Abstract
The transmission of free-living trematode stages is mediated by various environmental factors, of which the presence of ambient organisms within the host space is a potential major determinant. In two laboratory mesocosm experiments, we investigated the influence of four intertidal rocky shore species on transmission success of cercariae of the digenean trematodes Echinostephilla patellae (encysting in the tissue of blue mussels Mytilus edulis) and Parorchis acanthus (encysting on mussel shells). Encystment success of both parasite species was significantly lower in the presence of test organisms when compared to controls. Observations revealed that barnacles Austrominius modestus actively filtered cercariae, whereas the larvae were obstructed by the seaweeds Corallina officinalis and Fucus serratus. Anemones Actinia equina both physically disturbed and consumed cercariae. In a further laboratory experiment, grazing gastropods (Littorina littorea, Patella vulgata, and Gibbula umbilicalis) were found to significantly reduce the numbers of P. acanthus metacercariae in artificially prepared dishes by ingestion of cysts. Our results suggest that non-host organisms may play a key role in regulating the transmission of free-living trematode stages in rocky shore ecosystems, which is especially important with regard to the relative diversity and density of species in these habitats. The findings also emphasize the need to include parasites into marine food webs, since cercariae seem to be consumed by certain organisms to a considerable extent and could possibly represent an important energy source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Parasites form a vital part of biotic communities within marine intertidal ecosystems (Thomas et al. 1997; Mouritsen and Poulin 2002) and may account for a substantial proportion of the overall biomass (Kuris et al. 2008). They have to be considered as an important determinant in ecosystem functioning by directly affecting specific physiological host functions (Huxham et al. 1993; Mouritsen and Jensen 1994; Gorbushin 1997), thus altering host population dynamics (Desclaux et al. 2004; Fredensborg et al. 2005; Thieltges 2006b) and influencing community structure (Sousa 1991; Mouritsen and Poulin 2005). Digenean trematodes are the dominant metazoan parasites of macro-invertebrates inhabiting the littoral zone (Mouritsen and Poulin 2002). The life cycles of these endoparasites generally include the exploitation of different host organisms, which requires the development of free-living infective stages to ensure transmission to the next host. In the final or definitive host, adult stages of the parasite sexually reproduce and shed eggs with the host’s faeces into the water. From the eggs, free-living larval stages, ciliated miracidia, hatch and penetrate the first intermediate host, which is usually a gastropod. Within the first intermediate host, cercariae are produced in great numbers via asexual reproduction. On release, the short-lived larvae actively seek and penetrate a second intermediate host, in which they encyst and develop into metacercariae. In some trematode species, the cercariae encyst in the open on a substrate which forms the diet of the definitive host. Once the secondary host is ingested by the final host, the metacercariae develop into adult trematodes and the life cycle is completed (e.g., Fried 1997). Successful transmission between hosts is crucial for the maintenance of the reproductive cycle and hence parasite survival. Moreover, transmission rates determine infection levels in host organisms, with high parasite burdens having negative impacts on host fitness. Miracidia as well as cercariae represent highly sensitive stages in the parasitic life cycle. Once emerged from the host, they face an unpredictable environment in which they are exposed to diverse environmental factors that may affect their survival and the infection of the next host (Pietrock and Marcogliese 2003; Thieltges et al. 2008c). To understand the mechanisms leading to infection patterns in local host populations as well as the maintenance of complex life cycles in marine systems, knowledge of factors controlling transmission processes is crucial.
In freshwater systems, the regulatory function of a wide range of ambient organisms, including plants, in the transmission of trematode eggs (Kopp and Jokela 2007), miracidia (e.g., Chernin and Perlstein 1971; Upatham and Sturrock 1973; Bunnag et al. 1977), and cercariae (e.g., Oliver-Gonzales 1946; Knight et al. 1970; Christensen et al. 1980; Fernandez et al. 1991; Schotthoefer et al. 2007) has long been recognized and well documented (see Thieltges et al. 2008c for review). In contrast, research in marine intertidal systems has mainly focused on the importance of variable natural abiotic factors (e.g., temperature) as well as anthropogenic activities (e.g., pollution) (Mouritsen 2002; Pietrock and Marcogliese 2003; Poulin 2006; Thieltges and Rick 2006). Although it has been noted that hydroids growing on gastropod shells ingest emerging trematode cercariae (Køie 1975) and that the presence of algae may lower infection levels in marine fish (Bartoli and Boudouresque 1997), the ecological relevance of these particular interspecific interactions awaited detailed study. Only in recent years, the importance of biotic factors in the transmission of free-living endohelminth stages has been highlighted (Morley and Lewis 2004) and scientists have become increasingly aware that the presence of surrounding species within the host space may constitute a major determinant in marine parasite transmission processes. Present results of experimental laboratory and field studies suggest that various marine organisms co-occurring with specific parasite–host associations may limit trematode loads in the target host by preying on or filtering cercariae out of the water column, acting as alternative hosts or decoys (Hopper et al. 2008; Thieltges et al. 2008a, 2009; Kaplan et al. 2009).
