Abstract
The present study provides the first analysis of the feeding macroecology of territorial damselfishes (Perciformes: Pomacentridae), a circumtropical family whose feeding and behavioral activities are important in structuring tropical and subtropical reef benthic communities. The analyses were conducted from data collected by the authors and from the literature. A strong positive correlation was observed between bite rates and sea surface temperature (SST) for the genus Stegastes. A negative correlation was found between bite rates and mean body size for the genera Stegastes and Pomacentrus, but this relationship was not significant when all territorial pomacentrids were analyzed together. A negative correlation between body size and SST was observed for the whole group and for the genera Stegastes, and Pomacentrus. No relationship was found between territory size and feeding rates. Principal Components Analysis showed that differences in feeding rates accounted for most of the variability in the data. It also suggested that body size may be important in characterizing the different genera. In general, tropical species are smaller and have higher bite rates than subtropical ones. This study extended the validity of Bergmann’s rule, which states that larger species or larger individuals within species occur towards higher latitudes and/or lower temperatures, for an important group of reef fishes. The identification of large-scale, robust ecological patterns in the feeding ecology of pomacentrid fishes may establish a foundation for predicting large-scale changes in reef fish assemblages with expected future changes in global SST.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A major goal of macroecology is to study the general patterns of the ecological assembly and structure of biotas on a large scale (Brown 1995; Lomolino et al. 2006). Some of these patterns are surprisingly consistent; for example, there are “ecogeographic rules” (sensu Lomolino et al. 2006) in which recurring geographic gradients in the features of organisms occur across continental and/or marine realms (Gaston et al. 2008). Analyses of these gradients generally evaluate the relationships between geographical and/or environmental variables (e.g. latitude, temperature, humidity) and morphological aspects (e.g. body size, mass, color), and are the key to understanding patterns of diversity along large-scale gradients (Lomolino et al. 2006).
Most macroecological studies have been conducted on terrestrial organisms (see Brown 1995; Eeley and Foley 1999; Kelt and Van Vuren 2001), with less focus on attempting to understand the underlying rules for aquatic organisms (Rex et al. 1997; Roy and Martein 2001; Alimov 2003), including fishes (Sale 1978; Macpherson and Duarte 1994; Minns 1995; Smith and Brown 2002; Floeter et al. 2005, 2007). Bergmann’s rule, for example, which states that larger species or larger individuals within species (see Gaston et al. 2008) occur towards higher latitudes and/or lower temperatures, is well described for several vertebrate groups (e.g. mammals, birds, salamanders and turtles; Lomolino et al. 2006). However, there is little evidence that this pattern exists for marine fishes (see Discussions in Macpherson and Duarte 1994; Choat and Robertson 2002; Smith and Brown 2002). The present macroecological analysis is one of the first studies of its kind based on behavioral in situ observations of reef fishes (see Floeter et al. 2005, 2007).
Damselfishes as study models
In the last decades, territorial pomacentrids have been subject to a number of manipulation experiments (Robertson 1984, 1995; Ferreira et al. 1998; Ceccarelli et al. 2005), and have provided the foundation of many population and community theories. They are a widespread and abundant component of reef fish communities and are considered the numerically dominant herbivores on some reefs and in some habitats (Scott and Russ 1987; Ceccarelli 2007). They have been found to mediate algal diversity (Hixon and Brostoff 1983), affect coral zonation (Wellington 1982), and structure the benthic communities within their territories through their feeding and behavioral activities (Ferreira et al. 1998; Ceccarelli et al. 2001; Ceccarelli 2007). At Lizard Island, Great Barrier Reef, the family Pomacentridae was found to have the highest rate of body mass growth per week, showing their importance for trophic energy transmission on coral reefs (Depczynski et al. 2007).
Many studies have tried to understand the feeding and territorial activity of damselfishes in order to quantify their role in structuring reef communities (Brawley and Adey 1977; Robertson 1984, 1996; Ferreira et al. 1998; Letourneur 2000; Hata and Kato 2002; Menegatti et al. 2003; Alwany et al. 2005; Osório et al. 2006). The magnitude of their influence on coral reef communities depends on their density, size, territorial and feeding behaviors (Robertson 1984, 1996; Hixon and Webster 2002). These factors are often inter-related and influenced by environmental parameters such as water temperature (Polunin and Klumpp 1989; Ferreira et al. 1998). By addressing the nature of the relationships between feeding behavior, size, territory size and temperature we can begin to understand how the patterns of damselfish herbivory are organized on a global scale.
Damselfish aggression may be related to their territory size, or to the amount of algae inside their territories (Jan et al. 2003). However, it is known that the algal turf the damselfish feeds on does not always encompass the entire territory area (Jan et al. 2003; Ceccarelli, 2007). Therefore, it can be said that the relationship between territorial and feeding behaviors, territory size and algal turf abundance is still poorly understood (but see Hata and Kato 2004). Given the current lack of consensus, it is possible that clearer patterns exist between damselfish feeding activity and territoriality at larger scales. One may ask the question: do individuals defending smaller territories display higher feeding rates than the ones with larger territories?
