Abstract
Muscle tissue was collected for stable isotope analysis (SIA) from the main fish predators and their fish and cephalopod prey from oceanic waters off eastern Australia between 2004 and 2006. SIA of δ15N and δ13C revealed that the species examined could be divided into three main trophic groups. A “top predator” group consisted mainly of large billfish (Xiphias gladius and Tetrapturus audax), yellowfin (Thunnus albacares), bigeye (T. obesus) and southern bluefin (T. maccoyii) tunas and sharks; with mako (Isurus oxyrinchus) the highest. Below this tier was a second group composed of mid-trophic level fishes including albacore tuna (Thunnus alalunga), lancet fish (Alepisaurus ferox), mahi mahi (Coryphaena hippuris) and ommastrephid squid. Underlying both groups was a grouping of small fishes including myctophids, small scombrids and nomeids as well as surface fishes including macrorhamphosids. These groupings were based largely on mean animal size which showed a positive linear relation to δ15N (r2 = 0.58). Some species showed significant ontogenetic variation in either δ15N (swordfish, lancet fish, yellowfin and albacore tuna) or δ13C (mako shark). We also noted a consistent latitudinal change in δ15N and δ13C at ~28°S for the top predator species, particularly albacore and yellowfin tuna. The differences were consistent with a change from oligotrophic Coral Sea to nutrient rich Tasman Sea waters. These differences suggest that predatory fishes may have extended residence time in distinct regions off eastern Australia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
With the general requirement that oceanic fisheries be managed in an ecologically sustainable manner detailed information is needed on the way targeted species link with their prey and with other similar species (Worm and Duffy 2003). Central to this task is an understanding of the main food chain pathways. Stomach contents analysis (SCA) is the most common way for these interactions to be assessed. However, this is a time consuming and labour intensive process which is ultimately limited by the fact that SCA only recognises a single days’ feeding, and may be biased through differential digestion rates (e.g., MacDonald et al. 1982; James 1988). Biochemical techniques such as stable isotope analysis (SIA), which integrate feeding over an extended time period, have proven to be a valuable tool in determining trophic level for a range of predators and their prey (Peterson and Fry 1987). SIA can also provide a broader understanding of food chain links of top predators in pelagic ecosystems, temporally and spatially (Graham et al. 2007; Menard et al. 2007).
The stable isotopes of carbon and nitrogen have been used successfully in many lake and marine trophic studies to elucidate not only trophic positions, but also trophic shifts induced by disturbance and migration (e.g., Best and Schell 1996; Vander Zanden et al. 1999; Davenport and Bax 2002; Caraveo-Patino et al. 2007; Cherel and Hobson 2007; Sara and Sara 2007). The basic premise behind all these studies is that the carbon isotopic signature of predators is enriched relative to their food source by 1‰ or less (DeNiro and Epstein 1978; Fry and Sherr 1984) while nitrogen is enriched by −0.5 to 9‰ (mean ca. 3‰; DeNiro and Epstein 1981). Thus, with little trophic fractionation, carbon isotopic values are useful in establishing primary food sources while the potentially much larger fractionation observed with nitrogen isotopic composition means this isotope is more useful in establishing trophic position. However, results must be interpreted with caution as trophic enrichment is variable, even between individuals of the same species (DeNiro and Epstein 1981) which may well be due to ontogenetic shifts with age and/or size (Young et al. 2006; Sara and Sara 2007) and between tissue types of the same individual (Pinnegar and Polunin 1999; MacNeil et al. 2005, 2006). In addition, baseline signatures can vary both seasonally and spatially (Popp et al. 2007) and these variations will propagate up the food chain.
Davenport and Bax (2002) successfully used stable isotopes to investigate the trophic interactions on the continental shelf off south-eastern Australia and were able to clearly elucidate a trophic structure related to functional patterns in feeding. Studies by Young et al. (2006) and Sara and Sara (2007) utilised stable isotopes to investigate the trophic interactions of higher-order predators and demonstrated that trophic position appeared to be directly related to size for both swordfish and bluefin tuna, respectively. The results from these studies indicated that we may be able to investigate trophic interactions within the Australian Eastern Tuna and Billfish Fishery (ETBF) in a similar manner.
