Abstract
Variation in the timing and abundance of marine food resources is known to affect the breeding behaviour of many seabirds, constraining our understanding of the extent to which these behaviours vary in different parts of a species’ range. We studied incubation shifts of northern fulmars (Fulmarus glacialis) breeding at two colonies in Arctic Canada (High Arctic oceanographic zone) and one colony in the UK (Boreal oceanographic zone) between 2001 and 2005. Fulmars in Arctic Canada had longer incubation shifts than previously reported at more southern colonies, presumably because marine productivity is lower early in the breeding season in the Arctic. Shift durations were particularly long at one colony in years with abnormally late, extensive sea-ice cover, although at the other Arctic colony, where sea-ice cover is predictably late every year, the duration of shifts was shorter than expected. At the Boreal colony, incubation shifts were much longer than expected, similar to Arctic colonies, and likely attributable to poor marine food supplies in the North Sea in recent years. Collectively, our data suggest that fulmars can adjust their incubation rhythm to compensate for poor marine feeding conditions, although this may incur a cost to body condition or reproductive success.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oceans experience a variety of natural cycles and anthropogenic stressors, which cause changes in marine food webs (Aebischer et al. 1990; Pauly and Maclean 2003; ACIA 2005; Frederiksen et al. 2006). One way to track these effects is to monitor seabirds, which are often regarded as effective indicators of the condition of marine ecosystems (Cairns 1987; Furness and Camphuysen 1997; Frederiksen et al. 2007). Because these birds rely on resources from the ocean, changes in marine productivity or specific food supplies may be detected by monitoring seabird reproduction. For example, in response to local reductions in marine food supplies, breeding seabirds may exhibit lower colony attendance, fewer breeding attempts, delayed egg-laying, reduced clutch or egg size, reduced reproductive success, or altered behaviour during breeding (e.g. Schreiber 2002; Frederiksen et al. 2006). Even when marine conditions are “normal”, aspects of seabird breeding behaviour may differ between colonies where the type, distribution, and availability of principal prey items differ (e.g. Lewis et al. 2004, 2006; Wilson et al. 2005). Therefore, in situations where annual marine food supplies differ predictably, we might expect to see concordant differences or local adaptation in the typical behaviour of seabird conspecifics at their colonies.
One aspect of breeding behaviour that should be sensitive to food availability is the incubation rhythm of nesting pairs. For pelagic seabirds, and notably the petrels, incubation behaviour has been well-studied (Warham 1990). Incubation is energetically demanding for petrels, with one mate losing 2–20% of its body mass during an incubation shift (e.g. Warham 1990, Chaurand and Weimerskirch 1994; Chastel et al. 1995), while the other mate may travel long distances to find sufficient food to replenish energy stores before returning to the nest (Chaurand and Weimerskirch 1994; Weimerskirch 1995, 1998). Thus, incubation shift length is affected both by the time required to search for food and the physical condition of each mate. Reduced availability of food or increased effort to find food may result in longer incubation shifts in petrels (e.g. Johnstone and Davies 1990).
Some aspects of incubation have been studied in northern fulmars (Fulmarus glacialis), the only petrel found in the Boreal, Low Arctic and High Arctic oceanographic zones (Salomonsen 1965; Hatch and Nettleship 1998). Fulmars exhibit both individual and seasonal variation in incubation rhythms (Hatch 1990a, b). Some of this variation is likely attributable to age and breeding experience (Ollason and Dunnet 1978; Hatch 1990b), but annual conditions in the marine environment also appear to influence incubation scheduling (Hatch 1990b; Gaston et al. 2005).
We studied incubation rhythms of northern fulmars nesting at two colonies in the Canadian High Arctic, as well as one colony in temperate waters around the UK. The Arctic sites were near the northern limit of the species’ breeding range, where ambient temperatures and sea-ice cover present a markedly different environment from that experienced by the majority of fulmars breeding elsewhere in the North Atlantic or North Pacific oceans (Fisher 1952). At the Arctic colonies, extensive sea-ice can cover local foraging areas until ice breaks up, requiring that fulmars travel farther and incur higher energetic costs to find open water in which to feed. Consequently, we predicted that fulmars in the High Arctic would typically take longer incubation shifts than fulmars breeding in the Boreal oceanographic zone, where potential foraging areas were available close by breeding colonies. Moreover, our two Arctic colonies differed in the predictability with which sea ice covered local foraging areas. At Cape Vera, fulmars had to cross 200 km of sea ice throughout incubation and into chick-rearing each year, before they reached open water to feed (Mallory and Forbes 2007). In contrast, at Prince Leopold Island, sea ice cover of surrounding marine waters varied considerably among years (Gaston et al. 2005), and typically, there was open water adjacent to the colony through much of incubation. Hence we predicted that, in normal ice years, Cape Vera fulmars would have longer incubation shifts than those breeding on Prince Leopold Island, but that in years when ice conditions adjacent to Prince Leopold Island resembled those found every year at Cape Vera, shift lengths at the two colonies would be similar.