The influence of temperature on feeding activity and body size
Fishes, with few exceptions (e.g. Scombridae; Schmidt-Nielsen 2002), are thermo-dependent organisms whose metabolism is regulated according to the surrounding water temperature, which also influences their body size. It has been demonstrated that the herbivorous surgeonfish Acanthurus bahianus live longer and grow larger in colder waters when compared to tropical counterparts (Choat and Robertson 2002). The same pattern was observed for the Brazilian damselfish Stegastes fuscus: this species attains larger maximum sizes in temperate waters found along the southern Brazilian coast than in the warmer waters of northeastern Brazil (Floeter and Ferreira pers. obs.). It may be important to examine variations in body size with temperature and/or latitude in herbivorous reef fishes in general (e.g. Acanthuridae, Scarinae and some Pomacentridae), to better understand the magnitude of their roles as modifiers of the reef benthic community (Ceccarelli et al. 2005; Ceccarelli 2007) and as primary consumers in marine trophic webs (Horn 1989). We therefore ask: do damselfishes in colder waters grow larger than those in warmer waters?
The relationship between water temperature and fish metabolism tends to be one where metabolic rates increase as temperature rises (Clarke and Johnston 1999). Daily and seasonal temperature variation can therefore play an important role in the activity of reef fishes. Studies have reported that damselfishes and other herbivorous reef fishes increase their feeding activity either throughout the day or seasonally with increasing temperature (Polunin and Klumpp 1989; Ferreira et al. 1998). These relationships, however, were described for a single species at a time. In the present study, we investigated whether temperature was a relevant factor in damselfishes’ feeding activity at a global scale, with the question: do damselfishes inhabiting warmer waters display higher feeding rates compared to those inhabiting colder waters?
Fish metabolism is also related to their body mass/size, and smaller fishes generally have a higher specific metabolism (O2 consumption/mass/time) than larger ones (Yager and Summerfelt 1993). To maintain the faster metabolism, smaller fishes must display proportionally higher feeding rates than larger ones. Menegatti et al. (2003) observed a decrease in bite rates from juveniles to adults in Brazilian damselfish Stegastes fuscus, which may reflect the higher metabolic rate in juveniles. Here we test whether this relationship is applicable to a broad scale analysis with several species of damselfishes, where most reach different adult sizes and assess whether larger damselfishes have lower feeding rates than smaller ones.
A global scale macroecological study of territorial damselfishes can assist with the understanding of large scale patterns for this group or fishes, and will be useful to inform future manipulation experiments trying to predict the impacts of damselfishes on reefs.
Materials and methods
Database compilation
A database was compiled using existing literature and the authors’ data (Table 1) for herbivorous territorial damselfishes throughout the world (Fig. 1). It encompassed: (1) feeding rates, (2) body sizes (total length) as described in the literature or collected by the authors, (3) territory size and (4) sea surface temperature (SST). Only published data for adults collected between 11:00 h and 18:00 h were included based on herbivorous fish diurnal feeding activity (Zemke-White et al. 2002), except for Lison de Loma and Harmelin-Vivien (2002) and Lobel (1980), which contained data from earlier hours in the morning.
World map showing the places where data were collected by the authors (gray arrows) and from the literature (black arrows). 1 Réunion Island, 2 Sesoko Island, 3 Motupore Island, 4 Kimbe Bay, 5 Lizard Island, 6 Orpheus Island, 7 Magnetic Island, 8 Fanning Atoll, 9 Los Frailes, 10 Bocas del Toro, 11 San Blas, 12 Fernando de Noronha, 13 João Pessoa, 14 Tamandaré Reefs, 15 Guarapari, 16 Arraial do Cabo, 17 Porto Belo, 18 Florianópolis, 19 São Tomé. (a) Stegastes nigricans, (b) Plectroglyphidodon lacrymatus, (c) Neoglyphidodon nigroris, (d) Pomacentrus adelus, (e) P. bankanensis, (f) P. burroughi, (g) P. tripunctatus, (h) S. lividus, (i) Hemiglyphidodon plagiometopon, (j) P. chrysurus, (k) P. wardi, (l) S. apicalis, (m) Microspathodon dorsalis, (n) S. rectifraenum, (o) Microspathodon chrysurus, (p) S. adustus, (q) S. planifrons, (r) S. rocasensis, (s) S. fuscus, (t) S. imbricatus
Authors’ data
Atlantic data for Microspathodon chrysurus, Stegastes adustus, S. planifrons (Bocas del Toro, Panama) and S. imbricatus (São Tomé & Príncipe, Africa) were collected by CELF and SRF; S. fuscus data from the Brazilian coast were collected by DRB, SRF, CELF, DMBF and DFD using identical methods. The feeding activity and size (total length visually estimated with 1 cm precision) of individual damselfish were recorded during 5-minute observation periods; feeding rates were assessed according to the frequency of bites on the substratum during the observation periods and are expressed as bites per minute. To estimate territory size of S. adustus and S. planifrons (CELF and SRF), individual fish were observed for 15 min, and the boundaries in which patrolling activities took place were marked with a rope divided into 10 cm intervals with lead weights. The area was calculated by measuring the circumference of each territory and using the formula for a circle of equivalent circumference.