The ETBF is a longline fishery extending along the eastern seaboard of Australia to past the limits of the EEZ. There are now five species targeted by the fishery—swordfish (Xiphias gladius), albacore (Thunnus alalunga), yellowfin tuna (Thunnus albacares), bigeye tuna (Thunnus obesus) and striped marlin (Tetrapturus audax)—as well as a suite of bycatch species. In all, ~200 species have been recorded from the longline catch (CSIRO Marine and Atmospheric Research, unpublished data). It is an Australian Commonwealth fishery and as such is required to be managed with regard to ecosystem sustainability. We, therefore, need to define the overall structure of the ETBF ecosystem, and whether we are dealing with one or a number of interrelated systems. For example, does the presence of the Tasman front effectively separate the fishery into two different regions requiring different management strategies?
The broad objective of this study therefore, was to determine the isotopic signatures of the main fish species and their prey within the ETBF. We aimed to identify the main trophic groups in relation to fish length and point of capture within the region. Second, we aimed to test whether the region could be separated based on the isotopic structure of the constituent species.
Methods
White muscle tissue of predator species were collected at sea on tuna longliners operating off eastern Australia between latitudes 25 and 35°S between 2004 and 2006 (Tables 1, 2; Fig. 1; Young et al. 2006). Details of fish length, and position and date of capture were included with each sample. Squid prey species were collected from predator stomach contents in the laboratory. Fish prey species were collected from mid-water tows taken on research voyages in the area (Young et al. 2009). All samples were frozen at sea and then transported to the laboratory where they were thawed for analysis.
Stable isotopes were determined for predators and their prey following Davenport and Bax (2002). Muscle tissue was usually collected from below the dorsal fin in fish and sections of mantle were collected from cephalopods. Although it is common to remove lipids from samples prior to SIA or to adjust data for lipids (e.g., McConnaughey and McRoy 1979), lipid removal for these samples was deemed to be unwarranted. We wanted our data to be comparable to that of Davenport and Bax (2002) who similarly did not remove lipids due to most Australian fish and crustaceans averaging only about 1% lipid (Nichols et al. 1998), reflecting the generally oligotrophic waters surrounding the continent and white muscle, in particular, is lipid poor (Pinnegar and Polunin 1999). In addition, we randomly selected a subset of samples from the major groups and found no difference in carbon isotope signature between samples prior to or post-lipid removal. Samples were dried at 55°C overnight, before being ground, homogenised and weighed into tin cups for analysis. Samples were analysed using a Carlo Erba NA1500 CNS analyser interfaced via a Conflo II to a Finnigan Mat Delta S isotope ratio mass spectrometer, operating in the continuous flow mode. Combustion and oxidation were achieved at 1,090°C and reduction at 650°C. Samples were analysed at least in duplicate. Results are presented in standard δ notation:
where R = 15N/14N or 13C/12C. The standard for nitrogen is air and for carbon Vienna Pee Dee Bolemnite (VPDB). The reproducibility of the stable isotope measurements was 0.2‰ for carbon and 0.3‰ for nitrogen.
Data analysis
Statistical analysis and visualisation of stable isotope data were carried out using the Statistica software package (Statsoft Inc.) in four steps. Predators and prey were initially grouped using cluster analysis of mean δ13C and δ15N for each family (Ward’s minimum variance method). Second, the relative importance of factors potentially influencing these groupings (season, latitude, longitude, animal length, sea surface temperature and chlorophyll a) were assessed further using a general linear model (GLM). Data were analysed using a non-stepwise approach in order to allow an assessment of the relative significance for each parameter (β). Third, the significance of the relationship between size and δ15N was further investigated by using the method of Jennings et al. (2001). This method utilises a plot of δ15N to log10 (body length) to highlight any significant trends in isotopic signature with increasing body size. Fourth, latitudinal influence on predator isotopic signature was investigated by a moving mean and summed coefficient of variance analysis at each degree of latitude between 24 and 34°S. For the moving mean analysis, at each point of latitude the mean δ15N value was calculated for two groups of predators; those north (all samples caught at a latitude ≥ the target latitude) and those south (all samples caught at a latitude < the target latitude) of that position. The summed coefficient of variance represents the cumulative variance in the data as samples from each latitudinal band are added to the data pool.