Despite recent progress towards a better understanding of the role of biotic factors in the transmission ecology of free-living trematode stages in marine systems, there is still a lack of information regarding particular taxa and functional groups of species. Findings on the importance of ambient organisms are based on a limited number of host–parasite associations and exclusively originate from soft sediment ecosystems (Mouritsen and Poulin 2003; Krakau et al. 2006; Hopper et al. 2008; Thieltges et al. 2008a, 2009; Kaplan et al. 2009). There is no information available in this context for rocky shore habitats. However, species diversity as well as density is particularly high in these systems (Bertness 1999), with trematodes also being abundant (e.g., James 1968; Copeland et al. 1987; Galaktionov and Bustness 1995). Since the magnitude of the dilution effect largely depends on the abundance and diversity of surrounding organisms (Thieltges et al. 2008a, c), potential effects are likely to be pronounced. Furthermore, the composition of hard substrate communities, including parasites, is different to soft bottom ones and previous findings might not hold for rocky shore ecosystems.
In this study, we investigated if various benthic rocky shore organisms co-occurring in the host space affect the transmission of larval stages of two marine philophthalmid digeneans with different modes of transmission. The digenean Echinostephilla patellae utilizes common limpets Patella vulgata as first intermediate and blue mussels Mytilus edulis as second intermediate host (Prinz et al. 2009). The cercariae of Parorchis acanthus, emitted from dogwhelks Nucella lapillus, do not encyst as metacercariae in a secondary host but form a protective cyst in the open (Rees 1937), preferably on the shell of blue mussels (Prinz, personal observation). Various gulls Larus spp. and shorebirds like oystercatchers Haematopus ostralegus may act as final hosts. We conducted two laboratory mesocosm experiments to examine the influence of four non-host species on the encystment success of cercariae of the two trematode species: (1) the beadlet anemone Actinia equina, (2) the Australasian barnacle Austrominius modestus (formerly Elminius modestus), (3) the coral weed Corallina officinalis, and (4) the toothed wrack Fucus serratus. All of the organisms used in the experiments are widely distributed on intertidal hard bottoms of the eastern North Atlantic and naturally occur together with both primary gastropod hosts often in very high densities (Hayward et al. 1996). The specific feature of the life-history of P. acanthus not to penetrate and encyst inside a host but in the open makes not only the cercariae vulnerable to the influence of ambient species, but also the encysted metacercariae, which are directly exposed to the environment. Therefore, we investigated the impact of the grazing activity of three gastropod species (the common periwinkle Littorina littorea, the common limpet P. vulgata, and the topshell Gibbula umbilicalis) on settled metacercariae of P. acanthus in a further laboratory experiment.
Materials and methods
Organisms
Dogwhelks N. lapillus as a source for P. acanthus cercariae were obtained from Grab-all Bay, Crosshaven, Ireland (51°48′N, 8°16′W). Individuals infected with the parasite were isolated in the laboratory by observing release of cercariae when exposing snails to light in seawater at room temperature. Common limpets P. vulgata parasitized by E. patellae were collected from Garrettstown Strand, Ireland (51°38′N, 8°35′W) by identifying infected individuals through visual examination of the snails’ mantle blood vessels, in which cercariae accumulate before release from their host. Infected dogwhelks and limpets were maintained in the laboratory in separate tanks filled with constantly aerated seawater at 15°C and light applied from above, before the start of the experiments. Beadlet anemones A. equina (2.5–3.8 cm base diameter), coral weed C. officinalis (approximately 8 cm in height), and toothed wrack F. serratus (20–30 cm length) for the mesocosms as well as periwinkles L. littorea (19–23 mm shell height), limpets P. vulgata (20–25 mm shell length), and top shells G. umbilicalis (10–12 mm shell height) for the grazing experiment were also collected from Garrettstown Strand. Uninfected blue mussels M. edulis (45–50 mm shell length) used as target hosts for E. patellae and as a substrate for encystment for P. acanthus in the mesocosms were collected from Blackrock, Ireland (53°17′N, 6°09′W), where mussels were found to be free of trematodes by screening 100 individuals for metacercarial infections prior to the experiments. Australasian barnacles A. modestus (approximately 5 mm in base diameter) attached to small rocks were sampled from Monkstown, Ireland (51°51′N, 8°19′W). All organisms were collected during summer 2008 just prior to the conduction of the respective experiments.