Indo-West Pacific data for Stegastes apicalis (Magnetic Island, GBR, Australia), Pomacentrus chrysurus and Hemiglyphidodon plagiometopon (Orpheus Island, GBR Australia), P. burroughi, P. bankanensis, Plectroglyphidodon lacrymatus, Neoglyphidodon nigroris and S. lividus (Kimbe Bay, Papua New Guinea) and P. tripunctatus, P. wardi and P. adelus (two or more of the locations above) were collected by DMC using methods similar to those described above. Feeding observation periods were 15 min and included the assessment of territory boundaries. The boundaries were marked and measured at the end of the observation periods, and territory area estimated with the method described above. The fish were subsequently caught for gut content analysis (see Ceccarelli 2007), and total length measured in the laboratory.
Data standardization
All data were standardized to conduct the analyses. Feeding rates were transformed to bites per minute, sizes (cm) were transformed (when necessary) to total length and territory size to square meters (m2). We transformed standard lengths (SL) into total lengths (TL) using photographs of adults (Allen 1991) from which we obtained the ratio between SL and TL.
Sea surface temperature
The high resolution sea surface temperature (SST) analysis product used in the present study was developed by Reynolds et al. (2007) using optimum interpolation (OI) version 2 (https://doi.org/eclipse.ncdc.noaa.gov/pub/OI-daily/daily-sst.pdf). The analyses are global and have a spatial grid resolution of 0.25° and temporal resolution of 1 day. The analysis uses Advanced Very High Resolution Radiometer (AVHRR) infrared satellite SST data and in situ data from ships and buoys that include a large-scale adjustment of satellite biases. For this study, we used the data set between January 1985 and December 2005.
We obtained mean annual SST values over 21 years for each site from a time series of daily SST extracted from the global grids, summing 21 years of SST averages. In the same way the minimum and the maximum values of each month were extracted from the global grids to calculate the mean minimum and maximum SST for each site over 21 years. For the analyses we used the mean minimum data set as the minimum temperature may present physiological constraints to herbivorous fishes (Floeter et al. 2005). Moreover, the correlation between the minimum and the mean SST for all sites is highly significant (r2 = 0.99; p < 0.0001).
Data analyses
Regression analyses were conducted to verify the relationship and significance between the estimated macroecological parameters (Zar 1999). The genera Pomacentrus and Stegastes were analyzed separately, as they represent the two most diverse genera of herbivorous territorial pomacentrids (Allen 1991). Microspathodon spp. was added to the Stegastes analysis of bite rates against SST due to the phylogenetic proximity of these two genera (Quenouille et al. 2004; Cooper et al. 2008). One-way analysis of variance (ANOVA) was conducted to test whether feeding rates and body size are different between Stegastes and Pomacentrus.
A Principal Components Analysis (PCA) biplot was used to summarize the overall relationship between feeding rates, body size and SST. Body size data for the species Stegastes fuscus 2 and 4, which were not collected, were extrapolated based on the closest species data available. All data (bites/min, body size and SST) were log10 transformed to reduce the effect generated by different orders of magnitude in some of the values (i.e., bite rates ranged from approximately 6 to 13 bites/min, mean body size from 7 to 20 cm while SST ranged from 18 to 26°C). MVSP 3.1 for Windows software was used for the PCA.
Results
Feeding rates and body size versus SST
No correlation between bite rates and SST was found for the territorial Pomacentridae (Fig. 2). When the two most diverse genera of this group were analyzed separately, we observed a positive correlation for the genus Stegastes, but not for the genus Pomacentrus (Fig. 2). Data for S.nigricans 3 and S. lividus from Papua New Guinea were considered outliers and were therefore removed from these analyses (see Discussion for more details). ANOVA showed that Stegastes spp. have higher feeding rates than Pomacentrus spp. (F1,30 = 7.71; p < 0.01) which may explain why the mixing between different genera (Pomacentridae) was not significant.
Relationship between feeding rates (bites/min) and sea surface temperature (SST) in the territorial Pomacentridae and the genera Stegastes and Pomacentrus. For Stegastes and Pomacentrus graphs the black triangle represents S. adustus or P. adelus; white triangle = S. nigricans or P. wardi; black diamond = S. imbricatus or P. bankanensis; white diamond = S. lividus or P. burroughi; black circle = S. rectifraenum; white circle = S. planifrons or P. chrysurus; black square = S. fuscusor P. tripunctatus; white square = S. apicalis; gray square = S. rocasensis; gray circle = Microspathodon dorsalis; gray triangle = M. chrysurus. For the Pomacentridae graph, the white triangle represents Neoglyphidodon; black diamond = Hemiglyphidodon; white diamond = Plectroglyphidodon; black circle = Stegastes; white circle = Pomacentrus; gray triangle = Microspathodon
A negative correlation between body size and SST was observed for the territorial Pomacentridae as well as the genus Stegastes (Fig. 3). This correlation, however, was only marginally significant for the genus Pomacentrus (Fig. 3).