Results
A total of 244 predator and 115 prey samples were analysed for δ15N and δ13C (Tables 1, 2; Fig. 1). Mean δ15N values ranged from 10.1 (Alepisaurus ferox) to 15.1‰ (Isurus oxyrinchus); mean δ13C ranged from −14.1 (Carcharhinus falciformis) to −21.2‰ (Ruvettus pretiosus) indicating wide differences in the isotopic signals across a range of pelagic fishes in the study area. Similar differences were noted across a wide range of potential prey species (Table 2).
Within species differences in isotope signature were also noted for both predators and prey. For example, mako shark (I. oxyrinchus) had δ15N values ranging from 9.7 to 18.4‰ (diff. 8.7‰). Within species, differences were also noted for δ13C. For example, yellowfin tuna (T. albacares) exhibited a δ13C range from −24.7 to −16.0‰ (diff. 8.7‰). Of the smaller “prey” species that we sampled δ15N of myctophid fishes ranged from 8.4 to 12.3‰ (diff. 3.9‰) and had a δ13C range of −20.9 to −18.0‰ (diff. 2.9‰) (Table 2). Isotope values of the dominant squid group, family Ommastrephidae, had a δ15N signal ranging from 6.8 to 14.6‰ (diff. 7.8‰) but with a lower degree of variation in carbon, with a δ13C range of −18.2 to −17.0‰ (diff. 1.2‰).
Broad scale food web groupings
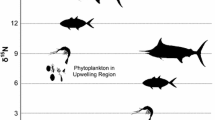
A dual isotope plot for all predators, selected fish and squid families (where n ≥ 5) is shown in Fig. 2. Three distinct groupings were apparent and these could be assigned as mid-level prey, mid-level predators and generalist top predators. Mako sharks (I. oxyrinchus) occupied the highest trophic position slightly above the generalist predators. In addition to the bi-plot, we also performed a cluster analysis on the same data (Fig. 3). The resulting dendrogram showed the same three groupings and also that the mid-level predators and mid-level prey were not as distinct from each other as these two groups were from the top-level predators. In particular, Alepisaurus spp. spanned these two groups as both mid-level prey when juvenile and a mid-level predator as adults.
Biplot of δ15N and δ13C of the main predators and prey off eastern Australia showing three broad trophic levels but also showing a wide variation in carbon across the species. (AL juv. Alepisaurus sp., ALB T. alalunga, ALX adult A. ferox, BET T. obesus, BR Bramidae, BSH P. glauca, DOL C. hippurus, GES G. serpens, LEC L. flavobrunneum, MA M. scolopax, MLS T. audax, MY Myctophidae, OM Omnastrephidae, SBF T. maccoyii, SC Scombridae, SMA I. oxyrinchus, SWO X. gladius, YFT T. albacares). BR and MLS are superimposed
Size relationships
There was a significant relationship between mean length and mean δ15N across all taxa analysed (r2 = 0.7; β = 0.760; SE = 0.162, P < 0.0005; Fig. 4) indicating that the above pattern may be partly explained by the mean size of the particular species. However, there was no similar relationship with δ13C indicating that other factors were important in the observed differences. Multiple linear regression of spatial, temporal and biotic variables confirmed that size was just one of a number of factors influencing isotope concentration.
Mean δ15N for individual species plotted against mean species length (mm) for main predator and prey groupings. (AL juv. Alepisaurus sp., ALB T. alalunga, ALX adult A. ferox, BET T. obesus, BR Bramidae, BSH P. glauca, DOL C. hippurus, GES G. serpens, LEC L. flavobrunneum, MA M. scolopax, MLS T. audax, MY Myctophidae, OM Omnastrephidae, SBF T. maccoyii, SC Scombridae, SMA I. oxyrinchus, SWO X. gladius, YFT T. albacares)
Grouping variables
Multiple regression analysis found that for δ15N the variables species, length, and longitude were significant in explaining 68% of the variability (r2 = 0.69, F = 8.9, P < 0.0001). For δ13C, 60% of the total variability could be explained using the variables species, year, latitude, longitude and length (r2 = 0.59, F = 6.0, P < 0.0001). We further examined the response of “species” to environmental factors using a GLM. No consistent environmental effects were found across the range of species tested, although length was a significant factor for the ‘top predator’, swordfish and albacore species in relation to δ15N (Table 3). Similarly, no overall patterns were detected for δ13C, although area of capture and ‘year” were important for some of the species (Table 4).
