Abstract
A benthological survey in the Benguela upwelling area off northern Namibia (located at 17.3°S and water depth ranging between 26 and 117 m) showed the concentration of dissolved oxygen and the accumulation of organic-rich sediments to control macrozoobenthic community patterns. In contrast to highly biodiverse nearshore areas with well-structured shell deposits of the brachiopod Discinisca tenuis (Sowerby 1847), the benthic community in the oxygen minimum zone (OMZ) decreased strongly in species numbers. Nevertheless, a well-established community ranging from 13 to 31 species persisted. Species densities (300–3,350 ind m−2) and biomass (4–109 g afdw/m2) were surprisingly high for areas with near bottom oxygen concentrations from 0.06 to 0.88 ml l−1. In contrast to OMZ’s of other upwelling areas, where the benthic macrofauna is generally dominated by small-bodied polychaetes, off Namibia larger key organisms like the bivalve Nuculana bicuspidata (Gould 1845) and the snail Nassarius vinctus (Marrett 1877) accounted for a large proportion of the macrozoobenthos >1 mm. This is supposed to have a distinct effect on the functional properties of the sediments.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Marine oxygen minimum zones (OMZ thereafter; <0.5 ml l−1) impinge upon the continental margins at depths of 100–1,000 m along much of the Eastern Pacific, Arabian Sea and off West Africa (Levin 2003; Cowie and Levin 2009). Studies at OMZ boundaries, where strong oxygen gradients occur over short distances can demonstrate effects of oxygen availability on the regulation of benthic communities and their influence on nutrient and carbon cycling (Levin et al. 2006). Research conducted by Gutierrez et al. (2000) and Smith et al. (2000) demonstrated the effects of low dissolved oxygen and high input of fresh organic matter on the bioturbation potential of macrofauna, to be one of the major functional processes occurring in OMZ areas. When conditions of dissolved oxygen concentrations are observed to be below 0.15 ml/l, physiological adaptation sets in (Gooday et al. 2009; Hughes et al. 2009), bioturbation may be reduced and chemosynthesis-based nutrition (via heterotrophy and symbiosis) becomes an important process (Levin 2003; Levin et al. 2009). Shelf sediments off the Namibian coast comprise extensive areas of diatomaceous mud, which supports minimal quantities of higher marine life although it contains high concentrations of organic matter and reduced sulphur (Sakko 1998). In the core of the OMZ’s off Namibia, the macrofauna species diversity has been observed to be reduced, whereas higher abundances and biomasses have been observed at the edge of the OMZ (Sanders 1969). Fluctuations of environmental conditions are an inherent feature of this extreme environment, which has been in existence for more than two million years (Shannon 1985). Clearly, species that have persisted under these conditions have evolved mechanisms for coping with the inherent variability of this environment (Sakko 1998).
Whereas several macrozoobenthic investigations from intertidal waters off Southwest Africa have been published (Penrith and Kensley 1970; Kensley and Penrith 1980; McLachlan 1985; Tarr et al. 1985; Donn and Cockroft 1989; Hammond and Griffiths 2006) the benthic communities in subtidal regions have been poorly studied (Sakko 1998). Sanders (1969), was one of the first researchers, who explored macrobenthic community structures (e.g. species number, abundance and diversity) off Walvis Bay impacted by the OMZ. Since then, similar research has been carried out in OMZ regions of the eastern Pacific Ocean and the Arabian Sea (Levin et al. 2000, 2002, 2009; Gallardo et al. 2004; Gooday et al. 2009; Hughes et al. 2009; Palma et al. 2005). But to date, there is still a lack of comprehensive studies, which deal with OMZ-related macrofauna distribution in shelf waters off Namibia or the Benguela upwelling area. Some grab stations at depths between 150 and 201 m, which contained macrofauna, mostly polychaetes (Paraprionospio sp.) were analysed by Gallardo et al. (1998). Some other megafauna species (i.e. Diopatra sp., Anthozoa, Decapoda, Asteroidea, and Holothuroidea) were sampled at one station in a depth of 178 m.