Relationship between body size and sea surface temperature (SST) in the territorial Pomacentridae and the genera Stegastes and Pomacentrus. See Fig. 2 for graphic representation of species
Feeding rates versus body size and territory size
There was no significant relationship between body size and bite rates for the territorial Pomacentridae (Fig.4). However, we found a negative correlation for both the genera Stegastes and Pomacentrus (Fig. 4). ANOVA showed that Stegastes spp. are significantly larger than Pomacentrus spp, (F1,25 = 4.40; p < 0.05) which indicates that combining different genera may not be appropriate.
Relationship between feeding rates (bites/min) and body size in the territorial Pomacentridae and the genera Stegastes and Pomacentrus. See Fig. 2 for graphic representation of species
None of the analyses showed a relationship between feeding rates and territory size: Pomacentridae (r2 = 0.14; F1,19 = 3.22; p = 0.08); Stegastes (r2 = 0.18; F1,7 = 1.63; p = 0.24); Pomacentrus (r2 = 0.18; F1,7 = 1.61; p = 0.24).
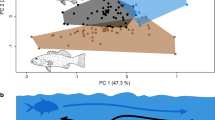
Principal components analysis
Differences in feeding rates (bites/min) explained most of the variability in the PCA (Axis 1: 74.40%; Fig. 5). Differences in body size and SST accounted for the other 22.75% (Axis 2) of the variation in the data. Larger species, such as subtropical fringe Stegastes spp., Hemiglyphidodon plagiometopon and Microspathodon dorsalis were characterized by having lower feeding rates than the smaller tropical Western Atlantic Stegastes spp. and the Indo-West Pacific S. nigricans. Despite the relatively large size of the tropical M. chrysurus, it has much higher feeding rates than its subtropical counterpart M. dorsalis. All species of Pomacentrus, Neoglyphidodon nigroris and Plectroglyphidodon lacrymatus are generally smaller than Stegastes spp.
Principal Components Analysis biplot of the overall relationship between sea surface temperature (SST), body size and bite rates for the territorial Pomacentridae. The length of the arrows indicates the relative importance of the three test factors. Circle sizes are proportional to body size, which is indicated by the horizontal bar. The species numbering code is based on Table 1
Discussion and conclusions
The influence of temperature on feeding
This study has highlighted general large-scale patterns in the relationships between temperature, body size and feeding activity in territorial pomacentrids. Temperature is a key factor in driving fish feeding activity. The higher temperatures found in the tropics are directly related to an increase in the metabolic rates of thermo-dependent organisms (Clarke and Johnston 1999; Schmidt-Nielsen 2002). Therefore, feeding activities must increase as well (Floeter et al. 2005). While there was a positive correlation between bite rates and SST in the genus Stegastes, this relationship was not significant in the territorial Pomacentridae and the genus Pomacentrus. The lack of a good range of SST data for the latter – data were collected at three Indo-Pacific locations, resulting in only two different temperatures (Ceccarelli, present paper) – made the analysis of macroecological patterns with an emphasis on temperature less robust for this genus. The combining of different genera for the analysis of territorial Pomacentridae may not be appropriate, as an ANOVA showed significant differences in feeding activity of both Stegastes and Pomacentrus.
Seasonal variations in temperature account for differences in feeding activities. Previous studies have demonstrated that the bite rates of a single damselfish species (e.g. Plectroglyphidodon lacrymatus, S. fuscus and S. rocasensis) within the same location can vary daily and seasonally with temperature (Polunin and Klumpp 1989; Ferreira et al. 1998; Souza 2007). Our study ascertains that temperature variations have a strong influence on feeding activity in the Stegastes genus. However, we do not know enough about how other oceanographic (e.g. hydrodynamics) and biological conditions (e.g. density–dependent interactions) can exert some influence on macroecological fish foraging patterns (Ferreira et al. 1998).
The influence of temperature on body size
Our results show that a negative correlation between body size and SST exists for Stegastes spp. and the Pomacentridae family, and it is marginally significant in the genus Pomacentrus, confirming the applicability of Bergmann’s rule to this group of fishes. Sampling Pomacentrus in a wider range of temperatures is likely to give a clearer view of this pattern.
Coral reefs have long been described as the richest marine ecosystem on the planet, with a complex three-dimensional structure, being compared only to tropical rain forests (Connell 1978). Being members of one of the most abundant and diverse reef fish families, territorial damselfishes display intense inter- and intraspecific competition (Robertson 1984, 1995; Ferreira et al. 1998; Ceccarelli et al. 2001; Hata and Kato 2002), may face high predation pressure (see Hixon and Webster 2002) and must deter intruding competitors (such as larger roving herbivores) from depleting the resources in their territories (Ceccarelli et al. 2005, 2006; Osório et al. 2006; Souza 2007). The cost of repelling intruders is expected to be high, and as smaller fishes allocate comparatively more energy to their metabolism than bigger fishes, the tropical environment may favor smaller and less aggressive individuals. In addition, the faster metabolism causes a larger degree of oxidative stress, which, together with possible higher predation rates, shortens their life span. This is corroborated by data showing smaller sizes and shorter life spans for reef fish in the tropics (Choat and Robertson 2002).