Even though the GLM showed no consistent relationship between length and the stable isotope values, an analysis of δ15N versus log10 (length) showed significant size-related effects indicating a trend towards more enriched δ15N values (higher trophic level) with increasing size (Fig. 5). This effect appears to be weak for individual species such as mako sharks and Alepisaurus spp. (Fig. 5), but there was still a significant overall difference between mean δ15N values for small and large fish. Swordfish and albacore tuna showed the most significant differences between large and small fish, but albacore display a trend opposite to that observed for the dataset as a whole (Fig. 5), becoming less enriched with increasing size. There was no significant difference in δ15N with size for the yellowfin tuna sampled.
Samples of predators were collected over a wide geographical range, covering 17° of latitude and 13° of longitude (Table 1; Fig. 1). Results from the GLM (Tables 3, 4) showed significant spatial variability in both carbon and nitrogen isotope concentration for some predator and prey groupings. Adequate spatial coverage was limited for an in-depth analysis. However, plots of moving means and summed coefficients of variance of isotope concentration for the groups “All predators”, swordfish, yellowfin and albacore tuna had a strong latitudinal component with breaks noticeable at 28 and 34°S for both δ15N (Fig. 6) and δ13C (Fig. 7).
Discussion
Trophic groupings
Three trophic groups were distinguished in pelagic waters off eastern Australia based on their mean δ15N signature, generally considered to be an indicator of trophic position (although see Popp et al. 2007). The first group was characterised by a general grouping of top predators composed of shark, tuna and billfish (Fig. 2). Of the top order grouping, only mako shark could be distinguished as a potentially higher predator. This agrees with results in the Northwest Atlantic which showed mako sharks sitting above a range of pelagic shark species including blue sharks (Estrada et al. 2003; MacNeil et al. 2005). The main group, however, which also included the tunas and billfish, could not be separated by δ15N. In contrast, swordfish that overlapped in length and area with yellowfin tuna in the Indian Ocean had a δ15N 0.7–2.8‰ higher than the latter (Menard et al. 2007). They reasoned this was due to differences in trophic ecology and behaviour of the two species in the region, particularly diving capability. One possibility for the lack of difference between the two species off eastern Australia was the samples were collected from similar areas so potentially they would be feeding on the same prey species. Lansdell and Young (2007) identified 11 prey species that were common to yellowfin tuna and swordfish from eastern Australian waters, including dominant prey such as Ommastrephes bartramii, which contributed significantly to the diet of both predators.
Below the top order group was a broad grouping of species with a smaller mean size composed of ommastrephids, albacore, adult Alepisaurus spp. and dolphin fish. This group consisted of species regarded primarily as piscivores and agrees with a similar grouping from the study off southeast Australia by Davenport and Bax (2002). That study reported a group of tertiary consumers, dominated by primary piscivores, with a mean δ15N signature of ca. 12.5‰ (this study 12.1‰). Below this group lay a base of mid-level prey species including myctophids, juvenile Alepisaurus spp., small scombrids and macrorhamphosids, which are generally zooplanktivorous.
The difference in the mean δ15N value of the top predators and mid-level predators (ca. 1.5‰) was less than the often quoted δ15N trophic fractionation of 3.4‰ (Post 2002) indicating overlap in the prey eaten by both groups. This is not surprising, particularly given that of the species examined, only lancet fish were analysed separately as larger (adults) and smaller (juveniles) fish. The effect of fish size on δ15N has been reported consistently (e.g., Jennings et al. 2001). Off eastern Australia, as swordfish length increased, diet changed from small fish such as myctophids with a low δ15N signature to squid with a higher δ15N (Young et al. 2006). The difference between the top predator and prey groupings, however, was very close to the difference of 3.4‰ suggested by Post (2002) and others, indicating that in broad terms the ‘top predator’ and ‘prey’ groups were the main trophic categories off eastern Australia. There is some evidence for this from recently completed multi-species diet comparisons in the region which showed significant dietary overlap between a range of fish top- and middle-order predators (Young et al. 2009).