Mesocosm experiments
In a first series of experiments, we studied the influence of non-host organisms on the infection success of E. patellae cercariae in blue mussels. In the laboratory, mesocosms were set up in plastic aquaria (30 × 20 × 20 cm) filled with 5 l of seawater and constantly aerated for the duration of the experiment to ensure continuous turbulent mixing of the water column and sufficient oxygen supply. The different treatments consisted of (1) three M. edulis as a control, (2) seven A. equina plus three M. edulis, (3) A. modestus densely attached to small rocks covering the bottom of the aquarium plus three M. edulis, (4) tufts of C. officinalis covering the bottom of the aquarium plus three M. edulis, and (5) two to three fronds with 14–18 branches each of F. serratus filling up the aquarium plus three M. edulis. The densities of non-host organisms in the mesocosms were selected in order to roughly meet the densities found in the field (Prinz, personal observation). The aquaria were placed in a constant temperature room at 15°C under artificial daylight in a completely randomized design. Each treatment was replicated six times. All organisms were kept in the experimental set ups for 24 h prior to the start of the experiments. To obtain E. patellae cercariae from the first intermediate host P. vulgata, isolated limpets were maintained dry in darkness for 1 h before being immersed in seawater under bright light to induce cercarial shedding. To each replicate, 500 E. patellae cercariae were added using a pipette. Cercariae produced by ten limpets were pooled and used in the experiments at a maximum age of 3 h to ensure infectivity. After 3 days, mussels were removed from the aquaria and transferred into fresh, aerated seawater for another 48 h to allow encystment of metacercariae to take place. The complete mussel tissues were subsequently removed from the shell and squashed between pressure glasses. Squashed preparations were screened and metacercariae counted under a stereomicroscope with transmitted light. In a second series of experiments, we investigated the impact of ambient organisms on the encystment success of P. acanthus cercariae on M. edulis. Mesocosms were set up in the same manner as described above, using five mussels as a substrate for encystment. Each treatment was replicated eight times. Since cercarial stages of P. acanthus rapidly encyst when in contact with any solid surface (e.g., the tip of a pipette), two infected primary hosts N. lapillus (26–29 mm shell height) were added to each tank instead of adding cercariae. To limit the variability in total cercarial output between the replicates, the experiment ran for 5 days. Mussels were subsequently removed from the tanks and valves were inspected for encysted metacercariae of P. acanthus under a stereomicroscope. Numbers of cysts per mussel were recorded. In addition to the mesocosms, potential parasite–organism interactions were studied in separate dishes filled with seawater containing the individual test species plus one M. edulis and approximately 100 E. patellae and P. acanthus cercariae, respectively. Observations were carried out under a stereomicroscope.
Grazing experiment
Clear plastic dishes measuring 85 mm in diameter were filled with approximately 200 ml of aerated seawater and experimentally prepared with 200 encysted P. acanthus metacercariae each by adding viable cercariae, awaiting encystment, and removing excess cysts under a stereomicroscope. Dishes were maintained at room temperature at approximately 20°C to allow formation of a biofilm, stimulating the gastropods to graze. After 1 week, the dishes were emptied and refilled with 200 ml of fresh seawater. Three individuals of L. littorea, P. vulgata, and G. umbilicalis, respectively, were subsequently added per dish. One treatment with P. acanthus cysts only served as a control. Each treatment was replicated ten times and constantly aerated for the duration of the experiment. All dishes were placed in a constant temperature room at 15°C in a completely randomized design and observed regularly. After 4 days, gastropods were removed from the dishes and the remaining cysts were counted under a stereomicroscope. Samples of gastropod faeces of each species were transferred to a microscope slide and examined under a light microscope for the presence of metacercariae. In addition to the experiments, individuals of all tested gastropod species were placed in separate dishes filled with aerated seawater and containing P. acanthus cysts, and observed under a stereomicroscope.
Statistical analysis
All statistical analyses were carried out using the statistical package SPSS for Windows and results considered significant at P < 0.05. Tests were preceded by evaluations of assumptions. For the E. patellae mesocosms, infection success (=infection rate) of cercariae was determined (% of added cercariae retrieved as metacercariae in all three M. edulis per replicate). For the P. acanthus mesocosms, mean number of metacercariae per mussel per replicate was calculated. For the grazing experiment, recovery rates were determined (% of cysts counted in the dishes at the end of the experiment). Differences in infection success of E. patellae and numbers of P. acanthus metacercariae in the mesocosms as well as recovery rates in the grazing experiment were analyzed with t-tests (control vs. treatment). Differences in shell length of the added M. edulis between the controls and the treatments in the mesocosm experiments were also tested with t-tests. Data on infection rates of E. patellae cercariae in M. edulis in the mesocosms as well as recovery rates of P. acanthus cysts in the grazing experiment were arcsine-transformed to meet the assumptions of parametric tests. Numbers of metacercariae in the P. acanthus mesocosms were log-transformed prior to the analyses. The rate of reduction of P. acanthus metacercariae due to gastropod grazing activity was calculated by setting the cyst recovery rate of the control to 100% and subsequent subtraction of the observed recovery rates in the different treatments.
