog (Mercenaria mercenaria)
One-day-old Northern quahog larvae were exposed to cultures of T-Iso (control), CP1 (with equal level of T-Iso), and C. polykrikoides bloom water from Peconic Bay (PB) and Old Fort Pond (OFP) for 24 h and 72 h (Fig. 4a). The CP1 culture caused mortalities in larvae from 9 to 59% in 24 h and 14 to 84% at 72 h at cell densities from 0.35 to 1.39 × 103 cells ml−1 (Fig. 4a). Bloom water from PB (C. polykrikoides cell density 0.79 × 103 ml−1) caused larval mortalities of 5–23% in 24 h and 27–97% in a 72-h exposure with different dilutions (Fig. 4a), while bloom water from OFP (C. polykrikoides cell density 1.79 × 103 ml−1) was less potent, causing mortalities of only 11% in 24 h and 23% in 72 h (Fig. 4a). Statistically, C. polykrikoides cell densities, the source of C. polykrikoides cells (CP1 culture vs. PB vs. OFP), and exposure time (24 vs. 72 h) all had significant treatment effects (Three-way ANOVA; P < 0.0001 for each; Fig. 4a): mortality of larvae during the 72-h exposure was significantly greater than those exposed for 24 h (post hoc comparison; P < 0.001; Fig. 4a); mortality of larvae exposed to PB bloom water was significantly greater than both other sources of C. polykrikoides cells (post hoc comparison; P < 0.001; Fig. 4a), while mortality of larvae exposed to CP1 was significantly greater than that of OFP bloom water (post hoc comparison; P < 0.001; Fig. 4a). Finally, the mortality of larvae exposed to even the lowest doses of C. polykrikoides cells was significantly greater than those in the T-Iso control (post hoc comparison; P < 0.001; Fig. 4a).
Northern quahog (Mercenaria mercenaria) larval bioassays for C. polykrikoides strain CP1 and C. polykrikoides bloom water collected from Peconic Bay (PB BW) and Old Fort Pond (OFP BW), New York, on September 5, 2008, showing relationship between larval mortality and cell density of C. polykrikoides. a Experiment using 24-h-old larvae exposed to CP1 culture (with addition of T-Iso) and bloom water. Exposure time of larvae to cultures and bloom water was 72 h, and cell density of T-Iso in control and CP1 culture was 1.1 × 104 cells ml−1; b experiment using larvae of 11-day-old and CP1 culture with addition of T-Iso. Exposure time was 72 h and cell density of T-Iso in control and CP1 culture was 1.6 × 104 cells ml−1. Error bars indicate SD of 6 replicates
Eleven-day-old Northern quahog larvae were also quite sensitive to C. polykrikoides (Fig. 4b). After a 24-h exposure, CP1 cultures at cell densities of 0.21 to 0.85 × 103 cells ml−1 caused larval mortalities from 31 to 71% (Fig. 4b). After 72 h of exposure, the mortalities increased but not significantly (Two-way ANOVA; P > 0.05; Fig. 4b). For this experiment, the density of C. polykrikoides cells had a significant treatment effect (Two-way ANOVA; P < 0.001). All densities of C. polykrikoides >0.42 × 103 cells ml−1 yielded larval mortality exceeding the control (post hoc comparison; P < 0.001; Fig. 4b).
