Abstract
The South Florida subpopulation of loggerhead sea turtles (Caretta caretta L.) nests with great fidelity on either the southeast or the southwest coast of Florida, USA. The hatchlings that emerge from those nests must swim in opposite directions and search for different surface currents to migrate away from continental shelf waters. In this laboratory study, we compared the pattern of swimming activity shown by the hatchlings from each coast over the first 6 days of migration. Turtles from both coasts were equally active during their “frenzy” period (the first 24 h of swimming) and during the daylight hours of the 5 days that followed (the “postfrenzy” period). However, the west coast turtles were significantly more active than the east coast turtles during the nocturnal portion of the postfrenzy period. This difference may be related to the greater distance southwest coast turtles must negotiate to locate surface currents for transport out of the Gulf of Mexico and into the Atlantic Ocean basin. These differing behavioral strategies may be genetically determined, as similar correspondence between activity and distance is well known among migratory populations of birds and fish and is often based upon inherited programs of endogenously driven activity. Alternatively, behavioral differences between the two nesting groups could be a manifestation of phenotypic plasticity that arises as the hatchlings respond to unique environmental cues on each coast.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Marine turtles are migratory during all stages of development (Hendrickson 1980; Carr 1987). Migration begins after the hatchlings emerge from underground nests on oceanic beaches, crawl to the water and swim offshore during a “frenzy” (hyperactive swimming period; Carr 1962) that lasts about 24 h (Wyneken and Salmon 1992). Days to weeks later, the turtles locate oceanic currents that transport them to distant “nursery” areas (reviewed by Musick and Limpus 1997; Bolten 2003a).
The geographic elements of marine turtle migratory movements are best understood in loggerheads (Caretta caretta L.) breeding in the northern hemisphere. Most nesting occurs on beaches located on the west side of the Atlantic and Pacific Ocean basins. During their migration, hatchlings are persistent but weak swimmers that ultimately depend upon gyre currents (Gulf Stream in the North Atlantic; Kuroshio in the North Pacific) for transport to nursery habitats located on the east side of the basin (Bowen et al. 1994, 1995; Bolten et al. 1998; Laurent et al. 1998; Bolten 2003b; Luschi et al. 2003). Later (10–12 years in the Atlantic; Bjorndal et al. 2000, 2001), the now larger juveniles cross the ocean in the opposite direction, often against prevailing currents (Luschi et al. 2003), and occupy coastal “developmental” (feeding) habitats, many of which are on the west side of each basin. Once there, turtles may migrate through coastal waters seasonally (Hopkins-Murphy et al. 2003) or return for brief periods to deeper water (McClellan and Read 2007).
Tagging and satellite tracking studies show that female loggerheads migrate with fidelity between coastal feeding areas and “natal” nesting beaches located in specific geographic regions (Limpus et al. 1992; Broderick et al. 2007). Regionally, nesting females can also be distinguished genetically as members of a “subpopulation” by their unique mitochondrial (mDNA) genetic haplotypes (Bowen et al. 1993) as all are descendents of a “founder” that originally nested in that region (Bowen et al. 1995; Encalada et al. 1998). The juvenile turtles in the nursery areas, however, are the offspring of all of the subpopulations and so are a mDNA “mixed stock” (Bolten et al. 1998; LaCasella et al. 2005; Bowen et al. 2005). Their proportional representation in the nursery is correlated with each subpopulation’s contribution in hatchling production (Bolten et al. 1998).
The convergence of genetically distinct individuals from varying geographic origins onto common foraging areas raises questions about mechanisms of natal dispersal. How do the juveniles from each subpopulation, whose nests are sometimes located on different beaches, reach the same nursery areas? Do the hatchlings migrating from each site differ behaviorally and if so, how do those differences develop in individuals and evolve among the subpopulations? To our knowledge, no published studies, using marine turtles as subjects, have addressed these issues.