During research cruises conducted off Angola and Namibia in 2004 and 2008 on board of German research vessels Alexander von Humboldt and Maria S. Merian, we investigated the macrozoobenthos distribution in the upper fringe of the Namibian OMZ. The aim of this study was to describe the community patterns in this specific habitat and therewith to complement Sanders’ (1969) results from the deeper areas (102–2,140 m off Walvis Bay). For the first time, the macro-infauna in this oxygen-reduced environment was compared with the highly diverse nearshore sediments. Following the oxygen gradient in bottom water from 26 to 117 m water depth, the change in diversity, abundance and biomass is shown. Our investigation focused on organisms >1 mm with a view on the assessment of functional properties. One major ecosystem-relevant function of soft bottom sediments has been defined as transport across the sediment/water interface and the exchange of matter between sediment and water (Norling et al. 2007). Bioturbation and biopumping activities of larger size classes of macrofauna contribute to an over proportional degree to these fluxes than smaller organisms.
The OMZ off Namibia differs from those beneath other upwelling regions by the close vicinity to the surface productive zone (~50 m). In this area, the supply of fresh organic matter to the sediment is not substantially reduced by water column decomposition. The vicinity to both sea surface and coast may as well affect the physical stability of the bottom water and may foster occasional intrusions of oxygenated water masses due to current or wave action. Under these conditions, we expected differences in composition, biomass and functional properties of the inhabiting macrofauna when compared with deeper and more stabile OMZs in other oceanic regions.
Materials and methods
Study area
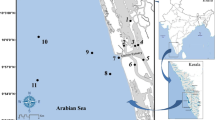
Namibia has a coastline of about 1,500 km extending in a north-south direction (Bianchi et al. 1999). The coastal waters off Namibia are part of the Benguela upwelling, one of the major upwelling systems of the world with large hypoxic and anoxic sublittoral areas, which affect many abundant species (Sakko 1998). Results from recent geological studies on the Namibian mud belt have revealed that the oxygen-reduced environment on the shelf has been sustained for millennia (West et al. 2004). Hydrological and biological patterns (i.e. pelagic fish larvae, zooplankton biomasses) of the Angola-Benguela Frontal Zone were recently described in detail by Postel et al. (2007), Mohrholz et al. (2008) and Bodungen et al. (2008). The sediments are predominantly of biogenic origin as a result of the high productivity in the upwelled waters of the Benguela system. Results presented in this study are from benthic samples and environmental data collected at 19 locations off northern Namibia. Sampling was conducted during March 2008 south of the Cunene, the border river to Angola (Fig. 1). In May 2004, a cruise along the Namibian and Angolan coast on a larger station grid was conducted. Data from this cruise were used for targeting the area studied in 2008.
Sampling
Triplicate benthic samples were taken with a 0.1 m2 van Veen grab at each station. Additional dredge hauls (containing a net mesh size of 5 mm) were taken for collection of larger, mobile or rare species. All samples were sieved through a 1-mm2 screen and animals were preserved on board in 4% buffered formaldehyde. Sorting procedures were conducted at the laboratory with a, stereomicroscope with 10–40× magnification. All macrofauna samples were identified to the lowest taxonomic level whenever possible. The nomenclature was checked following the World Register of Marine Species (WoRMS: http://www.marinespecies.org/index.php). Environmental variables such as salinity, temperature, chlorophyll fluorescence and oxygen concentrations in the water column down to the sediment boundary were recorded by means of a profiling CTD-system (Seabird, USA) with attached oxygen (Seabird) and fluorescence (Dr. Haardt, Germany) sensors and a 13-bottle sampling rosette. Oxygen sensors were calibrated by immediate potentiometric Winkler titration of three samples per watercolumn, including the closest position to the sediment, in the ships laboratory. Chlorophyll was determined fluorometrically following JGOFS-protocolls (UNESCO 1994). An additional sediment sample was taken to extract the upper surface sediment layer (≤20 mm) for analyses of median grain size (laser particle sizer Cilas 1180L), organic matter estimation by weight loss upon ignition (afdw) and direct determination of organic carbon and nitrogen content by means of an Hekatech (Germany) elemental analyzer (Euro EA). Table 1 displays characteristic values for bottom water and sediment variables of stations sampled during this study. At the near shore shell deposits elemental and grain size analyses were not possible due to the coarseness properties of the sediments.
Statistical analyses
For each benthic sample the abundance of species was counted. Following this, the replicate data were averaged to a total abundance per square metre at each station (no pooling).