Results
Mesocosm experiments
Infection success of E. patellae cercariae as well as numbers of P. acanthus metacercariae was significantly lower in all four treatments with added organisms when compared to controls (Table 1; Figs. 1, 2). No difference in mussel size was detected between the respective treatments and controls (P > 0.05). Observations revealed that E. patellae and P. acanthus cercariae were obstructed by the tested algae C. officinalis and F. serratus, representing a physical barrier and hindering the larvae reaching their target M. edulis. Both barnacles A. modestus and anemones A. equina were observed to ingest cercariae. Whilst barnacles were actively filtering cercariae out of the water column, larvae were trapped on the tentacles of the anemones. Moreover, cercariae were paralyzed and subsequently killed by contact with the scapus of A. equina, where they aggregated in considerable numbers.
Infection success (%) of Echinostephilla patellae in Mytilus edulis (mean + SE) in the mesocosm experiments (control and four different treatments containing the test organisms Actinia equina, Austrominius modestus, Corallina officinalis, and Fucus serratus; n = 6) with 500 added cercariae and three mussels per replicate
Number of encysted metacercariae of Parorchis acanthus per Mytilus edulis (mean + SE) in the mesocosm experiments (control and four different treatments containing the test organisms Actinia equina, Austrominius modestus, Corallina officinalis, and Fucus serratus; n = 8) with two added primary hosts Nucella lapillus and five mussels per replicate
Grazing experiment
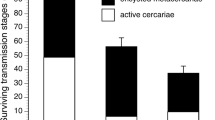
All of the tested gastropod species reduced the number of P. acanthus metacercariae in the dishes (Fig. 3). The cyst recovery rate was 5.9 ± 1.62% (mean ± SE) in the L. littorea treatment, 22.1 ± 3.01% in the P. vulgata treatment, 90.9 ± 1.61% in the dishes containing G. umbilicalis, and 98.2 ± 0.46% in the control. Differences between the gastropod treatments and the control were highly significant (Table 1). Observations of the gastropod grazing activity showed that individuals of all species took in metacercariae. Examination of the gastropods’ faeces revealed cysts in the faecal deposits of all snails, which were mostly still intact with parasites moving inside the cyst in the case of L. littorea and P. vulgata (Fig. 4). The cyst walls of metacercariae recovered in faeces of G. umbilicalis were partly disintegrated and no intact parasite could be found.
Discussion
All of the tested organisms were capable of significantly affecting larval transmission processes in the experimental systems by reducing cercarial encystment success and detaching or destroying metacercarial cysts, respectively.
The nature of the interactions leading to the observed effects varied in the different species. Thieltges et al. (2008c) identified six types of interference mechanisms with the potential to limit parasite transmission success. Two of these mechanisms could be directly observed in the mesocosm experiments in the present study: predation and physical disturbance. Barnacles A. modestus actively filtered and ingested cercarial stages. The relatively large cercariae of E. patellae (740 μm in body length) (Rees 1934) and P. acanthus (1 mm in body length) (Rees 1937) are within the size range of particles consumed by barnacle filter feeders (Barnes 1959; Crisp and Southward 1961), therefore forming a suitable prey item. In addition, cercariae may have also been physically disturbed by the barnacles’ filtering activity causing small-scale water turbulences, resulting in an increased cercarial energy use and thus lower transmission efficiency. Anemones A. equina trapped and ingested cercariae, but also formed a physical barrier limiting cercarial transmission to the mussels. Whilst the impact of barnacles has not been previously described, the regulatory function of anemones in relation to trematode abundance in the target host has been reported from New Zealand, where the mud flat anemone Anthopleura aureoradiata is known to consume trematode cercariae (Mouritsen and Poulin 2003; Hopper et al. 2008). The algae C. officinalis and F. serratus used in the mesocosms physically obstructed cercariae and hence prevented successful encystment in and on M. edulis, respectively. Christensen (1979) observed a similar effect of water plants in the transmission of freshwater schistosome cercariae. In our study, both tested trematodes were negatively affected by the presence of algae, lowering their encystment success. However, the mechanisms and consequences of the observed influence presumably vary in the two parasite species. Cercariae of E. patellae probably use up their limited energy reserves and die in trying to reach the mussel host, when obstructed by seaweeds. In this case, infective stages are irrevocably lost from the infective pool and the parasite’s life cycle cannot be completed. In contrast, contact by algae may trigger encystment of cercarial stages of P. acanthus on a surface less suitable for transmission than the valves of M. edulis, which obviously decreases the probability of successful transmission to the next host but does not render it impossible. The reduction of trematode loads in the secondary host due to the presence of plants in marine systems has only been previously reported by Bartoli and Boudouresque (1997). The authors explained the near-complete absence of digenean trematode infections in labrid fish Symphodus ocellatus by secondary metabolites produced by the algae and emitted into the environment or transmitted along the food web. This phenomenon has also been described for the transmission of free-living endohelminth stages in freshwater systems (Warren and Peters 1968). Toxic exudates synthesized by C. officinalis and F. serratus, negatively affecting the viability of cercariae, might represent a further mechanism of interference effective in the mesocosms, which remains to be elucidated.