Even before they enter the sea, North Atlantic loggerhead hatchlings possess a magnetic sense that guides their transport eastward across the ocean (Lohmann and Lohmann 2003). But hatchlings exiting different beaches may have to swim for different distances, and often in different directions either to locate gyre currents or to find other current systems that provide entry into the gyre. These considerations led us to hypothesize that hatchlings entering the sea from different rookery sites might initially differ in migratory behavior (orientation, activity, or both), but later converge in response to common geomagnetic (and possibly additional) cues once they become entrained within the gyre. Analogous differences in behavior are well known among the juveniles and adults of geographically distinct populations of migratory birds, fishes and insects (Dingle 1996; Dingle and Drake 2007).
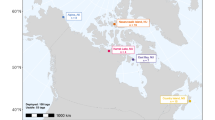
Here, we compare the migratory activity shown by loggerhead hatchlings from the South Florida subpopulation. Nesting females, with rare exceptions, deposit clutches on either the southeast (hereafter, east turtles) or southwest (west turtles) coast of the peninsula (Bjorndal et al. 1983; Lebuff 1990). East turtles swim toward the Gulf Stream (Witherington 2002) which is located relatively close to the coastline. West turtles, however, swim in the opposite direction (Wyneken and Salmon, unpublished data) toward the more distant Loop Current (Fig. 1; Molinari 1980; Sturges and Evans 1983). We therefore hypothesized that the west turtles might show greater activity during the early stages of migration than the east turtles. Our results provide support for that hypothesis.
Diagram of surface currents adjacent to peninsular Florida (adapted from http://www.noaa.gov, 2006). Hatchlings that emerge from each nesting beach (position near the white dots) swim toward the Loop Current on the west coast of Florida and toward the Gulf Stream on the East coast of Florida. The Loop Current flows into the Gulf Stream via the Florida Current. Dashed dotted line is the 200 m isocline
Materials and methods
Animals
Loggerhead hatchlings were obtained between July and early October. East turtles were studied during the summers of 1986, 1992 and 2006. They came from nests located between Broward and Brevard Counties (25°58′49″N–28°46′56″N). West turtles were studied in 1991 and 1992 and came from nests located between Longboat Key and Casey Key (27°23′44″N–27°7′25″N). Nests on each coast were monitored for several days prior to an anticipated hatchling emergence (~50–55 days after the deposition date). When a depression formed above the egg chamber indicating an emergence would occur that evening, we dug into sand above the nest and carefully removed up to four hatchlings nearest the sand surface, without disturbing the remaining turtles (which emerged on their own later that night).
Siblings were transported to the laboratory inside lightproof Styrofoam® boxes that contained a shallow layer of moist sand to prevent dehydration. Coolers were stored in a dark room at ambient temperatures (28–30°C) until just before midnight, when hatchlings were individually placed in harnesses and tethered so they could swim inside water-filled pools.
Activity
Swimming activity was measured using previously described methods (Wyneken and Salmon 1992) that are briefly summarized here. Each turtle swam inside a circular, plastic pool (1.6 m diameter × 25 cm deep, filled to a depth of 15 cm with local seawater) where it was tethered with a short length of monofilament line to a horizontally rotating lever arm (Fig. 2). Tethered hatchlings could swim in any direction, make shallow dives, and surface to breathe but could not contact either the side or bottom of the pool. When hatchlings swam, they pulled the arm down to complete an electrical (DC) circuit used to measure their time active; when they rested at the water surface, a counterweight raised the arm and broke the circuit. Activity data were stored on a computer or a chart recorder (Esterline-Angus, Model A620X; Indianapolis, Indiana) for 6 days (144 h).
Activity of the east turtles was quantified at the Harbor Branch Oceanographic Institution (Fort Pierce, FL, USA) and at the Florida Atlantic University marine laboratory (Boca Raton, FL, USA). West turtles were tested at the Mote Marine Laboratory (Sarasota, FL, USA). At both sites, air temperature was maintained between 28 and 30°C and photoperiod matched seasonal changes. Lighting was provided by a mixture of sunlight, supplemented when necessary by full spectrum fluorescent lamps, turned on and off with timers. Gradual changes in illumination during dawn–dusk transitions were provided by switching some of the lights on 30 min before local sunrise and switching some of them off 30 min after sunset. Maximum light intensities at the different sites varied by no more than ±15%.