Statistical methods were employed for the exclusion of species contributing to less than 1% to the species abundance. The initial number of 257 species had to be reduced (Legendre and Gallagher 2001) to exclude outliers species. This was done, successively by employing dominance as filter criteria and creating a final data matrix of 19 dominant species for the 19 stations (Table 2).
Multivariate analysis was conducted by superimposing the results of group averaged hierarchical clustering based on Bray-Curtis similarities of 4th-root transformed abundance data for 19 stations on non-metric multidimensional scaling (MDS) surface. By using this method, benthic assemblages could be assigned to clear clusters. A one-way analysis of variance (ANOVA) (Clarke and Corley 2006) and ANOSIM tests were conducted to assess the significance of this classification. Species responsible for classification were determined applying SIMPER exploratory analysis and visual re-examination of the modified data matrix whereupon benthic assemblages had been determined and described (PRIMER; Clarke and Warwick 1994).
Results
The environmental conditions for macrofauna were variable within the stations over the whole grid (Table 1). The oxygen bottom water concentrations varied between 0.06 and 0.83 ml l−1 over muddier stations. Values exceeding 1 ml l−1 were observed over the coastal bottom deposits. The onset of intense bottom water oxygen deficiency (<0.3 ml O2 l−1) was observed at approximately 80 m depth (Fig. 2). Chlorophyll concentrations in the whole water column were high with means between 5.5 and 7 μg Chl a l−1 in surface intermediate and bottom water. These values were more than 20 times higher than in the adjacent oceanic mixed layer, providing excellent food supply for the whole area. Organic carbon content of surface sediment (means: OM 8.5%, Corg 4% wt. of sediment dry mass) was in the lower range for the Namibian mud belt, for the centre of which values of up to 25% wt. of organic carbon are reported (Bremner 1981). The reason for this difference is rather supposed to be the higher input of mineralic matter from the near-by coast than a difference in the pelagic production regime and a resulting lower supply of organic matter.
At the deeper stations, the variability between the measured environmental variables, especially the sediment characteristics (e.g. organic content and grain size, details are provided in Table 1) was low, showing no clear direction and no significant correlation to macrofaunal densities (Spearman correlation).
Although the taxonomic determination and especially the correct nomenclature are not finally completed for all groups, we are able to present an extensive list of taxa. Especially, the final identification of some rare species will add to the knowledge of faunal biodiversity in this region. Two distinct communities, could be distinguished during the study. On the one hand, the characteristic OMZ community with very abundant gastropod and bivalve species (see later) and otherwise the shell community mainly characterised by the deposits of a brachiopod. Ninety-two species were observed at the deeper, low oxygen stations (Fig. 3a). The most dominating groups were the polychaetes, gastropods and amphipods. Very similar relations were found at the two shallower stations (Fig. 3b). Again the polychaetes, gastropods and amphipods dominated the community. However, the overall species diversity was 2.4 times higher than in the OMZ area. Altogether 257 taxa were observed in near and offshore waters off northern Namibia.
Macrozoobenthos composition in offshore waters off northern Namibia in 2008: a OMZ community including 16 stations with oxygen values between 0.06 and 0.88 ml l−1; b shell community including 2 stations with values between 1.19 and 1.21 ml l−1. The intermediate station BE06 was not considered. For definition of the communities the Bray-Curtis Similarity was used (Fig. 6)
A clear difference was observed between communities living in the OMZ and those situated nearshore in the shell deposits of the brachiopod Discinisca tenuis (Sowerby 1847) (Fig. 4). The first cluster is characterised by relative low species richness, relatively low abundance, high biomass and is mainly dominated by the bivalve Nuculana bicuspidata (Gould 1845) and the gastropod Nassarius vinctus (Marrett 1877). The second cluster is distinctly more diverse (Table 2, Fig. 5) with both high abundance and biomass values.
Between 13 and 31 species (altogether 92 taxa) are forming the “OMZ-community”. Fourteen species show a high frequency and contribute most to the abundance (Table 2). The most dominant species are: N. bicuspidata and N. vinctus the polychaetes Lumbrineris cf. coccinea (Renier 1804), Paraprionospio pinnata (Ehlers 1901) and Sabellaria eupomatoides Augener 1918. The elevated abundances (300–3,350 ind m−2) and high biomass (up to 109 afdw g m−2) are quite surprising for an area where the near bottom water oxygen concentrations range between 0.06 and 0.88 ml/l (Fig. 5).