Variability with size
Previous studies of pelagic food webs have shown that a large fraction of total variance in the natural abundance of stable isotopes in organisms, ranking from plankton to fishes, was due to individual organism size (Montoya et al. 1990; Fry and Quinones 1994). Also, studies of δ15N enrichment across size classes of fishes demonstrated that trophic positions within a community were largely determined by the size of the organisms while inter-specific relationships between maximum body mass and δ15N were of low relative importance (Jennings et al. 2001, 2002). The overall increase in trophic position was generally caused by the intra-specific accumulation of heavy isotopes with the growth in body mass (Rau et al. 1989; Lindsay et al. 1998; Jennings et al. 2002). Our study largely supports this perspective, with trophic position and δ15N mainly increasing with the mean size of the fish, both within and between species (Fig. 4). However, there were exceptions, we think related to the specific behavioural and physiological differences between top predator fishes. For example, the δ15N of albacore decreased with increasing size, apparently due to a shift in feeding from higher to lower trophic prey species. In a companion study we found that crab megalopa, which have a δ15N of 7.9 (Davenport and Bax 2002), made up over half of the diet of albacore caught north of 28°S (Young et al. 2009). South of this divide fish and squid were the major prey species which this and other studies in the area have shown to have higher δ15N values (Davenport and Bax 2002; Young et al. 2006).
Evidence for bio-regionalization from SIA
δ13C—we found a generally negative shift in δ13C of muscle tissue with increasing latitude, likely due to water temperature which is known to affect the solubility of CO2 and subsequently the δ13C of plankton (Rau et al. 1989). In this study, there was a ~9°C difference between northern (25°C) and southern (16°C) waters. Using the equation from Rau et al. (1989), this could equate to a difference of ca. 2‰ in the δ13C of phytoplankton irrespective of other factors. This is the same as the mean difference measured in this study between the samples collected in the northern area (−17‰) and those caught in the southern area (−19‰). Analysis of the stable isotope data using moving means and summed coefficients of variance (Fig. 6) clearly showed that for all predators combined and individual target species, latitude is an important correlate of carbon isotopic signature. Changes were observed at 28 and 34°S. The former is the approximate position of the Tasman front which separates warm oligotrophic waters of the Coral Sea from colder Tasman Sea waters of sub Antarctic origin. The northern region is also the site of a chain of seamounts frequently targeted by ETBF. The more southerly position at ~34°S has a highly variable oceanography that includes warm-core eddies and intrusions of rich Tasman Sea waters of more southerly origin. Also, the eddies in this region are generally associated with the shelf break and act to produce upwelling along the shelf edge (Young et al. 2001). The resulting productivity in the area attracts aggregations of yellowfin tuna, and was also the site for many years of a fishery for juvenile southern bluefin tuna (Hynd 1974, Shingu 1978).
As there is a period of months for muscle tissue to register differences in diet (Graham et al. 2007), the difference in isotopic signature is evidence of extended residence time in particular regions. Frequent migrations between north and south would cause a “blurring” of the isotopic signature of samples collected in the two regions. The difference in δ13C values for swordfish samples appear to shift in the opposite direction to that expected, becoming more enriched in the southern area. However, the difference may be length-related as the fish sampled north of 28°S were dominated by two large individuals with high δ13C values. A similar pattern was noted by Baird et al. (2008) for particulate organic matter who suggested this may have been the result of higher productivity in the Tasman Sea leading to draw-down of aqueous CO2 and thus enriched values of δ13C.
δ15N—individuals caught in the northern area were, on average, more depleted in their δ15N value compared with those in the south, a result also found by Baird et al. (2008). They found δ15N values for particulate organic matter ranging from ~6‰ north of the Tasman front to 10‰ in the south. Of the key species of interest, this effect was most significant in yellowfin tuna. The nitrogen isotopic signature of a predator is set not only by trophic position, but also by the signature at the base of the food chain. Thus, the differences observed could be due to factors affecting either or both of these parameters. The primary factor influencing the nitrogen isotopic signature of phytoplankton is the concentration and source of nutrients being utilised and this is particularly important in oligotrophic waters, such as those to the north of the Tasman front. We were not able to obtain direct measured values for particulate nitrogen in the study area. However, Davenport and Bax (2002) reported δ15N values for primary producers in south-eastern Australia (south of the Tasman front) of 6‰, consistent with oceanic NO3 −, which resulted in tertiary consumers having δ15N values of around 13‰, similar to those determined for the top predators caught in the southern region in this study. More recently, Baird et al. (2008) reported the same value from north of the Tasman front but with a much higher value (10‰) to the south.