This same study demonstrated that individual Acanthurus bahianus grow larger (and live longer) in colder waters (Choat and Robertson 2002). The authors suggested that lower temperatures reduce growth rates and/or increase life span, and that this pattern may be related to low recruitment rates in subtropical fringe areas, also leading to a longer life span. Although there are local ecological attributes to address when analyzing the body size of fishes (e.g. territorial behavior and competition; Robertson 1984, 1995, 1996; Choat and Robertson 2002; Hata and Kato 2004), we suggest that metabolic aspects were the main factor driving the observed patterns between body size and temperature in reef fishes on a macro scale analysis.
The influence of body size on feeding
There was a negative correlation between feeding rates and body size for both Stegastes and Pomacentrus, but not for the Pomacentridae combined. The difference in body size between Stegastes and Pomacentrus as showed by ANOVA indicates that combining the two genera may not be appropriate. Larger individuals tend to have lower feeding rates than smaller ones regardless temperature, as their specific metabolism (O2 consumption/mass/time)—and therefore their feeding activity—is expected to be lower (Yager and Summerfelt 1993).
Natural variations in body size exist between and within species and genera, and this can determine the behavioral activity of individual fish. Ecological interactions between different species may play a large role in determining individual body size: the presence of larger and dominant species (Robertson 1984), intra and interspecific competition and ability (Robertson 1995, 1996), density and distribution of territories (Meadows 2001) and different feeding and ‘farming’ patterns (extensive vs. intensive; Hata and Kato 2004). Despite all the ‘noise’ that may be caused by those interactions during the life of a damselfish, we demonstrate that macroecological patterns emerge when a large enough pool of species is analyzed.
The temperature-size axis in the PCA (Axis 1) separates the genus Pomacentrus from the other species considered in this study. This may be due to the geographic separation of sampling sites (Pomacentrus spp. were only sampled in the Indo-West Pacific), but potentially indicates the importance of size, rather than feeding rates, in characterizing the different genera. Sampling Pomacentrus spp. more widely will offer a clearer view of this pattern.
The PCA analysis grouped S. nigricans 3 with subtropical fringe species due to its larger size and very low feeding rate, even though it inhabits relatively warm waters (Kimbe Bay, PNG). In terms of SST, S. nigricans 3 is an “outlier” within the genus Stegastes, as a tropical Stegastes species would be expected to be smaller. A further contrast highlighted by the PCA is that Hemiglyphidodon plagiometopon is large, inhabits warm waters and displays a low feeding rate, while the size equivalent Microspathodon chrysurus, also living in warm waters, has a much higher feeding rate. Furthermore, M. dorsalis displays lower feeding rates than M. chrysurus, as would be expected for a subtropical species. Overall, it appears that within each genus there is a particular pattern for the relationship between feeding activity, body size and temperature that may or may not correspond to the pattern displayed by other genera.
Some studies show that macroecological patterns become clearer as the group analyzed becomes more restricted (Macpherson and Duarte 1994; Choat and Robertson 2002; Smith and Brown 2002). Among territorial damselfishes, territoriality may vary considerably spatially (e.g. density-dependent processes—not evaluated here; Hixon and Webster 2002), and between taxa (Ceccarelli et al. 2001). Stegastinae and Pomacentrinae are not closely related genera within Pomacentridae (Quenouille et al. 2004; Cooper et al. 2008) and have different demographic structures (Ceccarelli pers. obs.), which may be a determining factor in shaping species’ body size and therefore also influence their feeding activity. Pomacentrus spp. are smaller (as showed by ANOVA) and less aggressive than Stegastes spp. (D.M.B. Frensel, D.R. Barneche, S.R. Floeter, D.M. Ceccarelli, C.E.L. Ferreira, D.F. Dinslaken, in prep.), and may be able to inhabit coral reef systems by maintaining extensive, rather than intensive, algal farming (Hata and Kato 2004). In addition, we observed that Microspathodon spp. data added to the Stegastes analysis sustained the pattern observed, and we believe that this is due to their close phylogenetic relationship (Quenouille et al. 2004; Cooper et al. 2008). When Stegastes and Pomacentrus were analyzed separately, they presented the same pattern, albeit at different scales. As a consequence, no pattern is observed when the data are pooled for the family analysis.
Feeding activity and territory area
No relationship was observed between feeding activity and territory size. We hesitate to draw conclusions from this finding due to the different approaches to territory size measurement. The overall territory area patrolled by resident fish is often not the same as the feeding area. Much of the feeding activity tends to take place on a small patch of algae within the territory (Ceccarelli et al. 2001; Jan et al. 2003; Ceccarelli 2007). Furthermore, competition with surrounding individuals and the quality of the locally available food resources can influence both territory size and feeding activity. In turn, the level of competition is likely to be affected by the species composition of the surrounding fish community (Crossman et al. 2001; Purcell and Bellwood 2001; Russ 2003; Ceccarelli et al. 2006).