The “shell community” consists of more than 210 species. Only very few are characteristic and show both high frequenciesFootnote 1 and high abundances. The continuous occurrence of the brachiopod D. tenuis (very often dead shells) is conspicuous. The ophiuroid Ophiolepis cf. affinis is very common in this zone (Table 2). Furthermore, amphipods like Maera hirondellei Chevreux, 1900 and Melita zeylanica Stebbing, 1904 find optimal living conditions in these well-structured beds. The polychaetes Diopatra neapolitana capensis (Day 1960), Lumbrineris cf. coccinea (Renier 1804) and Polydora cf. normalis (Day 1957) settle on the surface or within the interstices of Discinisca shells with high abundances and biomasses (Table 2). The total abundances range between 9,500 and 14,200 ind. m−2 and the biomasses between 52 and 83 afdw g m−2, respectively (Fig. 5, above 1 ml l−1 oxygen).
A similarity dendrogram (Fig. 6), comparing the sites on the basis of abundances of the 19 recorded dominant species, clearly demonstrates that samples from the OMZ and the shell deposits form tight groups. At the 10% similarity level of the cluster analysis, the separation of two groups of sampling stations and at 35% one single site (BE06) was clearly distinctive. Results of hierarchal cluster analysis were supplemented by the MDS ordination (not shown in this publication but available upon request). In order to confirm the significance of the categorisation, a one-way ANOVA was used. The hypothesis of significant difference (p < 0.01) between the groups “OMZ community” and “Shell community” could be corroborated (global R = 1). Particularly, the clustering of the biomasses (not shown in figures) demonstrates both a high level of separation (only 15% similarity) of two well-distinguished communities and the interim position of the location BE06. The 16 remaining stations group at a similarity level of ca. 80%.
Dendrogram showing similarity of 19 sampled stations based on the abundance of 19 dominant species recorded (Table 2). The bottom water oxygen values of the different stations are indicated
The obvious differences between the communities supported by SIMPER analysis demonstrate, which species were responsible for characterising the infauna of the various stations. A complete list of species and their counts (ind m−1) of the five very low oxygen stations (<0.15 ml l−1) of the Namibian OMZ is presented in Table 3. The remarkable distribution of the gastropod N. vinctus and the bivalve N. bicuspidata heavily dominated the OMZ community (Fig. 7). The relative abundance of N. vinctus ranged between 26 and 65% and that of N. bicuspidata between 14 and 64%, respectively. In terms of biomass, these dominances were even more evident (Fig. 7b).
Discussion
This study was performed to increase the scarce database on macrobenthos distribution in the Namibian mud belt region. In contrast to this area, the littoral benthos off Southwestern Africa is much better investigated (McLachlan 1985; Penrith and Kensley 1970; Kensley and Penrith 1980; Tarr et al. 1985; Donn and Cockroft 1989; Hammond and Griffiths 2006). Off Namibia, Sanders (1969) investigated some macro-infaunal community parameters on one transect from the mud belt to the deep sea. Few other studies have dealt with megafauna and the impacts of trawl fishery on benthic biodiversity in the deep sea (Atkinson and Field 2008; Lange 2008; Ramos et al. 2008). The distribution of benthic ostracods in the Benguela upwelling system and its association with particular environmental characteristics was investigated by Dingle (1995) and Mangalo (2004) studied some dominant molluscs in the offshore benthos. The present study examines patterns in composition, abundance and biomass of benthic macro-infaunal communities within the OMZ and the nearshore shell deposits of the brachiopod D. tenuis in the northern Benguela area. The endemic disc-lamp shell D. tenuis occurs in large numbers and dense beds at the low water mark (and sublittoral). Clear formations can be observed by its deposits in the northern Benguela region (Hiller 1990; Sakko 1998). The associated macrobenthic fauna in these highly diverse subtidal waters differed extremely from the OMZ area.