Cochlodinium polykrikoides strain CPCB-10 was also highly toxic to Northern quahog larvae. For example, 0.40 × 103C. polykrikoides cells ml−1 killed 17% of 24-h larvae in 24 h and 20% in 72 h, while the corresponding mortalities of larvae exposed to the CPCB-10 culture with addition of T-Iso were 11% in 24 h and 24% in 72 h (Table 2). In contrast, control treatments displayed complete survival during this experiment (Table 2). While the addition of T-Iso to cultures did not alter the survival of larvae exposed to CPCB-10, mortalities of the larvae were significantly higher in both treatments with CPCB-10 compared to the control (post hoc comparison; P < 0.001; Table 2), and mortalities were significantly higher after 72 h compared to the 24-h exposure (post hoc comparison; P < 0.001; Table 2). Older larvae (11 days) were similarly susceptible to CPCB-10 as cell densities of 0.86 × 103 cells ml−1 caused 59 and 54% mortalities without and with the addition of T-Iso after a 24-h exposure and significantly higher mortalities (97 and 80%) after the 72-h exposure (post hoc comparison; P < 0.001; Table 2). The mortality of the 11-days larvae was significantly higher in all treatments with C. polykrikoides CPCB-10 compared to the control (post hoc comparison; P < 0.001; Table 2). Finally, C. polykrikoides strain CPPB17 caused significantly greater mortality in 24-h Northern quahog larvae compared to control treatments (post hoc comparison; P < 0.001; Table 2).
Effects of growth stage and enzymes on toxicity of C. polykrikoides to bivalve larvae
Cochlodinium polykrikoides cultures in exponential growth were significantly more toxic to the 24-h Northern quahog larvae after a 72-h exposure (96% mortality) than late exponential (39% mortality) and stationary stages (31% mortality; post hoc comparison; P < 0.001; Fig. 5), while the later two showed similar toxicity (post hoc comparison; P > 0.05). Additions of the enzymes peroxidase (final concentration 1.25 μg ml−1) and catalase (final concentration 0.5 U ml−1) to the culture of CP1 with a cell density of 1.74 × 103 cells ml−1 significantly reduced the mortality of 10-day bay scallop larvae from 85 to 27 and 61%, respectively, during a 72-h exposure (post hoc comparison, P < 0.001; Fig. 6a). The addition of peroxidase was more effective in mitigating the toxicity of CP1 to scallop larvae than catalase (post hoc comparison; P < 0.05). Peroxidase (1.25 μg ml−1) and catalase (0.5 U ml−1) also significantly reduced the mortality of 24-h Northern quahog larvae in 24 and 72-h exposures to CP1 (Two-way ANOVA, post hoc comparison, P < 0.001; Fig. 6b), with a more significant mitigation effect observed during the 24-h exposure compared to the 72-h exposure (Two-way ANOVA, post hoc comparison, P < 0.001; Fig. 6b). Again, the addition of peroxidase was more effective than catalase in mitigating the toxicity in a 24-h exposure (post hoc comparison, P < 0.01; Fig. 6b). The mortality of Northern quahog larvae in the filtrate of CP1 culture (3 and 6% mortalities in 24 and 72-h exposures, respectively) was nearly identical to that in the T-Iso control (4 and 8% mortalities in 24 and 72-h exposures, respectively; ANOVA post hoc comparison, P > 0.9) and was significantly lower than for the exposure to the whole culture (81 and 96% mortalities in 24 and 72-h exposures, respectively, post hoc comparison, P < 0.001; Fig. 6b).