Studies have shown that larger fishes defend larger territories (Sale 1978; Letourneur 2000). The size of the ‘feeding patch’ may be also proportional to the feeding activity and/or the size of the occupant, but this assumption has yet to be investigated. Although our study does not analyze this aspect, we suggest that these patches may vary between taxa or even between locations for a single species.
Conclusions
On the basis of these results, we extend the validity of Bergmann’s rule to territorial damselfishes, a numerically and ecologically important group of reef fishes. This ecogeographic rule applies to the territorial damselfish group in this study despite phylogenetic differences and variations in diet, aggression and sampling methods. Territorial damselfishes inhabiting warmer waters are smaller and have higher feeding rates than their subtropical counterparts. The identification of large-scale, robust ecological patterns underpins the understanding of reef fish community macroecology. Identifying these relationships for other reef fish groups may establish a foundation for predicting future large-scale changes in reef fish assemblages with expected changes in SST.
References
Alimov AF (2003) Territoriality in aquatic animals and their sizes. Biol Bull 30:79–86. doi:https://doi.org/10.1023/A:1022075829401
Allen GR (1991) Damselfishes of the World. Aquariums Systems, Melle
Alwany M, Thaler E, Stachowitsch M (2005) Territorial behaviour of Acanthurus sohal and Plectroglyphidodon leucozona on the fringing Egyptian Red Sea reefs. Environ Biol Fishes 72:321–334. doi:https://doi.org/10.1007/s10641-004-2587-0
Arias-Gonzalez JE, Done TJ, Page CA, Cheal AJ, Kininmonth S, Garza-Perez JR (2006) Towards a reefscape ecology: relating biomass and trophic structure of fish assemblages to habitat at Davies Reef, Australia. Mar Ecol Prog Ser 320:29–41. doi:https://doi.org/10.3354/meps320029
Brawley SH, Adey WH (1977) Territorial behavior of threespot damselfish (Eupomacentrus planifrons) increases reef algal biomass and productivity. Environ Biol Fishes 2:45–51. doi:https://doi.org/10.1007/BF00001415
Brown JH (1995) Macroecology. University of Chicago Press, Chicago
Ceccarelli DM (2007) Modification of benthic communities by territorial damselfish: a multi-species comparison. Coral Reefs 26:853–866. doi:https://doi.org/10.1007/s00338-007-0275-1
Ceccarelli DM, Jones GP, McCook LJ (2001) Territorial damselfishes as determinants of the structure of benthic communities on coral reefs. Oceanogr Mar Biol Annu Rev 39:355–389
Ceccarelli DM, Jones GP, McCook LJ (2005) Foragers versus farmers: contrasting effects of two behavioural groups of herbivores on coral reefs. Oecologia 145:445–453. doi:https://doi.org/10.1007/s00442-005-0144-y
Ceccarelli DM, Jones GP, McCook LJ (2006) Impacts of simulated overfishing on the territoriality of coral reef damselfish. Mar Ecol Prog Ser 309:255–262. doi:https://doi.org/10.3354/meps309255
Choat JH, Robertson DR (2002) Age-based studies on coral reef fishes. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 57–80
Clarke A, Johnston NM (1999) Scaling of metabolic rate with body mass and temperature in teleost fish. J Anim Ecol 68:893–905. doi:https://doi.org/10.1046/j.1365-2656.1999.00337.x
Connell JH (1978) Diversity in tropical rain forests and coral reefs. Science 199:1302–1310. doi:https://doi.org/10.1126/science.199.4335.1302
Cooper WJ, Smith LL, Westneat MW (2008) Exploring the radiation of a diverse reef fish family: phylogenetics of the damselfishes (Pomacentridae), with new classifications based on molecular analyses of all genera. Mol Phyl Evol (in press)
Crossman DJ, Choat JH, Clements KD, Hardy T, McConochie J (2001) Detritus as food for grazing fishes on coral reefs. Limnol Oceanogr 46:1596–1605
Depczynski M, Fulton CJ, Marnane MJ, Bellwood DR (2007) Life history patterns shape energy allocation among fishes on coral reefs. Oecologia 153:111–120. doi:https://doi.org/10.1007/s00442-007-0714-2
Eeley H, Foley RA (1999) Species richness, species range size and ecological specialisation among African primates: geographical patterns and conservation implications. Biodivers Conserv 8:1033–1056. doi:https://doi.org/10.1023/A:1008831320469