The relatively depleted δ15N values of predators we observed in the northern area could potentially be due to several reasons. First, predators could be feeding at a lower trophic position, which is possible given that myctophids were more prevalent in the diet of many of the species from this region (Young et al. 2009). Second, predator isotope values could be directly influenced by regional variations in the δ15N of the primary producers on which the food web is based. Oligotrophic waters such as those north of the front largely rely on regenerated nutrients for primary production, which in turn can lead to a depleted δ15N value. In addition, nitrogen fixation can also be an important process in nutrient poor waters, which will also cause a trend to more depleted δ15N values (Mahaffey et al. 2005). At times, northern Australian waters are known to exhibit high rates of nitrogen fixation due to the common presence of Trichodesmium sp. (Revelante et al. 1982) and unicellular diazotrophs (Montoya et al. 2004). Thus, it would seem likely that the most probable cause of the slightly depleted δ15N values in samples from the northern area can be directly attributed to variation in phytoplankton isotope values.
Future research
While good stomach content data will continue to be necessary, this study has shown that stable isotopes can add to our understanding of trophic interactions, and also provide valuable information on spatial patterns and residence times for top predators in the region. In future, multiple-tissue sampling may utilise differences in tissue metabolism to discern trophic dynamics with a minimum of sampling effort (Kurle and Worthy 2002) and over different time scales. There is clearly a need in this study to identify the δ15N signature at the base of area-specific food chains and while this is difficult utilising conventional filtering techniques, especially in oligotrophic waters, techniques such as compound-specific isotope analysis of compounds such as amino acids have the potential to provide this information from the target species themselves (McClelland and Montoya 2002).
References
Baird ME, Timko PG, Middleton JH, Mullaney TJ, Cox DR, Suthers IM (2008) Biological properties across the Tasman Front off southeast Australia. Deep Sea Res Part I Oceanogr Res Pap 55:1438–1455. doi:https://doi.org/10.1016/j.dsr.2008.06.011
Best PB, Schell DM (1996) Stable isotopes in Southern Right Whale (Eubalaena australis) baleen as indicators of seasonal movements, feeding and growth. Mar Biol (Berl) 124:483–494. doi:https://doi.org/10.1007/BF00351030
Caraveo-Patino J, Hobson K, Soto L (2007) Feeding ecol gray whales inferred from stable-carbon nitrogen isotopic anal baleen plates. Hydrobiologia 586:17–25
Cherel Y, Hobson KA (2007) Geographical variation in carbon stable isotope signatures of marine predators: a tool to investigate their foraging areas in the southern ocean. Mar Ecol Prog Ser 329:281–287. doi:https://doi.org/10.3354/meps329281
Davenport SR, Bax NJ (2002) A trophic study of a marine ecosystem off southeastern Australia using stable isotopes of carbon and nitrogen. Can J Fish Aquat Sci 59:514–530. doi:https://doi.org/10.1139/f02-031
DeNiro MJ, Epstein S (1978) Influence of diet on the distribution of carbon isotopes in animals. Geochim Cosmochim Acta 42:495–506. doi:https://doi.org/10.1016/0016-7037(78)90199-0
DeNiro MJ, Epstein S (1981) Influence of diet on the distribution of nitrogen isotopes in animals. Geochim Cosmochim Acta 45:341–351. doi:https://doi.org/10.1016/0016-7037(81)90244-1
Estrada JA, Rice AN, Lutkavage ME, Skomal GB (2003) Predicting trophic position in sharks of the north–west Atlantic Ocean using stable isotope analysis. J Mar Biol Assoc UK 83:1347–1350. doi:https://doi.org/10.1017/S0025315403008798