Northern quahog (Mercenaria mercenaria) larval bioassays for C. polykrikoides strain CP1, showing effect of growth stage on toxicity of CP1 culture to 24-h-old larvae. Exposure time of larvae to cultures was 72 h, and cell density of T-Iso control was 7.95 × 103 cells ml−1. Error bars indicate SD of 6 replicates
a Bay scallop (Argopecten irradians) (10-day-old) and b Northern quahog (Mercenaria mercenaria) (24 h old) larval bioassays for C. polykrikoides strain CP1, showing effect of addition of peroxidase (final concentration 1.25 μg ml−1) and catalase (final concentration 0.5 U ml−1) to the whole culture on toxicity. The T-Iso culture (7.75 × 103 and 7.95 × 103 cells ml−1 for scallop and clam, respectively) and, for clam larvae only, the filtrate of CP1 culture through 0.22-μm mesh (with and without T-Iso) were used as negative controls, while the initial cultures of CP1 were used as positive controls. Error bars indicate SD of 6 replicates
Discussion
This study has explicitly demonstrated the highly toxic activity of Cochlodinium polykrikoides cultures and bloom water from the northeast coast of North America to larvae of three bivalve species: the Eastern oyster, the bay scallop, and the Northern quahog (Crassostrea virginica, Argopecten irradians, and Mercenaria mercenaria). The toxicity of C. polykrikoides cultures (CP1, CPCB-10, and CPPB17) and bloom water (Great Peconic Bay, Shinnecock Bay, New York, USA) to bivalve larvae was dependent on cell densities (both in cultures and in bloom water), growth stage of C. polykrikoides (i.e. cultures in exponential growth were more toxic than that at later stages), exposure time of larvae to cells (i.e. longer exposure caused higher mortality), the age of larvae (i.e. younger larvae were more susceptible), and the relative abundance of C. polykrikoides (i.e. the presence of other microalgae decreased toxicity). The dependence of toxicity on the cell density, growth stage, exposure time, age of larvae, and presence of other microalgae is consistent with previous investigations, which have characterized the toxicity of this species to finfish (Tang and Gobler 2009). The greater toxicity of exponential stage cultures and cultures with higher cell abundances described here is also consistent with the findings of Kim et al. (1999) who investigated Asian strains of C. polykrikoides. The greater resistance of older larvae of scallops and Northern quahog to the toxicity of C. polykrikoides may be due to their more calcified shells (Carriker 2001) and/or ingestion of C. polykrikoides cells during incubations (thus reducing the density of toxic cells; Jeong et al. 2004). However, this finding differs from the toxic effect of Karlodinium veneficum to Eastern oyster larvae, which were more sensitive at later stages (Stoecker et al. 2008). Since older larvae consume more prey, the differences in toxicity between C. polykrikoides and K. veneficum to older larvae suggests that K. veneficum toxicity involves an intracellular toxin which is consumed in higher quantities by older individuals (i.e. karlotoxin; Stoecker et al. 2008), whereas C. polykrikoides toxicity does not. The significant reduction in mortalities of bay scallop and Northern quahog larvae by the removal of C. polykrikoides cells (i.e. culture filtrate) or the addition of the enzymes peroxidase and catalase to a CP1 culture was also consistent with previous investigations of juvenile finfish (Tang and Gobler 2009). These findings suggest that a similar toxic mechanism is responsible for killing finfish and bivalve larvae and that toxicity is maximized by close contact between viable cells and victim organisms and may involve highly labile toxins, such as reactive oxygen species which can be scavenged by peroxidase and catalase (Kim et al. 1999; Tang and Gobler 2009). However, further study is warranted to establish the mortality mechanism associated with C. polykrikoides.
Previously, a field study using seawater containing high abundances of C. polykrikoides cells (>104 cells ml−1) demonstrated this bloom water could rapidly kill Eastern oyster larvae (Crassostrea virginica; Ho and Zubkoff 1979). However, the levels of mortality of oyster larvae (~90%) exposed to 104 cells ml−1 were similar to our observations of cultures and some blooms at order of magnitude lower densities. Ho and Zubkoff (1979) suggested that spatial competition was the cause of adverse effects that the bloom water posed to oyster larvae. However, this hypothesis would not account for the mitigation effect of additional microalgae or scavenging enzymes (peroxidase and catalase) on the toxicity of C. polykrikoides cultures, suggesting other factors, such as reactive toxins, contribute toward larval mortality.