Hatchlings from different nests were tested as they became available. On days 4–6 of observations turtles were fed bits of raw shrimp at different times of the day. Within a few days after making these measurements the turtles were transported by boat 10–15 km offshore and released according to Florida Fish and Wildlife Conservation Commission guidelines (2002).
Data analysis and statistics
We quantified the total time each turtle was active and converted those times into proportions of the available light and dark period. This procedure made it possible to standardize for seasonal differences in day length.
Proportions were arcsine transformed to promote normality and the variance assessed for uniformity. Two-way, repeated-measures ANOVAs (Zar 1999) were used to analyze the proportion of time spent active during each (24 h) day, during the diurnal period, and during the nocturnal period. When differences were found, post hoc Tukey tests were used to identify the source(s) (Zar 1999). The null hypothesis of no difference between the nesting groups was rejected when P ≤ 0.01.
Results
Data were obtained for 37 east coast turtles from 10 nests and for 30 west coast turtles from 8 nests.
Daily (24 h) activity levels shown by the groups differed significantly (coast, Table 1) as the west coast turtles were generally more active (Fig. 3). Differences were also apparent across the 6 days (period, Table 1) as in both groups, activity during the first 24 h of swimming (the frenzy period) exceeded activity shown over the following 5 days (the postfrenzy period; Fig. 3). Differences as a function of interactions between coast and period, however, did not differ statistically between the two groups (Table 1).
Proportion of time turtles actively swam over each 24 h period for 6 days. Above, west coast turtles (n = 30); below, east coast turtles (n = 37). Stars indicate that activity on that day exceeds (by P ≤ 0.01) activity during days (those below the horizontal bar) that follow. Brackets are the SE of the mean
During the diurnal period, there were no statistical differences in activity between the turtles from the two coasts (Table 2). However, both groups were significantly more active during day 1 than during most of the days that followed (Day, Table 2; Fig. 4).
Arcsine transformed proportions of diurnal (open bars, left) and nocturnal (solid bars, right) activity shown by the hatchlings from each coastal nesting group. Format and symbols, as in Fig. 3
Turtles from the two coasts differed significantly in nocturnal activity by coast, by night, and as a function of the interaction between coast and night (Table 3). Activity was highest in both groups during night 1 and lower during subsequent evenings (Fig. 4). Nocturnal activity in the east turtles declined over successive evenings but in the west turtles there was no further decline after night 2 (Fig. 4).
Discussion
Hatchlings swimming offshore from Florida’s east and west coast are members of the same (Southern Florida) subpopulation, and presently cannot be distinguished genetically (Bowen et al. 1993). They show identically high levels of swimming activity during their frenzy period but as this study shows the west turtles are more active than the east turtles during the postfrenzy period. This difference between the groups occurs because the west turtles swim more actively at night.
One possibility is that each nesting group responded to some difference associated with the laboratories we used to house our pools. We minimized this possibility by controlling for the most obvious variables (light levels and spectral composition; air and water temperatures; pool size, shape and color) that might have induced the two nesting groups to behave differently. Some differences obviously remained (e.g., room color and/or shape) but if detected visually should have induced differences in behavior between the nesting groups when the laboratory was illuminated. No such effects were evident during the day (Table 2).
Another possible explanation for these differences is that they occurred by chance during those times when the two nesting groups were studied during different years. The nature of both the similarities and the differences between the two groups makes this explanation unlikely. Even though the two groups were sometimes studied during different years, they failed to differ in either frenzy period activity or postfrenzy activity during the day. The highly specific differences shown by the two groups during the dark period are difficult to attribute to some factor that varied annually, and affected overall migratory vigor. A more plausible hypothesis is that the difference we observed is shaped by natural selection because of its important survival benefits.
Other variables, left free to vary, may have affected the turtles’ behavior. These include the possibility of genetic differences (currently unknown), habitat differences during development (such as nest sand composition, which can affect growth, size and yolk reserves at hatching, and hatchling behavior; Carthy et al. 2003), and differences between the seawater (Atlantic Ocean vs. Gulf of Mexico) used to fill the pools (which might trigger different migratory responses). Our data do not permit us to distinguish between the causal factor(s).