In our study, we identified grazing by intertidal gastropods as an additional, previously unknown biotic factor with the potential to negatively affect transmission in marine trematode species whose cercariae encyst in the open. Trematodes with this specific mode of transmission are especially vulnerable, since the cercarial as well as the metacercarial stage is subject to interference by ambient organisms. In addition to the negative effect of grazing on trematode survival, L. littorea and P. vulgata may also serve as paratenic or transport hosts for P. acanthus, since the metacercariae inside the cysts were mostly still alive after having passed the gastropods’ digestive system. The phenomenon of intertidal snails serving as paratenic hosts for trematodes has been reported from various host–parasite systems; trematode metacercariae that were ingested by gastropod predators and scavengers, respectively, feeding on barnacles (McCarthy et al. 1999), cockles (McFarland et al. 2003) and crabs (Latham et al. 2003) remained within the paratenic hosts for several days. They retained their viability after passage through the gastropods’ digestive tract, which was proven by subsequent experimental excystment showing high survival rates. If this also applies to P. acanthus metacercariae, L. littorea and P. vulgata could act as temporary transport hosts, opening up alternative transmission routes to the final bird host, for example, the oystercatcher Haematopus ostralegus which feeds on limpets and periwinkles (Hulscher 1996). The likelihood of G. umbilicalis acting as a transport host for P. acanthus metacercariae can be ruled out, since no intact cysts could be observed in the faeces of the topshell. This might be attributable to the comparatively small size of this gastropod species, not capable of ingesting metacercariae whole, therefore destroying the protective cyst wall enclosing the parasite on feeding.
Our study, showing a dilution effect of different functional groups of species on trematode transmission, supports recent findings on the importance of ambient species in various intertidal host–parasite systems (Hopper et al. 2008; Thieltges et al. 2008a, 2009; Kaplan et al. 2009). To be able to quantify the effect and to compare the relative influence of the interfering species, it would have been necessary to standardize the density or biomass, respectively, of the non-host organisms in the treatments. This should be addressed in future experiments. However, with regard to previous studies, our results indicate that species-specific traits might be of relevance. Hopper et al. (2008) could not detect a significant reduction of metacercarial load of the marine trematode Maritrema novaezealandensis in stalk-eyed mud crabs Macrophthalmus hirtipes in the presence of barnacles Balanus sp. and algae Enteromorpha spp. in laboratory mesocosms. In contrast, they observed that accumulation of metacercariae in the crustacean second intermediate host was lowered when crabs and primary gastropod hosts were kept together in tanks with anemones A. aureoradiata preying on cercariae. It can be assumed that, besides differences in the characteristics of the added organisms, cercarial size and behaviour play an important role concerning the impact of co-occurring species. Previous studies revealed that the amount of larval intake by benthic predators largely depends on the body dimensions and swimming behaviour of the cercariae, and varies considerably in different trematode species (Kaplan et al. 2009; Prinz, personal observation). This stresses the necessity of further studies investigating size-selective feeding preferences of various species in different systems to allow more general conclusions.
The observed reduction of encystment success in the barnacle treatment deserves special attention, since there is increasing evidence that invasive species may play an important role in the transmission of marine trematode larvae, for example, by acting as alternative hosts, decoys or predators of infective stages (Krakau et al. 2006; Thieltges et al. 2009). The Australasian barnacle A. modestus is a highly successful invader, having continuously expanded its range since its introduction and now being widespread in European coastal waters (Crisp 1958; Hayward et al. 1996). Although sessile and not capable of moving inside the water column like mobile benthic crustacean predators of cercariae (e.g., Carcinus maenas and Crangon crangon, Thieltges et al. 2008a), they occur in much higher densities and are very efficient cercarial consumers (this study), being able to exhibit a feeding rate about double that of native intertidal barnacle species (Crisp 1958). Given the potential impact of invasive species on native parasite–host systems (Bartoli and Boudouresque 1997; Kopp and Jokela 2007; Thieltges et al. 2009; this study), it seems worthwhile to further investigate if A. modestus is a particularly efficient cercarial predator possibly outcompeting native filter feeders with major consequences for trematode populations. The observation that marine organisms may consume cercariae to a considerable extent, confirms that cercarial stages may form a potentially important food supply to filter feeders and predators (Thieltges et al. 2008a, b; Kaplan et al. 2009) and emphasizes the necessity to incorporate parasites into marine food webs (Lafferty et al. 2006, 2008; Thieltges et al. 2008b).