Ferreira CEL, Gonçalves JEA, Coutinho R, Peret AC (1998) Herbivory by the dusky damselfish Stegastes fuscus (Cuvier, 1830) in a tropical rocky shore: effects on the benthic community. J Exp Mar Biol Ecol 229:241–264. doi:https://doi.org/10.1016/S0022-0981(98)00056-2
Floeter SR, Behrens MD, Ferreira CEL, Paddack MJ, Horn MH (2005) Geographical gradients of marine herbivorous fishes: patterns and processes. Mar Biol 147:1435–1447. doi:https://doi.org/10.1007/s00227-005-0027-0
Floeter SR, Vázquez DP, Grutter AS (2007) The macroecology of marine cleaning mutualisms. J Anim Ecol 76:105–111. doi:https://doi.org/10.1111/j.1365-2656.2006.01178.x
Gaston KJ, Chown SL, Evans KL (2008) Ecogeographical rules: elements of a synthesis. J Biogeogr 35:483–500. doi:https://doi.org/10.1111/j.1365-2699.2007.01772.x
Hata H, Kato M (2002) Weeding by the herbivorous damselfish Stegastes nigricans in nearly monocultural algae farms. Mar Ecol Prog Ser 237:227–231. doi:https://doi.org/10.3354/meps237227
Hata H, Kato M (2004) Monoculture and mixed-species algal farms on a coral reef are maintained through intensive and extensive management by damselfishes. J Exp Mar Biol Ecol 313:285–296. doi:https://doi.org/10.1016/j.jembe.2004.08.009
Hixon MA, Brostoff WN (1983) Damselfish as keystone species in reverse: intermediate disturbance and diversity of reef algae. Science 230:511–513. doi:https://doi.org/10.1126/science.220.4596.511
Hixon MA, Webster MS (2002) Density dependence in reef fish populations. In: Sale PF (ed) Coral reef fishes: dynamics and diversity in a complex ecosystem. Academic Press, San Diego, pp 303–325
Horn MH (1989) Biology of marine herbivorous fishes. Oceanogr Mar Biol Annu Rev 27:167–272
Jan R-Q, Ho C-T, Shiah F-K (2003) Determinants of territory size of the dusky gregory. J Fish Biol 63:1589–1597. doi:https://doi.org/10.1111/j.1095-8649.2003.00270.x
Kelt DA, Van Vuren DH (2001) The ecology and macroecology of mammalian home range area. Am Nat 157:637–645. doi:https://doi.org/10.1086/320621
Letourneur Y (2000) Spatial and temporal variability in territoriality of a tropical benthic damselfish on a coral reef (Reunion Island). Environ Biol Fishes 57:377–391. doi:https://doi.org/10.1023/A:1007658830339
Lison de Loma T, Harmelin-Vivien M (2002) Summer fluxes of organic carbon and nitrogen through a damselfish resident, Stegastes nigricans (Lacepède, 1803), on a coral reef flat at La Réunion (Indian Ocean). Mar Freshw Res 53:169–174. doi:https://doi.org/10.1071/MF01145
Lobel PS (1980) Herbivory by damselfishes and their role in coral reef community ecology. Bull Mar Sci 30:273–289
Lomolino MV, Riddle BR, Brown JH (2006) Biogeography. Sinauer, Massachusetts
Macpherson E, Duarte CM (1994) Patterns in species richness, size, and latitudinal range of East Atlantic fishes. Ecography 17:242–248. doi:https://doi.org/10.1111/j.1600-0587.1994.tb00099.x
Meadows DW (2001) Centre-edge differences in behaviour, territory size and fitness in clusters of territorial damselfish: patterns, causes, and consequences. Behaviour 138:1085–1116. doi:https://doi.org/10.1163/156853901753287154
Menegatti JV, Vescovi DL, Floeter SR (2003) Interações agonísticas e forrageamento do peixe-donzela, Stegastes fuscus (Peciformes: Pomacentridae). Nat On Line 1:45–50
Minns CK (1995) Allometry of home range size in lake and river fishes. Can J Fish Aquat Sci 52:1499–1508. doi:https://doi.org/10.1139/f95-144
Montgomery WL (1980) Comparative feeding ecology of two herbivorous damselfishes (Pomacentridae: Teleostei) from the Gulf of California, Mexico. J Exp Mar Biol Ecol 47:9–24. doi:https://doi.org/10.1016/0022-0981(80)90134-3
Osório RM, Rosa IL, Cabral H (2006) Territorial defense by the Brazilian damsel Stegastes fuscus (Teleostei: Pomacentridae). J Fish Biol 69:233–242. doi:https://doi.org/10.1111/j.1095-8649.2006.01095.x
Polunin NVC, Klumpp DW (1989) Ecological correlates of foraging periodicity in herbivorous reef fishes of the Coral Sea. J Exp Mar Biol Ecol 126:1–20. doi:https://doi.org/10.1016/0022-0981(89)90121-4
Purcell S, Bellwood DR (2001) Spatial patterns of epilithic algal and detrital resources on a windward coral reef. Coral Reefs 20:117–125. doi:https://doi.org/10.1007/s003380100150
Quenouille B, Bermingham E, Planes S (2004) Molecular systematics of the damselfishes (Teleostei : Pomacentridae): Bayesian phylogenetic analyses of mitochondrial and nuclear DNA sequences. Mol Phylogenet Evol 31:66–88. doi:https://doi.org/10.1016/S1055-7903(03)00278-1