Ecological correlates
Nesting beaches are often located in proximity to offshore currents that transport neonates to nursery areas (Collard and Ogren 1990), although distances between the coast and those currents can vary. For hatchlings emerging from southeast Florida nesting beaches, those distances are relatively short (5–30 km, depending upon nesting beach latitude; NOAA National Data Buoy Center 2006). Even so, it may take weeks or even months for the turtles to become entrained within the gyre (Witherington 2002; Bolten 2003a).
On the west coast of Florida, loggerhead hatchlings must swim toward the Loop Current which could most directly transport them south to the Florida Straits, where they gain entry into the Atlantic Ocean (Fig. 1). To complete this phase of migration, the west turtles must negotiate a distance exceeding 200 km. The Loop Current also varies more capriciously than the Gulf Stream in its geographic position relative to the coast, and in its northward intrusion into the Gulf of Mexico (Molinari 1980; Sturges and Evans 1983; He and Weisburg 2003). Once entrained within the Loop Current, the hatchlings also face the possibility of transport west by warm-core rings or eddies that separate from the main current, then travel across the Gulf of Mexico (Collard and Ogren 1990; Gyory et al. 2005). A few turtles that strand on the west side of the Gulf of Mexico may have suffered that fate (Plotkin 1996).
Few neonates strand on eastern Gulf beaches, leading to the hypothesis that normally the turtles are transported around the Florida peninsula to the Atlantic Ocean. However, the evidence is weak and based, in part, upon doubts that hatchling loggerheads can survive winter temperatures in the Gulf of Mexico. The number of west hatchlings that recruit to the eastern Atlantic nursery population is unknown, first because they cannot be distinguished genetically from the more numerous east coast turtles and second, because proportionally so few hatchlings are produced on Florida’s west coast (Bolten 2003a).
On the other hand, differences in postfrenzy swimming activity between the east and west coast turtles suggest, albeit indirectly, that each nesting group can show behavioral differences that might promote survival under conditions that can vary during offshore migration. Distance may not be the only factor responsible for those behavioral differences. Coastal waters in the eastern Gulf of Mexico are more productive (as measured by chlorophyll a levels) and the continental shelf extends farther offshore than on the opposite Florida coastline (Fig. 5). For both reasons, the Gulf of Mexico is likely to support greater densities of predaceous seabirds and fishes (Rhyther 1969; Waide et al. 1999). Many of the seabirds and fishes (tarpon, barracuda, sharks, dolphin and snappers) are documented hatchling predators (Caldwell 1959; Hughes 1974; Witham 1974; Stancyk 1982; Stewart and Wyneken 2004). West coast hatchlings may increase their nocturnal postfrenzy swimming activity to avoid detection and to minimize their exposure time to these predators, many of which are diurnally active.
Coastal chlorophyll a concentrations adjacent to peninsular Florida (December 1999–December 2006). Concentrations in Florida are higher and more extensive in offshore waters adjacent to the southwest than on the southeast coast. Image and data are from the GES-DISC Interactive Online Visualization and Analysis Infrastructure (Giovanni), NASA Goddard Earth Sciences (GES), Data and Information Services Center (DISC); NASA, 2006
Among other migratory species, selection promotes comparable differences in behavior (as well as morphology and physiology) among populations migrating different distances (Åkesson and Hedenström 2007; Pulido 2007). For example, Blackcap warbler (Sylvia atricapilla) populations show fidelity to different overwintering and breeding sites (Berthold and Querner 1982; Berthold 1988; review: Berthold 1993). Each population has an endogenous program of migration that differs in its duration and onset time. Birds migrating over greater distances show longer periods of migratory activity (Berthold and Querner 1982; Gwinner and Helm 2003) and hybridization experiments demonstrate that differences among populations are inherited (Berthold and Helbig 1992). Selection experiments indicate that population responses can evolve rapidly (Pulido et al. 1996).