As expected, few macrofaunal species were found in the hypoxic mud belt at depths between 50 and 120 m. However, a small assemblage of highly specialised animals appears to cope physically, physiologically and to some extent in their behaviour with this hostile environment where anoxia and exposure to hydrogen sulphide regularly occurs. Particularly in areas, where bottom water is not totally anoxic/sulphidic, surface-dwelling fauna consisted of an established macrofaunal community. Although existing literature often consents to the hypothesis that both diversity and biomass within the OMZ would be lower than beneath the OMZ, in the present study (similar to Gallardo et al. 2004 from Chilean waters) this was only true for diversity. This study’s macrofaunal densities are lower than those at upwelling areas of Peru and Chile (Levin et al. 2002; Gallardo et al. 2004) but are similar to those found by Palma et al. (2005) at the continental margin of Chile. Contrary to studies from OMZ’s under other upwelling areas (see review by Levin 2003), where generally small-bodied polychaetes dominate, in waters off Namibia two molluscs species reach up to 90% of the abundance and biomass, respectively (Fig. 7). We have to point out that in all these studies sediments were sieved through either 300 or 500 μm meshes, whereas we used a 1 mm2 mesh sieve. So, these findings may be partially biased by the use of coarser sieves, which may have resulted in the loss of soft-bodied small taxa (e.g. polychaetes) during our sieving procedure. Some of the species or genera found in the OMZ of Southwest America were found in the Benguela region too. P. pinnata, Cirratulus sp. and Lumbrineris sp. were frequently observed in Namibian and South American upwelling areas as well (Table 2). The biomass in Namibia is, however, a manifold of that in Chilean waters due to the dominance of mollusc species. Up to 109 g ash-free dry weight per m2 (equates 1.4 kg wet weight and 827 g dry weight, respectively) were measured (Fig. 5d). In comparison, approximately 65 g m−2 wet weight was published by Levin et al. (2002), Gallardo et al. (2004) and Palma et al. (2005) for the OMZ off Chile and Peru although the surface-dwelling gastropod Astyris permodesta (Dall 1890) is present in the Santa Barbara Basin and in a Basin off Peru as well (Bernard et al. 2000; Levin et al. 2003).
Lutjeharms and Meeuwis (1987) partitioned the Benguela system into six upwelling cells based on different surface water conditions. The central upwelling proceeds in the vicinity of Luderitz with declines to the north and south. Our investigation area was situated in the northernmost of these cells (Namib cell). The far northern region of the Namib province coincides with the area of transition between the temperate and the tropical biogeographical provinces (Sakko 1998). The front between the cool waters of the Benguela system and the warm Angolan Current waters oscillates seasonally in the vicinity of the Cunene River mouth (Shannon et al. 1987; Mohrholz et al. 2008). Since the Angola Basin is a primary source of advected oxygen-poor water (Mohrholz et al. 2008), the effects of dysoxia will be greater in the northern cells than in the southern Benguela region, which is more affected by oceanic, oxygen-rich, advected Antarctic Intermediate Water. Upwelling in the Benguela as well shows a clear seasonal cycle with a maximum for the northern Benguela region during late austral winter to spring (Shannon 1985). The bottom water generally becomes hypoxic to anoxic during the late austral summer (sampling time of this study) to autumn when hypoxic, nutrient-rich South Atlantic central water (SACW) from the Angola Gyre is transported into the Benguela Current area, whereas more intense ventilation occurs during late winter to spring when fresher Eastern South Atlantic Central water (ESACW) is drawn onto the shelf (Mohrholz et al. 2008).