Although we did not detect differences in toxicity among different clonal isolates of C. polykrikoides to bivalve larvae, there were differences among different growth stages of cultures and between blooms from different locations. For example, bloom water from the Peconic Estuary with 0.8 × 103 cells ml−1 of C. polykrikoides was significantly more lethal to Northern quahog larvae than bloom water from Old Fort Pond with more than twice the C. polykrikoides density (1.8 × 103 cells ml−1). These differences may be a function of the physiological activity of cells at different growth stages, or mitigation by the presence of other plankton, or both factors. The presence of other microalgae can significantly reduce the toxic effects of C. polykrikoides cultures to finfish (Tang and Gobler 2009) and larvae (present study). In the Peconic Estuary bloom water, there were eight-times fewer non-C. polykrikoides microalgae (0.2 × 103 cells ml−1) present compared to Old Fort Pond (1.6 × 103 cells ml−1), suggesting that other microalgae were more likely to mitigate the toxicity of the Old Fort Pond bloom. Furthermore, the lower toxicity of the Old Fort Pond bloom might also reflect a lower growth rate (or late growth stage) of that population since the cell densities found there (1.8 × 103 cells ml−1) were close to the maximum obtained in culture and thus may have been in a later, and thus less toxic, stage of growth. Altered physiological activity of cells may also account for the low toxicity of Asian C. polykrikoides cultures to larval Pacific oysters (Crassostrea gigas) reported by Matsuyama et al. (2001), where a cell density of 3.0 × 104 cells ml−1 caused only 5% mortality. Although the cell abundances used by Matsuyama et al. (2001) were ~10-times higher than those used in the present study, they were achieved by centrifugation, a process that causes this species to lose viability and toxicity (Tang and Gobler 2009). Since the toxicity of C. polykrikoides can vary widely depending on the viability and physiological status of cultures (Fig 5; Tang and Gobler 2009), cell dosage and median lethal concentration (LC50) concept are not ideal indices of C. polykrikoides toxicity.
The toxic effects of C. polykrikoides on bivalve larvae seem to be more severe than most harmful/toxic dinoflagellates such as Alexandrium tamarense (Yan et al. 2001, 2003), Prorocentrum minimum (Wikfors and Smolowitz 1995; Stoecker et al. 2008), Karlodinium veneficum (Stoecker et al. 2008), Pfiesteriashumwayae (Shumway et al. 2006), and Karenia brevis (Leverone et al. 2006), as compared in Table 3 on a per cell basis. The most toxic strains of Pfiesteria piscicida, however, caused 100% mortality in larval bay scallops (A. irradians) at cell densities of 5 × 103 cells ml−1 after only 1-h exposure (Springer et al. 2002), indicating P. piscicida is more lethal to scallop larvae than C. polykrikoides.
The cell abundances of C. polykrikoides used in our experiments (≤2.2 × 103 cells ml−1) were equivalent to levels found in blooms in US estuaries (Gobler et al. 2008). Since the bivalve species used in our study spawn in months (June to October; Barber and Blake 1983; Hesselman et al. 1989; Thompson et al. 1996; Tettelbach et al. 2002) overlapping with the occurrence of C. polykrikoides blooms on the east coast of North America (July to October; Nuzzi 2004; Gobler et al. 2008), our results suggest that blooms of C. polykrikoides may have severe negative impacts on Eastern oyster, bay scallop, and Northern quahog populations. Since many wild populations of bivalves, including the species studied here, have experienced precipitous declines (Jackson et al. 2001; Kemp et al. 2005; Lotze et al. 2006; Myers et al. 2007), future management and restoration planning should consider the temporal and spatial range of C. polykrikoides and other harmful algal blooms.