Fry B, Quinones RB (1994) Biomass spectra and stable-isotope indicators of trophic level in zooplankton of the northwest Atlantic. Mar Ecol Prog Ser 112:201–204. doi:https://doi.org/10.3354/meps112201
Fry B, Sherr EB (1984) δ13C measurements as indicators of carbon flow in marine and freshwater ecosystems. Contrib Mar Sci 27:13–47
Graham BS, Grubbs D, Holland K, Popp BN (2007) A rapid ontogenetic shift in the diet of juvenile yellowfin tuna from Hawaii. Mar Biol (Berl) 150:647–658. doi:https://doi.org/10.1007/s00227-006-0360-y
Hynd JS (1974) Unusual bluefin tuna season in N.S.W. Aust Fish 33(4):9–11
James AG (1988) Are clupeid microphagists herbivorous or omnivorous? A review of the diets of some commercially important clupeids. S Afr J Mar Sci 7:161–177
Jennings S, Pinnegar JK, Polunin NVC, Boon TW (2001) Weak-cross species relationships between body size and trophic level belie powerful size-based trophic structuring in fish communities. J Anim Ecol 70:934–944. doi:https://doi.org/10.1046/j.0021-8790.2001.00552.x
Jennings S, Warr KJ, Mackinson S (2002) Use of size-based production and stable isotope analyses to predict trophic transfer efficiencies and predator–prey body mass ratios in food webs. Mar Ecol Prog Ser 240:11–20. doi:https://doi.org/10.3354/meps240011
Kurle CM, Worthy GAJ (2002) Stable nitrogen and carbon isotope ratios in multiple tissues of the northern fur seal Callorhinus ursinus: implications for dietary and migratory reconstructions. Mar Ecol Prog Ser 236:289–300. doi:https://doi.org/10.3354/meps236289
Lansdell M, Young JW (2007) Pelagic cephalopods from eastern Australia: species composition, horizontal and vertical distribution determined from the diets of pelagic fishes. Rev Fish Biol Fish 17:125–138. doi:https://doi.org/10.1007/s11160-006-9024-8
Lindsay DJ, Minagawa M, Mitani I, Kawaguchi K (1998) Trophic shift in the Japanese anchovy Engraulis japonicus in its early life history stages as detected by stable isotope ratios in Sagami Bay, Central Japan. Fish Sci 64:403–410
MacDonald JS, Waiwood KG, Green RH (1982) Rates of digestion of different prey in Atlantic cod (Gadus morhua), ocean pout (Macrozoarces americanus), winter flounder (Pseudopleuronectes americanus), and American plaice (Hippoglossoides platessoides). Can J Fish Aquat Sci 39:651–659. doi:https://doi.org/10.1139/f82-094
MacNeil MA, Skomal GB, Fisk AT (2005) Stable isotopes from multiple tissues reveal diet switching in sharks. Mar Ecol Prog Ser 302:199–206. doi:https://doi.org/10.3354/meps302199
MacNeil MA, Drouillard KG, Fisk AT (2006) Variable uptake and elimination of stable nitrogen isotopes between tissues in fish. Can J Fish Aquat Sci 63:345–353. doi:https://doi.org/10.1139/f05-219
Mahaffey C, Michaels AF, Capone DG (2005) The conundrum of marine N2 fixation. Am J Sci 305:546–595. doi:https://doi.org/10.2475/ajs.305.6-8.546
Mcclelland JW, Montoya JP (2002) Trophic relationships and the nitrogen isotopic composition of amino acids in plankton. Ecology 83:2173–2180
McConnaughey T, McRoy CP (1979) Food-web structure and the fractionation of carbon isotopes in the Bering Sea. Mar Biol (Berl) 53:257–262. doi:https://doi.org/10.1007/BF00952434
Menard F, Lorrain A, Potier M, Marsac F (2007) Isotopic evidence of distinct feeding ecologies and movement patterns in two migratory predators (yellowfin tuna and swordfish) of the western Indian Ocean. Mar Biol (Berl) 153:141–152. doi:https://doi.org/10.1007/s00227-007-0789-7
Montoya JP, Horrigan SG, McCarthy JJ (1990) Natural abundance of 15N in particulate nitrogen and zooplankton in the Chesapeake Bay. Mar Ecol Prog Ser 65:35–61. doi:https://doi.org/10.3354/meps065035