Morphological and behavioral differences also occur among Pacific salmon populations that differ in the distances they migrate up rivers to spawn. Sockeye salmon (Oncorhynchus nerka) that migrate farther store more energy reserves prior to migration, possess larger, more streamlined and hydrodynamically efficient body shapes, and use their energy reserves more efficiently for locomotion than populations that spawn at sites nearer to the ocean (Crossin et al. 2004). Behavioral differences also characterize populations of other salmonids (Coho, O. kisutch; Chinook, O. tshawytscha) where travel costs vary (Taylor and McPhail 1985; Brett 1995; Jonsson and Jonsson 2003). The young migrating from streams located different distances from the sea also show population-specific variation in migratory schedules and in orientation behavior (Hinch et al. 2003).
Morphological and behavioral variation in hatchlings
Recent studies document that hatchling phenotypes, even among nests that develop on the same beach, are variable. Differences can arise as a consequence of nest temperature, the moisture content of the sand surrounding the eggs, and their interactions (review: Ackerman 1997) and result in differences in body size, differences in swim speed and in rates of juvenile growth (review: Carthy 2003). Such differences can in theory (Burgess et al. 2006) or in practice (Gyuris 2000) affect survival and thus can serve as the raw material for selection.
When comparisons are made between hatchlings from different subpopulations phenotypic differences are more pronounced. For example, loggerheads (Stokes et al. 2006) and green turtles (Glen et al. 2003) from different Atlantic subpopulations differ in size and mass. Green turtles from the Atlantic and Pacific ocean basins show even more striking differences manifested by variation in allometry (body size relative to flipper size), shell color, and shell shape (Pritchard 1997; Wyneken et al. 1999). These more profound changes reflect the effects of longer periods of isolation and genetic divergence on developmental programs.
We document in this study that there can be significant behavioral differences even within what is currently considered a single subpopulation of loggerhead sea turtles, and that those behavioral contrasts resemble those found among other migratory animals facing similar ecological pressures (most generally, differences in migration “costs”). The next step is to determine how those differences develop. One possibility is that they are the consequence of a flexible genetic system that responds differently when organisms are exposed to each coastal environment (e.g., the turtles show phenotypic plasticity; Agrawal 2001). Alternatively, the two nesting groups may have developed genetic differences that best promote their survival during migration away from each coastline. We plan further experiments designed to distinguish between these alternatives.
References
Ackerman RA (1997) The nest environment and the embryonic development of sea turtles. In: Lutz PL, JA Musick (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 83–106
Agrawal AA (2001) Phenotypic plasticity in the interactions and evolution of species. Science 294:321–326. doi:https://doi.org/10.1126/science.1060701
Åkesson S, Hedenström A (2007) How migrants get there: migratory performance and orientation. Bioscience 57:123–133. doi:https://doi.org/10.1641/B570207
Berthold P (1988) Evolutionary aspects of migratory behavior in European warblers. J Evol Biol 1:195–209. doi:https://doi.org/10.1046/j.1420-9101.1998.1030195.x
Berthold P (1993) Bird migration: a general survey. Oxford University Press, Oxford
Berthold P, Helbig AJ (1992) The genetics of bird migration: stimulus, timing, and direction. Ibis 134:35–40