References
Anderson DM, Glibert PM, Burkholder JM (2002) Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries 25:562–584
Arnold WS (2008) Application of larval release for restocking and stock enhancement of coastal marine bivalve populations. Rev Fish Sci 16:65–71
Barber BJ, Blake NJ (1983) Growth and reproduction of the bay scallop, Argopecten irradians (Lamarck) at its southern distributional limit. J Exp Mar Biol Ecol 66:247–256
Briceji VM, Shumway SE (1998) Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer kinetics and biotransformation. Rev Fish Sci 6:315–383
Bricelj VM, MacQuarrie SP (2007) Effects of brown tide (Aureococcus anophagefferens) on hard clam Mercenaria mercenaria larvae and implications for benthic recruitment. Mar Ecol Prog Ser 331:147–159
Bruno JF, Stachowicz JJ, Bertness MD (2003) Inclusion of facilitation into ecological theory. Trends Ecol Evol 18:119–125
Carriker MR (2001) Embryogenesis and organogenesis of veligers and early juveniles. In: Kraeuter JN, Castagna M (eds) Biology of the hard clam. Elsevier Science, New York, pp 77–115
Carroll JC, Gobler CJ, Peterson BP (2008) Resource limitation of eelgrass in New York estuaries: light limitation and nutrient stress alleviation by hard clam. Mar Ecol Prog Ser 369:39–50
Cragg SM (2006) Development, physiology, behaviour, and ecology of scallop larvae. In: Shumway SE, Parsons GJ (eds) Scallops: biology, ecology, and aquaculture. Elsevier B. V, Amsterdam, pp 45–122
Curtiss CC, Langlois GW, Busse LB, Mazzillo F, Silver MW (2008) The emergence of Cochlodinium along the California coast (USA). Harmful Algae 7:337–346
Doblin MA, Blackburn SI, Hallegraeff GM (1999) Growth and biomass stimulation of the toxic dinoflagellate Gymnodinium catenatum (Graham) by dissolved organic substances. J Exp Mar Biol Ecol 236:33–47
Gallager SM, Stoecker DK, Bricelj VM (1989) Effects of the brown tide alga on growth, feeding physiology and locomotory behavior of scallop larvae (Argopecten irradians). In: Cosper EM, Bricelj MV, Carpenter EJ (eds) Novel phytoplankton blooms. Springer, Berlin, pp 511–541
Gobler CJ, Berry DL, Anderson OR, Burson A, Koch F, Rodgers BS, Moore LK, Goleski JA, Allam B, Bowser P, Tang YZ, Nuzzi R (2008) Characterization, dynamics, and ecological impacts of harmful Cochlodinium polykrikoides blooms on eastern Long Island, NY, USA. Harmful Algae 7:293–307
Gosselin LA, Qian PY (1997) Juvenile mortality in benthic marine invertebrates. Mar Ecol Prog Ser 146:265–282
Heisler J, Glibert P, Burkholder J, Anderson DM, Cochlan W, Dennison W, Dortch Q, Gobler CJ, Heil C, Humphries E, Lewitus A, Magnien R, Marshall H, Sellner K, Stockwell D, Stoecker D, Suddleson M (2008) Eutrophication and harmful algal blooms: a scientific consensus. Harmful Algae 8:3–13
Hesselman DM, Barber BJ, Blake NJ (1989) The reproductive cycle of adult hard clams, Mercenaria spp. in the Indian River Lagoon, Florida. J Shellfish Res 8:43–49
Ho MS, Zubkoff PL (1979) The effects of a Cochlodinium heterolobatum bloom on the survival and calcium uptake by larvae of the Eastern oyster, Crassostrea virginica. In: Taylor DL, Seliger HH (eds) Toxic dinoflagellate blooms. Elsevier, New York, pp 409–412
Hoagland P, Anderson DM, Kaoru Y, White AW (2002) The economic effects of harmful algal blooms in the United States: estimates, assessment issues, and information needs. Estuaries 25:819–837
Iwataki M, Kawami H, Mizushima K, Mikulski CM, Doucette GJ, Relox JR Jr, Anton A, Fukuyo Y, Matsuoka K (2008) Phylogenetic relationships in the harmful dinoflagellate Cochlodinium polykrikoides (Gymnodiniales, Dinophyceae) inferred from LSU rDNA sequences. Harmful Algae 7:271–277