Our findings suggest that also in rocky shore environments, organisms within the host space exhibit an important regulatory function in trematode transmission processes, with negative consequences for the parasite by removing infective stages from the system and decreasing trematode population size, and positive effects for the host by lowering parasite burden and mitigating detrimental influences of metacercarial infections (Desclaux et al. 2004; Thieltges 2006a). The results generally support the idea of biodiversity as being of major relevance regarding the potential limitation of negative effects of trematodes on host organisms (Thieltges et al. 2008c). Since the influence of different interference mechanisms is assumed to be additive and of particular relevance to host populations in complex communities exhibiting high abundances (Thieltges et al. 2008a, c), the impact of non-host organisms on trematode transmission may be particularly profound in intertidal rocky shore ecosystems, where species diversity and densities are generally high (Bertness 1999). Furthermore, other biotic factors like the presence of alternative hosts or decoys may add to the dilution effect in these systems and complex interactions between species as well as with abiotic factors are likely to occur. This stresses the need for further studies to assess the significance of ambient organisms in altering trematode transmission processes in rocky shore ecosystems, particularly under natural conditions.
References
Barnes H (1959) Stomach contents and microfeeding of some common cirripedes. Can J Zool 37:231–236
Bartoli P, Boudouresque C-F (1997) Transmission failure of parasites (Digenea) in sites colonized by the recently introduced invasive alga Caulerpa taxifolia. Mar Ecol Prog Ser 154:253–260. doi:10.3354/meps154253
Bertness MD (1999) The ecology of Atlantic shorelines. Sinauer Associates Inc, Sunderland
Bunnag T, Rabelo de Freitas J, Scott HG (1977) The predatory activity of Lebistes reticulatus (Peters, 1859) on Schistosoma mansoni miracidia in laboratory experiments. Trop Geogr Med 29:411–414
Chernin E, Perlstein JM (1971) Protection of snails against miracidia of Schistosoma mansoni by various aquatic invertebrates. J Parasitol 57:217–219. doi:10.2307/3278017
Christensen NO (1979) Schistosoma mansoni: interference with cercarial host-finding by various aquatic organisms. J Helminthol 53:7–14
Christensen NO, Frandsen F, Nansen P (1980) The interaction of some environmental factors influencing Schistosoma mansoni cercarial host-finding. J Helminthol 54:203–205
Copeland MR, Montgomery WI, Hanna REB (1987) Ecology of a digenean infection, Cercaria patellae in Patella vulgata near Portavogie Harbour, Northern Ireland. J Helminthol 61:315–328
Crisp DJ (1958) The spread of Elminius modestus Darwin in north-west Europe. J Mar Biol Assoc UK 37:483–520. doi:10.1017/S0025315400023833
Crisp DJ, Southward AJ (1961) Different types of cirral activity of barnacles. Phil Trans R Soc Lond B 243:271–307. doi:10.1098/rstb.1961.0003
Desclaux C, de Montaudouin X, Bachelet G (2004) Cockle Cerastoderma edule population mortality: role of the digenean parasite Himasthla quissetensis. Mar Ecol Prog Ser 279:141–150. doi:10.3354/meps279141
Fernandez J, Goater TM, Esch GW (1991) Population dynamics of Chaetogaster limnaei limnaei (Oligochaeta) as affected by a trematode parasite in Helisoma anceps (Gastropoda). Am Midl Nat 125:195–205
Fredensborg BL, Mouritsen KN, Poulin R (2005) Impact of trematodes on host survival and population density in the intertidal gastropod Zeacumantus subcarinatus. Mar Ecol Prog Ser 290:109–117. doi:10.3354/meps290109
Fried B (1997) An overview of the biology of trematodes. In: Fried B, Graczyk TK (eds) Advances in trematode biology. CRC Press, Boca Raton, pp 1–30
Galaktionov K, Bustness JO (1995) Species composition and prevalence of seabird trematode larvae in periwinkles at two littoral sites in north-Norway. Sarsia 80:187–191