Berthold P, Querner U (1982) Partial migration in birds: experimental proof of polymorphism as a controlling system. Experientia 38:805. doi:https://doi.org/10.1007/BF01972282
Bjorndal KA, Meylan AB, Turner BJ (1983) Sea turtle nesting at Melbourne Beach, Florida, I. Size, growth and reproductive biology. Conservation 26:65–77. doi:https://doi.org/10.1016/0006-3207(83)90049-6
Bjorndal KA, Bolten AB, Martins HR (2000) Somatic growth model of juvenile loggerhead sea turtles Caretta caretta: duration of the pelagic stage. Mar Ecol Prog Ser 202:265–272. doi:https://doi.org/10.3354/meps202265
Bjorndal KA, Bolten AB, Koike B, Schroeder BA, Shaver DJ, Teas WG, Witzell WN (2001) Somatic growth function for immature loggerhead sea turtles in southeastern US waters. Fish Bull (Wash D C) 99:240–246
Bolten AB (2003a) Variation in sea turtle life history patterns: neritic vs. oceanic developmental stages. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 243–257
Bolten AB (2003b) Active swimmers-passive drifters: the oceanic juvenile stage of loggerheads in the Atlantic system. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Books, Washington, pp 63–78
Bolten AB, Bjorndal KA, Martins HR, Dellinger T, Biscoito MJ, Encalada SE, Bowen BW (1998) Trans-Atlantic developmental migrations of loggerhead sea turtles demonstrated by mtDNA sequence analyses. Ecol Appl 8:1–7. doi:https://doi.org/10.1890/1051-0761(1998)008[0001:TDMOLS]2.0.CO;2
Bowen BW, Avise JC, Richardson JI, Meylan AB, Margaritoulis D, Hopkins-Murphy SR (1993) Population structure of loggerhead turtles (Caretta caretta) in the northwestern Atlantic Ocean and Mediterranean Sea. Conserv Biol 7:834–843. doi:https://doi.org/10.1046/j.1523-1739.1993.740834.x
Bowen BW, Kamezaki N, Limpus CJ, Hughes GR, Meylan AB, Avise JC (1994) Global phylogeography of the loggerhead turtle (Caretta caretta) as indicated by mitochondrial DNA haplotypes. Evol Int J Org Evol 48:1820–1828. doi:https://doi.org/10.2307/2410511
Bowen BW, Abreu-Grobois FA, Balazs GH, Kamezaki N, Limpus CJ, Ferl RJ (1995) Trans-Pacific migrations of the loggerhead sea turtle demonstrated with mitochondrial DNA markers. Proc Natl Acad Sci 92:3731–3734
Bowen BW, Bass AL, Soares L, Toonen RJ (2005) Conservation implications of complex population structure: lessons from the loggerhead turtle (Caretta caretta). Mol Ecol 14:2389–2402
Brett JR (1995) Energetics. In: Groot C, Margolis L, Clarke WC (eds) Physiological ecology of Pacific Salmon. University of British Columbia Press, Vancouver, pp 3–68
Broderick AC, Coyne MC, Fuller WJ, Glen F, Godley BJ (2007) Fidelity and over-wintering of sea turtles. Proc R Soc B 274:1533–1538
Burgess E, Booth D, Lanyon J (2006) Swimming performance of hatchling green turtles is affected by incubation temperature. Coral Reefs 25:341–349. doi:https://doi.org/10.1007/s00338-006-0116-7
Carthy RR, Foley AM, Matsuzawa Y (2003) In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Books, Washington DC, pp 144–154
Carr AF (1962) Orientation problems in the high seas travel and terrestrial movements of marine turtles. Am Sci 50:359–374
Carr AF (1987) New perspectives on the pelagic stage of sea turtle development. Conserv Biol 1:103–121. doi:https://doi.org/10.1111/j.1523-1739.1987.tb00020.x
Caldwell DK (1959) The loggerhead turtles of Cape Romain, South Carolina. Bull Fla State Mus 4:319–348
Collard S, Ogren LH (1990) Dispersal scenarios for pelagic post-hatchling sea turtles. Bull Mar Sci 47:223–243
Crossin GT, Hinch SG, Farrell AP, Higgs DA, Lotto AG, Oakes JD, Healey MD (2004) Energetics and morphology of sockeye salmon: effects of upriver migratory distance and elevation. J Fish Biol 65:788–810. doi:https://doi.org/10.1111/j.0022-1112.2004.00486.x
Dingle H (1996) Migration: the biology of life on the move. Oxford University Press, New York