The observed hypoxic conditions during our study and the impressive colonisation of the OMZ by the dominating bivalve N. bicuspidata and the gastropod N. vinctus cannot be a short-term and local event. The long-lived species seem to be adapted to the recurrence of nearly anoxic and hypoxic conditions in this region and the sediment properties witness the continuous existence of these conditions over historical time-scales. This view is encouraged by the findings of Mangalo (2004) and our own results from the 2004 expedition. The biomass of the macrofauna community does not decline in the observed oxygen gradient (Fig. 2) between 50 and 120 m water depth (0.88–0.06 ml O2 l−1). This suggests that the Nuculana-Nassarius-community found within the OMZ gets at least a certain discontinuous low supply of oxygen to convert the abundant organic food to biomass. It as well needs adaptational capabilities to overcome times of extremely low oxygen concentrations. These will be related to either an increase or a decrease of bioturbation and/or pumping activities and therefore will affect the functional role of the macrofauna in this area. This community is probably rather representative for the fringes of the upwelling cells of the northern Benguela (Cunene River to Walvis Bay, 17° to 23°S) than for the centre with severe anoxia and high hydrogen sulphide concentrations. These results concord with Bailey (1991), who observed that box core samples taken in the vicinity of Walvis Bay have frequently been devoid of infauna, confirming the semi-permanence of strong anoxia (Gallardo et al. 1998). On the ecological level further studies are necessary to clarify (1) which are the limiting environmental conditions for the distribution of this Nuculana-Nassarius-community (2) Its distribution in terms of water depth and the lateral distribution towards the southern upwelling cells. (3) Its effects on sediment/water exchange especially for nitrate and oxygen, and finally (4) if the shift in benthic diversity patterns observed between the fringe and the centre of the upwelling area is similar to results reported from the OMZ of the Eastern Pacific and the Arabian Sea (Levin 2003).
From our observations at the upper fringe of the Namibian OMZ the generalisation of Levin (2003): “In contrast to the Foraminifera, calcified invertebrates are absent or only weakly calcified where oxygen levels fall below 0.3 m l−1” cannot be supported in view of the high molluscs biomass at the extremely low oxygen concentrations. Our study rather backs Mullins et al. (1985), who under the Californian upwelling found fringes of OMZ’s to be centres of macrofauna abundance and activity. Probably, it is not the absolute environmental conditions that are determining the structure of macrofauna communities in OMZ’s margins but rather the steepness and the stability of the gradients at the fringes. In general, food availability remains a significant determinant of animal abundance and biogenic structure depth for assemblages evolving under permanent severe hypoxia (Levin et al. 2009). However, contrary to Oman and Peruvian conditions the Benguela OMZ starts directly beneath the euphotic zone and extremely fresh material is supplied to the sediment. This promotes high oxygen reduction rates but at the same time offers the possibility for occasional oxygen replenishment by current-, tide- or even wave-induced turbulent processes. Like in many other environmental interfaces it may as well be that the transport dynamics of reactants are of primary importance for the structural and functional properties of the biology in this gradient system.
Notes
Data collected during as part of 2004 cruise, containing additional near shore stations are included in this assessment.
References
Atkinson L, Field J (2008) The impact of the demersal hake trawl fishery on benthic biodiversity in southern Africa. World Conference on Marine Biodiversity in Valencia, 11–15 Nov 2008, Book of Abstracts
Bailey GW (1991) Organic carbon flux and development of oxygen deficiency on the modern Benguela continental shelf south of 22°S: spatial and temporal variability. Geol Soc Spec Publ 58:171–183
Bernard JM, Buck KR, Farmer MA, Bowser SS (2000) The Santa Barbara Basin is a symbiosis oasis. Nature 403:77–80