Montoya JP, Holl CM, Zehr JP, Hansen A, Villareal TA, Capone DG (2004) High rates of N2 fixation by unicellular diazotrophs in the oligotrophic pacific ocean. Nature 430:1027–1031. doi:https://doi.org/10.1038/nature02824
Nichols P, Mooney B, Virtue P, Elliott N (1998) Nutritional value of Australian fish: oil, fatty acid and cholesterol of edible species. Final report, FRDC Project 95/122
Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Annu Rev Ecol Syst 18:293–320. doi:https://doi.org/10.1146/annurev.es.18.110187.001453
Pinnegar JK, Polunin NVC (1999) Differential fractionation of 13C and 15N among fish tissues: implications for the study of trophic interactions. Funct Ecol 13:225–231. doi:https://doi.org/10.1046/j.1365-2435.1999.00301.x
Popp BN, Graham BS, Olson RJ, Hannides CCS, Lott MJ, Lopez-Ibarra GA, Galvan-Magafia F, Fry B (2007) Insight into the trophic ecology of yellowfin tuna Thunnus albacares, from compound specific nitrogen isotope analysis of proteinaceous amino acids. In: Dawson TE, Siegwolf R (eds) Stable isotopes as indicators of ecological change. Academic press, Elsevier
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83:703–718
Rau GH, Takahashi T, Des Marais DJ (1989) Latitudinal variations in plankton δ13C: implications for CO2 and productivity in past oceans. Nature 341:516–518. doi:https://doi.org/10.1038/341516a0
Revelante N, Williams WT, Bunt JS (1982) Temporal and spatial distribution of diatoms, dinoflagellates and Trichodesmium in waters of the Great Barrier Reef. J Exp Mar Biol Ecol 63:27–45. doi:https://doi.org/10.1016/0022-0981(82)90048-X
Sara G, Sara R (2007) Feeding habits and trophic levels of bluefin tuna Thunnus thynnus of different size classes in the Mediterranean Sea. J Appl Ichthyol 23:122–127. doi:https://doi.org/10.1111/j.1439-0426.2006.00829.x
Shingu C (1978) Ecology and stock of Southern Bluefin Tuna. Japan Association of Fishery Resources Protection. Fisheries Study 31 (in Japanese). English translation in: CSIRO Division of Fisheries and Oceanography, Report No. 131 (1981)
Vander Zanden MJ, Casselman JM, Rasmussen JB (1999) Stable isotope evidence for the food web consequences of species invasions in lakes. Nature 401:464–467. doi:https://doi.org/10.1038/46762
Worm B, Duffy JE (2003) Biodiversity, productivity and stability in real food webs. Trends Ecol Evol 18:628–632. doi:https://doi.org/10.1016/j.tree.2003.09.003
Young JW, Lamb TD, Bradford R, Clementson L, Kloser R, Galea H (2001) Yellowfin tuna (Thunnus albacares) aggregations along the shelf break of southeastern Australia: links between inshore and offshore processes. Mar Freshw Res 52:463–474. doi:https://doi.org/10.1071/MF99168
Young JW, Lansdell MJ, Riddoch S, Revill AT (2006) Feeding ecology of broadbill swordfish, Xiphias gladius, off eastern Australia in relation to physical and environmental variables. Bull Mar Sci 79:793–810
Young JW, Lansdell MJ, Hobday AJ, Dambacher JD, Cooper S, Griffiths SP, Kloser R, Nichols PD, Revill A (2009) Determining ecological effects of longline fishing in the Eastern Tuna and Billfish Fishery. FRDC Final Report 2004/063, pp 310
Acknowledgments
This study was completed as part of Fisheries Research and Development Account Grant 2004/63. We thank Dr. Karen Evans and Thor Carter for collection of samples and Rebecca Esmay for sample preparation. The manuscript was improved by suggestions from Drs. Alistair Hobday and Rhys Leeming and the constructive and insightful comments of three anonymous reviewers.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Revill, A.T., Young, J.W. & Lansdell, M. Stable isotopic evidence for trophic groupings and bio-regionalization of predators and their prey in oceanic waters off eastern Australia. Mar Biol 156, 1241–1253 (2009). https://doi.org/10.1007/s00227-009-1166-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-009-1166-5