Dingle H, Drake VA (2007) What is migration? Bioscience 57:113–121. doi:https://doi.org/10.1641/B570206
Encalada SE, Bjorndal KA, Bolten AB, Zurita JC, Schroeder BA, Possardt E, Sears J, Bowen BW (1998) Population structure of loggerhead turtle (Caretta caretta) nesting colonies in the Atlantic and Mediterranean as inferred from mitochondrial DNA control region sequences. Mar Biol (Berl) 130:567–575. doi:https://doi.org/10.1007/s002270050278
Florida Fish and Wildlife Conservation Commission (2002) Sea Turtle Conservation Guidelines Myfwc.org Tallahassee Florida
Glen F, Broderick AC, Godley BJ, Hays GC (2003) Incubation environment affects phenotype of naturally incubated green turtle hatchlings. J Mar Biol Assoc UK 83:1183–1186. doi:https://doi.org/10.1017/S0025315403008464h
Gwinner E, Helm B (2003) Circannual and circadian contributions to the timing of avian migration. In: Berthold P, Gwinner E, Sonnenschein E (eds) Avian migration. Springer, Berlin, pp 81–95
Gyory J, Mariano AJ, Ryan EH (2005) The gulf stream: ocean surface currents. http://oceancurrents.rsmas.miami.edu/atlantic/gulf-stream.html
Gyuris E (2000) The relationship between body size and predation rates on hatchlings of the green turtle (Chelonia mydas): is bigger better? In: Pilcher NJ, Ali L (eds) Sea turtles of the Indo-Pacific. Asean Academic Press, London, pp 143–147
He R, Weisburg RH (2003) A loop current intrusion case study on the west Florida shelf. J Phys Oceanogr 33:465–477. doi:https://doi.org/10.1175/1520-0485(2003)033<0465:ALCICS>2.0.CO;2
Hendrickson JR (1980) The ecological strategies of sea turtles. Am Zool 20:597–608
Hinch SG, Cooke SJ, Healey MC, Farrell AP (2003) Behavioural physiology of fish migrations: salmon as a model approach. In: Sloman KA, Wilson RW, Balshine S (eds) Behaviour and physiology of fish. Elsevier, London, pp 239–295
Hopkins-Murphy SR, Owens DW, Morphy TM (2003) In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Books, Washington DC, pp 79–92
Hughes GR (1974) The sea turtles of South East Africa. II. The biology of the Tongaland loggerhead turtle Caretta caretta L. with comments on the leatherback turtle Dermochelys coriacea L. and the green turtle Chelonia mydas L. in the study region. S Afr Assoc Mar Biol Res Investig Rep 36:1–96
Jonsson N, Jonsson B (2003) Energy allocation among developmental stages, age groups and types of Atlantic salmon (Salmo salar) spawners. Can J Fish Aquat Sci 60:506–516. doi:https://doi.org/10.1139/f03-042
LaCasella EL, Dutton PH, Epperly SP (2005) Genetic stock composition of loggerheads (Caretta caretta) encountered in the northeast Atlantic distant (NED) longline fishery using mtDNA analysis. NOAA-NMFS-SEFSC Tech Memo. National Technical Information Service, Springfield, Virginia
Lebuff CR Jr (1990) The loggerhead turtle in the eastern Gulf of Mexico. Caretta Research Inc, Sanibel
Laurent L et al (1998) Molecular resolution of the marine turtle stock composition in fishery bycatch: a case study in the Mediterranean. Mol Ecol 7:1529–1542. doi:https://doi.org/10.1046/j.1365-294x.1998.00471.x
Limpus CJ, Miller JD, Parmenter CJ, Reiner D, McLachian N, Webb R (1992) Migration of green (Chelonia mydas) and loggerhead (Caretta caretta) turtles to and from Eastern Australian rookeries. Aust J Wild Res 19:347–358. doi:https://doi.org/10.1071/WR9920347
Lohmann KJ, Lohmann CMF (2003) Orientation mechanisms of hatchling loggerheads. In: Bolten AB, Witherington BE (eds) Loggerhead sea turtles. Smithsonian Books, Washington DC, pp 44–62
Luschi P, Hays GC, Papi F (2003) A review of long-distance movements by marine turtles, and the possible role of ocean currents. Oikos 103:293–302. doi:https://doi.org/10.1034/j.1600-0706.2003.12123.x
Molinari RL (1980) Current variability and its relation to sea-surface topography in the Caribbean Sea and the Gulf of Mexico. Mar Geol 3:409–436