Bianchi G, Carpenter KE, Roux J-P, Molloy FJ, Boyer D, Boyer HJ (1999) Field guide to the living marine resources of Namibia. FAO, Rome, p 265
von Bodungen B, John H-C, Lutjeharms JRE, Mohrholz V, Veitch J (2008) Hydrographic and biological patterns across the Angola–Benguela Frontal Zone under undisturbed conditions. J Mar Syst 74:189–215
Bremner JM (1981) Sediments on the continental margin off south west Africa between latitudes 17° and 25°S. Mar Geosci Unit Bull 10:233. Department of Geology, University of Cape Town, South Africa
Clarke KR, Corley RN (2006) PRIMER v6: user manual/tutorial PRIMER-E, Plymouth
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Cowie GL, Levin LA (2009) Benthic biological and biogeochemical patterns and processes across an oxygen minimum zone (Pakistan Margin, NE Arabian Sea). Deep-Sea Res II. doi:https://doi.org/10.1016/j.dsr2.2008.10.001
Dingle RV (1995) Continental shelf upwelling and benthic Ostracoda in the Benguela System (southeastern Atlantic Ocean). Mar Geol 122:207–225
Donn TE, Cockroft AC (1989) Macrofaunal community structure and zonation of two sandy beaches on the central Namib coast, South West Africa (Namibia). Madoqua 16:129–135
Gallardo VA, Klingelhoeffer E, Arntz W, Graco M (1998) First report of the bacterium Thioplaca in the Benguela ecosystem off Namibia. J Mar Biol Assoc UK 78:1007–1010
Gallardo VA, Palma M, Carrasco FD, Gutiérrez D, Levin LA, Cañete JI (2004) Macrobenthic zonation caused by the oxygen minimum zone on the shelf and slope off central Chile. Deep-Sea Res II 51:2475–2490
Gooday AJ, Levin LA, Aranda da Silva A, Bett BJ, Cowie GL, Dissard D, Gage JD, Hughes DJ, Jeffreys R, Lamont PA, Larkin KE, Murty SJ, Schumacher S, Whitcraft C, Woulds C (2009) Faunal responses to oxygen gradients on the Pakistan Margin: a comparison of foraminiferans, macrofauna and megafauna. Deep-Sea Res II. doi:https://doi.org/10.1016/j.dsr2.2008.10.003
Gutierrez D, Gallardo V, Mayor S, Neira C, Vasquez C, Sellanes J, Rivas M, Soto A, Carrasco F, Baltazar M (2000) Effects of dissolved oxygen and fresh organic matter on the bioturbation potential of macrofauna in sublittoral sediments off Central Chile during the 1997–1998 El Nino. Mar Ecol Prog Ser 202:81–99
Hammond W, Griffiths C (2006) Biogeographical patterns in the fauna associated with southern African mussel beds. Afr Zool 41:123–130
Hiller N (1990) The southern African recent brachiopod fauna. In: MacKinnon DI, Lee DE, Campbell JD (eds) Brachiopods through time. Balkema, Rotterdam, pp 439–445
Hughes DJ, Levin LA, Lamont PA, Packer M, Feeley K, Gage JD (2009) Macrofaunal communities and sediment structure across the Pakistan margin oxygen minimum zone, North-East Arabian Sea. Deep-Sea Res II. doi:https://doi.org/10.1016/j.dsr2.2008.05.030
Kensley BF, Penrith MJ (1980) The constitution of the intertidal fauna of rocky shores of South West Africa. Part III. The north coast from Cape Frio to the Kunene River. Cimbebasia A 5:201–214
Lange L (2008) Benthic invertebrate biodiversity and biogeography patterns of South Africa. World Conference on Marine Biodiversity in Valencia, 11–15 Nov 2008, Book of Abstracts
Legendre P, Gallagher ED (2001) Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280
Levin LA (2003) Oxygen minimum zone benthos: adaptation and community response to hypoxia. Ocean Mar Biol Annu Rev 41:1–45
Levin LA, Gage JD, Martin C, Lamont PA (2000) Macrobenthic community structure within and beneath the oxygen minimum zone, NW Arabian Sea. Deep-Sea Res II 47:189–226
Levin LA, Gutiérrez D, Rathburn A, Neira C, Sellanes J, Muñoz P, Gallardo V, Salamanca M (2002) Benthic processes on the Peru margin: a transect across the oxygen minimum zone during the 1997–98 El Niño. Prog Oceanogr 53:1–27
Levin LA, Rathburn AE, Gutierrez D, Muñoz P, Shankle A (2003) Bioturbation by symbiont-bearing annelids in near-anoxic sediments: implications for biofacies models and paleo-oxygen assessment. Palaeo 199:129–140
Levin LA, Cowie G, Woulds C, Lamont P, Whitcraft C (2006) Oxygen minimum zone influence on benthos: boundary effects, threshold and bioturbation. Geophys Res Abstr 8:1