Jackson JBC, Kirby MX, Berger WH, Bjorndal KA, Botsford LW, Bourque BJ, Bradbury RH, Cooke R, Erlandson J, Estes JA, Hughes TP, Kidwell S, Lange CB, Lenihan HS, Pandolfi JM, Peterson CH, Steneck RS, Tegner MJ, Warner RR (2001) Historical overfishing and the recent collapse of coastal ecosystems. Science 293:629–638
Jeong HJ, Song JY, Lee CH, Kim ST (2004) Feeding by larvae of the mussel Mytilus galloprovincialis on red-tide dinoflagellates. J Shellfish Res 23:185–195
Jin D, Thunberg E, Hoagland P (2008) Economic impact of the 2005 red tide event on commercial shellfish fisheries in New England. Ocean Coast Manag 51:420–429
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303:1–29
Kim HG (1998) Cochlodinium polykrikoides blooms in Korean coastal waters and their mitigation. In: Reguera B, Blanco J, Fernandez ML, Wyatt T (eds) Harmful algae. Xunta de Galicia and intergovernmental oceanographic commission of UNESCO, Spain, pp 227–228
Kim CS, Lee SG, Kim HG, Jung J (1999) Reactive oxygen species as causative agents in the ichthyotoxicity of the red tide dinoflagellate Cochlodinium polykrikoides. J Plankton Res 21:2105–2115
Leverone JR, Blake NJ, Pierce RH, Shumway SE (2006) Effects of the dinoflagellate Karenia brevis on larval development in three species of bivalve mollusc from Florida. Toxicon 48:75–84
Lotze HK, Lenihan HS, Bourque BJ, Bradbury RH, Cooke RG, Kay MC, Kidwell SM, Kirby MX, Peterson CH, Jackson JBC (2006) Depletion, degradation, and recovery potential of estuaries and coastal seas. Science 312:1806–1809
Matsuyama Y, Usuki H, Uchida T, Kotani Y (2001) Effects of harmful algae on the early planktonic larvae of the oyster Crassostrea gigas. In: Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RJ (eds) Proceedings of the ninth international conference of harmful algal blooms, IOC, UNESCO, Paris, pp 411–414
Matthiessen GC (2001) Oyster culture. Blackwell Publishing, Connecticut, pp 47–74
Myers RA, Baum JK, Shepherd TD, Powers SP, Peterson CH (2007) Cascading effects of the loss of apex predatory sharks from a coastal ocean. Science 315:1846–1850
Newell RIE, Koch EW (2004) Modeling seagrass density and distribution in response to changes in turbidity stemming from bivalve filtration and seagrass sediment stabilization. Estuaries 27:793–806
Nuzzi R (2004) Cochlodinium polykrikoides in the Peconic Estuary. Harmful Algae News 27:10–11
Officer CB, Smayda TJ, Mann R (1982) Benthic filter feeding—a natural eutrophication control. Mar Ecol Prog Ser 9:203–210
Padilla DK, Doall MH, Gobler CJ, Hartson A, O’Boyle K (2006) Brown tide alga, Aureococcus anophagefferens, can affect growth but not survivorship of Mercenaria mercenaria larvae. Harmful Algae 5:736–748
Reise K (2002) Sediment mediated species interactions in coastal waters. J Sea Res 48:127–141
Schneider DW, Stoeckel JA, Rehmann CR, Blodgett KD, Sparks RE, Padilla DK (2003) A developmental bottleneck in dispersing larvae: implications for spatial population dynamics. Ecol Lett 6:352–360
Shumway SE (1990) A review of the effects of algal blooms on shellfish and aquaculture. J World Aquac Soc 21:65–104
Shumway SE, Burkholder JM, Springer J (2006) Effects of the estuarine dinoflagellate Pfiesteria shumwayae (Dinophyceae) on survival and grazing activity of several shellfish species. Harmful Algae 5:442–458
Smaal AC, Prins TC (1993) The uptake of organic matter and the release of inorganic nutrients by bivalve suspension feeder beds. In: Dame RF (ed) Bivalve filter feeders in estuarine and coastal ecosystem processes. Springer, Berlin, pp 271–298