McClellan CM, Read AJ (2007) Complexity and variation in loggerhead sea turtle life history. Biol Lett 3:592–594. doi:https://doi.org/10.1098/rsbl.2007.0355
Musick JA, Limpus CJ (1997) In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 137–159
NOAA National Data Buoy Center (2006) October 3. Florida and eastern Gulf of Mexico Recent Marine Data. http://www.ndbc.noaa.gov/maps/Florida.shtml. Accessed 3 Dec 2006
Plotkin P (1996) Occurrence and diet of juvenile loggerhead sea turtles, Caretta caretta, in the Northwestern Gulf of Mexico. Chelonian Conserv Biol 2:78–80
Pritchard PCH (1997) Evolution, phylogeny, and current status. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 1–28
Pulido F (2007) The genetics and evolution of avian migration. Bioscience 57:165–174. doi:https://doi.org/10.1641/B570211
Pulido F, Berthold P, van Noordwijk AJ (1996) Frequency of migrants and migratory activity are genetically correlated in a bird population: evolutionary implications. Proc Natl Acad Sci USA 93:14642–14647. doi:https://doi.org/10.1073/pnas.93.25.14642
Rhyther JH (1969) Photosynthesis and fish production in the sea. Science 166:72–76. doi:https://doi.org/10.1126/science.166.3901.72
Stancyk SE (1982) Non-human predators of sea turtles and their control. In: Bjorndal KA (ed) Biology and conservation of sea turtles. Smithsonian Institution, Washington DC, pp 19–38
Stewart KR, Wyneken J (2004) Predator risk to loggerhead hatchlings at a high-density nesting beach in Southeast Florida. Bull Mar Sci 74:325–335
Stokes L, Wyneken J, Crowder LB, Marsh J (2006) The influence of temporal and spatial origin on size and early growth rates in captive loggerhead sea turtles (Caretta caretta) in the United States. Herpetol Conserv Biol 1:71–80
Sturges W, Evans JC (1983) On the variability of the loop current in the Gulf of Mexico. J Mar Res 41:639–653
Taylor EB, McPhail D (1985) Variation in body morphology among British Columbia stocks of coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 42:2020–2028
Waide RB et al (1999) The relationship between productivity and species richness. Annu Rev Ecol Syst 30:257–300. doi:https://doi.org/10.1146/annurev.ecolsys.30.1.257
Witham R (1974) Neonate sea turtles from the stomach of a pelagic fish. Copeia 1974:548. doi:https://doi.org/10.2307/1442555
Witherington BE (2002) Ecology of neonate loggerhead turtles inhabiting lines of downwelling near a Gulf Stream front. Mar Biol (Berl) 140:843–853. doi:https://doi.org/10.1007/s00227-001-0737-x
Wyneken J, Salmon M (1992) Frenzy and postfrenzy swimming activity in loggerhead, green, and leatherback hatchling sea turtles. Copeia 1992:478–484. doi:https://doi.org/10.2307/1446208
Wyneken J, Balazs GH, Murakawa SKK, Anderson Y (1999) Size differences in hind limbs and carapaces of hatchling green turtles (Chelonia mydas) from Hawaii and Florida. USA Chelonian Conserv Biol 3:491–495
Zar JH (1999) Biostatistical analysis. Prentice Hall, New Jersey
Acknowledgments
Portions of this study were completed by SVM as a Masters thesis in the Department of Biological Sciences at Florida Atlantic University. Financial support was provided by the Nelligan Fund. The study was approved by the State (Florida Fish and Wildlife Conservation Commission, Turtle Permits No. 73 and 173) and by the University Institutional Animal Care Committee (Protocol A06-08).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Lewison.
Rights and permissions
About this article
Cite this article
Wyneken, J., Madrak, S.V., Salmon, M. et al. Migratory activity by hatchling loggerhead sea turtles (Caretta caretta L.): evidence for divergence between nesting groups. Mar Biol 156, 171–178 (2008). https://doi.org/10.1007/s00227-008-1074-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-1074-0