Gorbushin AM (1997) Field evidence of trematode-induced gigantism in Hydrobia spp. (Gastropoda: Prosobranchia). J Mar Biol Assoc UK 77:785–800. doi:10.1017/S0025315400036195
Hayward P, Nelson-Smith T, Shields C (1996) Seashore of Britain and Europe. Harper Collins Publishers, London
Hopper JV, Poulin R, Thieltges DW (2008) Buffering role of the intertidal anemone Anthopleura aureoradiata in cercarial transmission from snails to crabs. J Exp Mar Biol Ecol 367:153–156. doi:10.1016/j.jembe.2008.09.013
Hulscher JB (1996) Food and feeding behaviour. In: Goss-Custard JD (ed) The oystercatcher: from individuals to populations. Oxford University Press, Oxford, pp 7–29
Huxham M, Raffaelli D, Pike A (1993) The influence of Cryptocotyle lingua (Digenea: Platyhelminthes) infections on the survival and fecundity of Littorina littorea (Gastropoda: Prosobranchia). J Exp Mar Biol Ecol 168:223–238. doi:10.1016/0022-0981(93)90262-M
James BL (1968) The distribution and keys of species in the family Littorinidae and of their digenean parasites, in the region of Dale, Pembrokeshire. Field Stud 2:615–650
Kaplan AT, Rebhal S, Lafferty KD, Kuris AM (2009) Small estuarine fishes feed on large trematode cercariae: lab and field investigations. J Parasitol 95:477–480. doi:10.1645/GE-1737.1
Knight WB, Ritchie LS, Liard F, Chiriboga J (1970) Cercariophagic activity of guppy fish (Lebistes reticulatus) detected by cercariae labeled with radioselenium (75SE). Am J Trop Med Hyg 19:620–625
Køie M (1975) On the morphology and life-history of Opechona bacillaris (Molin, 1859) Looss, 1907 (Trematoda, Lepocreadiidae). Ophelia 13:63–86. doi:10.1007/BF00927847
Kopp K, Jokela J (2007) Resistant invaders can convey benefits to native species. Oikos 116:295–301. doi:10.1111/j.2006.0030-1299.15290.x
Krakau M, Thieltges DW, Reise K (2006) Native parasites adopt introduced bivalves of the North Sea. Biol Invasions 8:919–925. doi:10.1007/s10530-005-4734-8
Kuris AM, Hechinger RF, Shaw JC, Whitney KL, Aguirre-Macedo L, Boch CA, Dobson AP, Dunham EJ, Fredensborg BL, Huspeni TC, Lorda J, Mababa L, Mancini FT, Mora AB, Pickering M, Talhouk NL, Torchin ME, Lafferty KD (2008) Ecosystem energetic implications of parasite and free-living biomass in three estuaries. Nature 454:515–518. doi:10.1038/nature06970
Lafferty KD, Dobson AP, Kuris AM (2006) Parasites dominate food web links. Proc Natl Acad Sci USA 103:11211–11216. doi:10.1038/nature06970
Lafferty KD, Allesina S, Arim M, Briggs CJ, De Leo G, Dobson AP, Dunne JA, Johnson PTJ, Kuris AM, Marcogliese DJ, Martinez ND, Memmott J, Marquet PA, McLaughlin JP, Mordecai EA, Pascual M, Poulin R, Thieltges DW (2008) Parasites in food webs: the ultimate missing links. Ecol Lett 11:533–546. doi:10.1111/j.1461-0248.2008.01174.x
Latham ADM, Fredensborg BL, McFarland LH, Poulin R (2003) A gastropod scavenger serving as paratenic host for larval helminth communities in shore crabs. J Parasitol 89:862–864. doi:10.1645/GE-73R
McCarthy HO, Irwin SWB, Fitzpatrick SM (1999) Nucella lapillus as a paratenic host for Maritrema arenaria. J Helminthol 73:281–282. doi:10.1017/S0022149X99000463
McFarland LH, Mouritsen KN, Poulin R (2003) From first to second and back to first intermediate host: the unusual transmission route of Curtuteria australis (Digenea: Echinostomatidae). J Parasitol 89:625–628. doi:10.1645/0022-3395(2003)089[0625:FFTSAB]2.0.CO;2
Morley NJ, Lewis JW (2004) Free-living endohelminths: the influence of multiple factors. Trends Parasitol 20:114–115. doi:10.1016/j.pt.2003.12.002
Mouritsen KN (2002) The Hydrobia ulvae–Maritrema subdolum association: influence of temperature, salinity, light, water-pressure and secondary host exudates on cercarial emergence and longevity. J Helminthol 76:341–347. doi:10.1079/JOH2002136
Mouritsen KN, Jensen KT (1994) The enigma of gigantism: effect of larval trematodes on growth, fecundity, egestion and locomotion in Hydrobia ulvae (Pennant) (Gastropoda: Prosobranchia). J Exp Mar Biol Ecol 181:53–66. doi:10.1016/0022-0981(94)90103-1