Levin LA, Whitcraft CR, Mendoza GF, Gonzalez JP, Cowie G (2009) Oxygen and organic matter thresholds for benthic faunal activity on the Pakistan margin oxygen minimum zone (700–1100 m). Deep-Sea Res II. doi:https://doi.org/10.1016/j.dsr2.2008.05.032
Lutjeharms JRE, Meeuwis JM (1987) The extent and variability of the south-east Atlantic upwelling. S Afr J Mar Sci 5:51–62
Mangalo M (2004) Actuopaläontologische Untersuchungen an Mollusken aus dem Auftriebsgebiet vor Namibia. Diploma Thesis, University of Munich, p 1–77
McLachlan A (1985) The ecology of two sandy beaches near Walvis Bay. Madoqua 14:155–163
Mohrholz V, Bartholomae CH, van der Plas AK, Lass HU (2008) The seasonal variability of the northern Benguela undercurrent and its relation to the oxygen budget on the shelf. Cont Shelf Res 28:424–441
Mullins HT, Thompson JB, McDougall K, VercoutereT L (1985) Oxygen-minimum zone edge effects: evidence from the central California coastal upwelling system. Geology 13(7):491–494
Norling K, Rosenberg R, Hulth S, Grémare A, Bonsdorff E (2007) Importance of functional biodiversity and species-specific traits of benthic fauna for ecosystem functions in marine sediment. Mar Ecol Prog Ser 332:11–23
Palma M, Quiroga E, Gallardo VA, Arntz W, Gerdes D, Schneider W, Hebbeln D (2005) Macrobenthic animal assemblages of the continental margin off Chile (22° to 42°S). J Mar Biol Assoc UK 85:233–245
Penrith MJ, Kensley BF (1970) The constitution of the intertidal fauna of rocky shores of South West Africa. 1. Lüderitzbucht. Cimbebasia A 9:191–239
Postel L, da Silva AJ, Mohrholz V, Lass H-U (2007) Zooplankton biomass variability off Angola and Namibia investigated by a lowered ADCP and net sampling. J Mar Syst 68:143–166
Ramos A, Ramil F, González-Porto M, de Matos-Pita SS, Balguerías E, Hernández E, Salmerón F, García-Isach E, Burgos C, Sánz JL, Tello O, Cristobo J, Faraj A, Mesfoui H, Holtzhausen H, Meiners C (2008) Megabenthos biodiversity in Atlantic continental margins off West Africa. World Conference on Marine Biodiversity in Valencia, 11–15 Nov 2008, Book of Abstracts
Sakko AL (1998) The influence of the Benguela upwelling system on Namibia’s marine biodiversity. Biodivers Conserv 7:419–433
Sanders HL (1969) Benthic marine diversity and the stability-time hypothesis. Brookhaven Symp Biol 22:71–81
Shannon LV (1985) The Benguela ecosystem. Part I. Evolution of the Benguela, physical features and processes. Ocean Mar Biol Annu Rev 23:105–182
Shannon LV, Agenbag JJ, Buys MEL (1987) Large- and meso-scale features of the Angola-Benguela front. S Afr J Mar Sci 5:11–34
Smith CR, Levin LA, Hoover DJ, McMurtry G, Gage JD (2000) Variation in bioturbation across the oxygen minimum zone in the northwest Arabian Sea. Deep-Sea Res II 47:227–257
Tarr JG, Griffiths CL, Bally R (1985) The ecology of three sandy beaches on the Skeleton Coast of South Africa. Madoqua 14:295–304
UNESCO (1994) Protocols for the joint global ocean flux study (JGOFS) core measurements. Intergovernmental Oceanographic Commission and Scientific Committee on Oceanic Research, Bergen, Norway
West S, Jansen JHF, Stuut J-B (2004) Surface water conditions in the Northern Benguela Region (SE Atlantic) during the last 450 ky reconstructed from assemblages of planktonic foraminifera. Mar Micropal 51:321–344
Acknowledgments
We thank Dr. U. Struck (Berlin), Dr. V. Mohrholz (Rostock), U. Hehl (Rostock), M. Römer (Hamburg) and C. Berg (Rostock) for assistance during sampling at RV Maria S. Merian. We are thankful to the team of Physical Oceanography of our Institute (e.g. S. Krüger) for assistance in CTD measurements. We wish also to thank I. Glockzin and A. Hagenmeier (both Rostock) for analysis of benthic samples in the laboratory. Provision of additional taxonomical assistance was provided by W. Massier (Swakopmund), Prof. M. Hollmann (Bochum) and M. Huber (Zürich), we are mostly grateful to their support and expertise. We would like to express our gratitude to Silvana Birchenough (Suffolk) for helping to smooth our English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Zettler, M.L., Bochert, R. & Pollehne, F. Macrozoobenthos diversity in an oxygen minimum zone off northern Namibia. Mar Biol 156, 1949–1961 (2009). https://doi.org/10.1007/s00227-009-1227-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1227-9