Springer J, Shumway SE, Burkholder JM, Glasgow HB (2002) Interactions between two commercially important species of bivalves and the toxic estuarine dinoflagellate, Pfiesteria piscicida. Mar Ecol Prog Ser 245:1–10
Stoecker DK, Adolf JE, Place AR, Glibert PM, Meritt DW (2008) Effects of the dinoflagellates Karlodinium veneficum and Prorocentrum minimum on early life history stages of the Eastern oyster (Crassostrea virginica). Mar Biol 154:81–90
Sunda WG, Graneli E, Gobler CJ (2006) Positive feedback and the development and persistence of ecosystem disruptive algal blooms. J Phycol 42:963–974
Tang YZ, Gobler CJ (2009) Characterization of the toxicity of Cochlodinium polykrikoides isolates from Northeast US estuaries to finfish and shellfish. Harmful Algae 8:454–462
Tettelbach ST, Smith CF, Wenczel P, Decort E (2002) Reproduction of hatchery-reared and transplanted wild bay scallops, Argopecten irradians irradians, relative to natural populations. Aquac Int 10:279–296
Thompson RJ, Newell RIE, Kennedy VS, Mann R (1996) Chapter 9. Reproductive processes and early development. In: Kennedy VS, Newell RIE, Eble AF (eds) The Eastern oyster Crassostrea virginica. Maryland Sea Grant College, College Park, pp 335–370
Wall CC, Peterson BJ, Gobler CJ (2008) Facilitation of seagrass Zostera marina productivity by suspension-feeding bivalves. Mar Ecol Prog Ser 357:165–174
Whyte JNCI, Haigh N, Ginther NG, Keddy LJ (2001) First record of blooms of Cochlodinium sp. (Gymnodiniales, Dinophyceae) causing mortality to aquacultured salmon on the west coast of Canada. Phycologia 40:298–304
Wikfors GH, Smolowitz RM (1995) Experimental and histological studies of 4 life-history stages of the Eastern oyster, Crassostrea virginica, exposed to a cultured strain of the dinoflagellate Prorocentrum minimum. Biol Bull 188:313–328
Yan T, Zhou M, Fu M, Wang Y, Yu R, Li J (2001) Inhibition of egg hatching success and larvae of the scallop, Chlamys farreri, associated with exposure to cells and cell fragments of the dinoflagellate Alexandrium tamarense. Toxicon 39:1239–1244
Yan T, Zhou M, Fu M, Yu R, Wang Y, Li J (2003) Effects of the dinoflagellate Alexandrium tamarense on early development of the scallop Argopecten irradians concentricus. Aquaculture 217:167–178
Acknowledgments
We are thankful for the useful comments of two anonymous reviewers. We acknowledge support from the Suffolk County Department of Health Services, Office of Ecology and the New Tamarind Foundation. We thank Greg Doucette for providing a culture of CPCB-10 and Don Anderson for agreeing to share the culture. We thank Gary Wikfors at Milford laboratory, NOAA Fisheries Northeast Fisheries Science Center, Connecticut, for providing the oyster larvae, Gregg Rivara at the Cornell Cooperative Extension shellfish hatchery facility in Southold, New York for providing the Northern quahog larvae, and N. S. Fisher for supplying the culture of T-Iso. We also thank Alejandra M. Marcoval, Stephanie C. Talmage, and Michael H. Doall for technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Grassle.
Rights and permissions
About this article
Cite this article
Tang, Y.Z., Gobler, C.J. Cochlodinium polykrikoides blooms and clonal isolates from the northwest Atlantic coast cause rapid mortality in larvae of multiple bivalve species. Mar Biol 156, 2601–2611 (2009). https://doi.org/10.1007/s00227-009-1285-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1285-z