Rex MA, Etter RJ, Stuart CT (1997) Large-scale patterns of species richness in the deep-sea benthos. In: Ormond RG, Gage JD (eds) Marine biodiversity: patterns and process. Cambridge University Press, Cambridge, pp 94–116
Reynolds RW, Smith TM, Liu C, Chelton DB, Casey KS, Schlax MG (2007) Daily high-resolution blended analyses for sea surface temperature. J Clim 20:5473–5496. doi:https://doi.org/10.1175/2007JCLI1824.1
Robertson DR (1984) Cohabitation of competing territorial damselfishes on a Caribbean coral reef. Ecology 65:1121–1135. doi:https://doi.org/10.2307/1938320
Robertson DR (1995) Competitive ability and the potential for lotteries among territorial reef fishes. Oecologia 103:180–190. doi:https://doi.org/10.1007/BF00329078
Robertson DR (1996) Interspecific competition controls abundance and habitat use of territorial Caribbean damselfishes. Ecology 77:885–899. doi:https://doi.org/10.2307/2265509
Robertson DR, Lassig B (1980) Spatial distribution patterns and coexistence of a group of territorial damselfishes from the Great Barrier Reef. Bull Mar Sci 30:187–203
Roy K, Martein KK (2001) Latitudinal distribution of body size in northeastern Pacific marine bivalves. J Biogeogr 28:485–493. doi:https://doi.org/10.1046/j.1365-2699.2001.00561.x
Russ GR (2003) Grazer biomass correlates more strongly with production than with biomass of algal turfs on a coral reef. Coral Reefs 22:63–67
Sale PF (1978) Coexistence of coral reef fishes—a lottery for living space. Environ Biol Fishes 3:85–102. doi:https://doi.org/10.1007/BF00006310
Schmidt-Nielsen K (2002) Animal physiology: adaptation & environment. Cambridge University Press, Cambridge
Scott FJ, Russ GR (1987) Effects of grazing on species composition of the epilithic algal community on coral reefs of the central Great Barrier Reef. Mar Ecol Prog Ser 39:293–304. doi:https://doi.org/10.3354/meps039293
Smith KF, Brown JH (2002) Patterns of diversity, depth range and body size among pelagic fishes along a gradient of depth. Glob Ecol Biogeogr 11:313–322. doi:https://doi.org/10.1046/j.1466-822X.2002.00286.x
Souza AT (2007) Uso de habitat, comportamento alimentar e territorial de Stegastes rocasensis (Emery, 1972) (Pomacentridae: Teleostei) em Fernando de Noronha—PE. MSc thesis in Biological Sciences (Zoology), Universidade Federal da Paraíba, João Pessoa, Brazil
Waldner RE, Robertson DR (1980) Patterns of habitat partitioning by eight species of territorial Caribbean damselfishes (Pisces: Pomacentridae). Bull Mar Sci 30:171–186
Wellington GM (1982) Depth zonation of corals in the Gulf of Panama: control and facilitation by resident reef fishes. Ecol Monogr 52:223–241. doi:https://doi.org/10.2307/2937329
Wilson S, Bellwood DR (1997) Cryptic dietary components of territorial damselfishes (Pomacentridae, Labroidei). Mar Ecol Prog Ser 153:299–310. doi:https://doi.org/10.3354/meps153299
Yager TK, Summerfelt RC (1993) Effects of fish size and feeding frequency on metabolism of juvenile walleye. Aquac Eng 12:19–36. doi:https://doi.org/10.1016/0144-8609(93)90024-6
Zar JH (1999) Biostatiscal analysis. Prentice Hall, Upper Saddles River
Zemke-White LW, Choat JH, Clements K (2002) A re-evaluation of the diel feeding hypothesis for marine herbivorous fishes. Mar Biol 141:571–579
Acknowledgments
We would like to thank D. Wilhelm Filho and the three reviewers for corrections and insightful comments; R.F. Yamashita for Tamadaré Reef data; H. Andrade for statistical advice. Financial support was provided by CNPq (D.R. Barneche Bolsista PIBIC do CNPq - Brazil), National Geographic Society (grant 7937–05 to S.R. Floeter), Smithsonian Tropical Research Institute (STV grants to S.R. Floeter and C.E.L. Ferreira), Universidade Federal de Santa Catarina (Funpesquisa grant 239–2006 to S.R. Floeter) and IEAPM (C.E.L. Ferreira). Data collection on Australian and PNG reefs was supported by GBRMPA and ARC grants to D.M. Ceccarelli and G.P. Jones.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.D. Connell.
Rights and permissions
About this article
Cite this article
Barneche, D.R., Floeter, S.R., Ceccarelli, D.M. et al. Feeding macroecology of territorial damselfishes (Perciformes: Pomacentridae). Mar Biol 156, 289–299 (2009). https://doi.org/10.1007/s00227-008-1083-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1083-z