Mouritsen KN, Poulin R (2002) Parasitism, community structure and biodiversity in intertidal ecosystems. Parasitology 124:S101–S117. doi:10.1017}S0031182002001476
Mouritsen KN, Poulin R (2003) The mud flat anemone-cockle association: mutualism in the intertidal zone? Oecologia 135:131–137. doi:10.1007/s00442-003-1183-x
Mouritsen KN, Poulin R (2005) Parasites boost biodiversity and change animal community structure by trait-mediated indirect effects. Oikos 108:344–350. doi:10.1111/j.0030-1299.2005.13507.x
Oliver-Gonzales J (1946) The possible role of the guppy Lebistes reticulatus on the biological control of Schistosomiasis mansoni. Science 104:605. doi:10.1126/science.104.2712.605-b
Pietrock M, Marcogliese DJ (2003) Free-living endohelminth stages: at the mercy of environmental conditions. Trends Parasitol 19:293–299. doi:10.1016/S1471-4922(03)00117-X
Poulin R (2006) Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 132:143–151. doi:10.1017/S0031182005008693
Prinz K, Kelly TC, O’Riordan RM, Culloty SC (2009) Infection of Mytilus edulis by the trematode Echinostephilla patellae (Digenea: Philophthalmidae). J Helminthol (in press)
Rees FG (1934) Cercaria patellae Lebour, 1911, and its effect on the digestive gland and the gonads of Patella vulgata. Proc Zool Soc Lond 45:45–53
Rees FG (1937) The anatomy and encystment of Cercaria purpurae Lebour, 1911. Proc Zool Soc Lond B 107:65–73
Schotthoefer AM, Labak KM, Beasley VR (2007) Ribeiroia ondatrae cercariae are consumed by aquatic invertebrate predators. J Parasitol 93:1240–1243. doi:10.1645/GE1129R.1
Sousa WP (1991) Can models of soft-sediment community structure be complete without parasites? Am Zool 31:821–830. doi:10.1093/icb/31.6.821
Thieltges DW (2006a) Effect of metacercarial trematode infections (Renicola roscovita) on growth in intertidal blue mussels (Mytilus edulis). Mar Ecol Prog Ser 319:129–134. doi:10.3354/meps319129
Thieltges DW (2006b) Parasite induced summer mortality in the cockle Cerastoderma edule by the trematode Gymnophallus choledochus. Hydrobiologia 559:455–461. doi:10.1007/s10750-005-1345-4
Thieltges DW, Rick J (2006) Effect of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Dis Aquat Org 73:63–68. doi:10.3354/dao073063
Thieltges DW, Bordalo MD, Caballero Hernández A, Prinz K, Jensen KT (2008a) Ambient fauna impairs parasite transmission in a marine parasite-host system. Parasitology 135:1111–1116. doi:10.1017/S0031182008004526
Thieltges DW, de Montaudouin X, Fredensborg BL, Jensen KT, Koprivnikar J, Poulin R (2008b) Production of marine trematode cercariae: a potentially overlooked path of energy flow in benthic systems. Mar Ecol Prog Ser 372:147–155. doi:10.3354/meps07703
Thieltges DW, Jensen KT, Poulin R (2008c) The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 135:407–426. doi:10.1017/S0031182007000248
Thieltges DW, Reise K, Prinz K, Jensen KT (2009) Invaders interfere with native parasite–host interactions. Biol Invasions 11:1421–1429. doi:10.1007/s10530-008-9350-y
Thomas F, Cezilly F, de Meeüs T, Crivelli A, Renaud F (1997) Parasitism and ecology of wetlands: a review. Estuaries 20:646–654
Upatham ES, Sturrock RF (1973) Field investigations on the effect of other aquatic animals on the infection of Biomphalaria glabrata by Schistosoma mansoni miracidia. J Parasitol 59:448–453. doi:10.2307/3278770
Warren KS, Peters PA (1968) Cercariae of Schistosoma mansoni and plants: attempt to penetrate Phaseolus vulgaris and Hedychium coronarium produces a cercaricide. Nature 217:647–648. doi:10.1038/217647a0
Acknowledgments
K. Prinz received funding from the Irish Research Council for Science, Engineering and Technology (IRCSET) under the National Development Plan.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by F. Bulleri.
Rights and permissions
About this article
Cite this article
Prinz, K., Kelly, T.C., O’Riordan, R.M. et al. Non-host organisms affect transmission processes in two common trematode parasites of rocky shores. Mar Biol 156, 2303–2311 (2009). https://doi.org/10.1007/s00227-009-1258-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1258-2