Materials and methods
Physical data
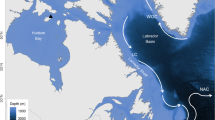
Research was conducted at two breeding colonies in Arctic Canada (Cape Vera and Prince Leopold Island) in the High Arctic oceanographic zone, and one colony in the UK (Eynhallow, Orkney) in the Boreal oceanographic zone (Fig 1). At Prince Leopold Island (74°N, 90°W), field crews observed fulmar breeding ecology between 16 June–21 August 2001, 1 June–25 August 2002, and 31 May–21 August 2003 (see also Gaston et al. 2005), while at Cape Vera (76°15′N, 89°15′W), northern Devon Island, observations were made during 26 May–22 August 2003, 14 May–9 August 2004, and 20 April–10 August 2005 (incubation data were collected only in 2005). On Eynhallow (59°08′N, 3°08′W) research on breeding ecology has been conducted since 1950 (Dunnet 1991; Thompson and Ollason 2001), but specific research on incubation was carried out from 3 June to 8 July 2003, 30 May to 14 July 2004, and 18 May to 8 July 2005.
The three northern fulmar colonies examined in this study were located in Arctic Canada (Cape Vera, Prince Leopold Island) and the UK (Eynhallow). Thick lines separate the High Arctic, Low Arctic and Boreal oceanographic zones (after Salomonsen 1965). In Arctic Canada, the North Water Polynya is indicated by “1” and Lancaster Sound is shown by “2”
At Cape Vera, nearby Jones Sound remains ice-covered from October through July of the following year, although there is open water immediately beside the colony in the Hell Gate–Cardigan Strait Polynya (Smith and Rigby 1981). However, breeding fulmars at Cape Vera do not feed in the local polynya, and instead fly to foraging areas >200 km to the east (Mallory et al. 2008). Prince Leopold Island is at the junction of Parry Channel (Lancaster Sound and Barrow Strait) and Prince Regent Inlet. Ice typically covers this region from October through at least April of the following year, but the annual extent of ice cover varies substantially (Gaston and Nettleship 1981; Gaston et al. 2005). Both colonies are situated on extensive, sedimentary cliffs rising to 300 m or more above sea level, with the majority of fulmars nesting along the upper third of the cliff faces (Gaston et al. 2006). Waters around Eynhallow are ice-free throughout the year, and potential open water foraging areas are found <1 km from nest sites that are found among the grassy turfs, old buildings and low-lying cliffs around the island (Dunnet et al. 1963). Approximate colony sizes during the study period were 11,000 apparently occupied sites (AOS) at Cape Vera and 22,000 AOS at Prince Leopold Island (Gaston et al. 2006). In both cases the nearest fulmar colonies were >100 km away. Eynhallow formed a much smaller colony (≤125 breeding pairs), but was one of many local colonies within the Orkney archipelago, where >90,000 AOS occurred within 50 km of the study site in 2000 (Mitchell et al. 2004).
We used weather data collected at the Resolute Bay airport weather station (approximately 125 km west of both Arctic field sites; http://www.weatheroffice.ec.gc.ca; accessed 24 January 2008) as indicative of general weather conditions near the Arctic colonies during incubation. Summary data for Eynhallow were acquired from the North Isles Weather Archive http://www.northisles-weather.co.uk (accessed 24 January 2008). Ice conditions near the Arctic colonies were obtained from the Environment Canada-Canadian Ice Service climate archives http://ice-glaces.ec.gc.ca (accessed 24 January 2008).
Biological data
Northern fulmars are long-lived, monogamous petrels that lay a single egg, and do not replace the egg if it is lost (Hatch and Nettleship 1998). At the Arctic colonies, fulmars are polymorphic for plumage colour, with birds ranging in colour on the head and body from pure white to dull slate grey, as well as differing in bill markings (Hatch and Nettleship 1998). Consequently, individual members of a pair could be distinguished if pair members were of contrasting morphs. The presence of these distinguishable pairs was identified at the start of each season by observing both pair members together at the site. Once identified, the colour morph of the sitting bird was recorded each day during breeding checks, allowing us to detect incubation changeovers to within ±1 day. If an exchange took place during a period when >1 day elapsed between observations (due to inclement weather), the exchange was estimated to have taken place half way between the two observations. Data were not used if >3 days had elapsed between observations.
At the Boreal colony on Eynhallow, all birds were light-phase morphs, but approximately 60–70% of study pairs could be distinguished visually from colour rings that had been applied previously (Dunnet 1991). Most data were collected in the 2004 season, when daily breeding checks allowed us to detect incubation changeovers to within ±1 day. The incubation patterns of a subset of these birds were also recorded using data loggers (Francis Scientific Instruments, Cambridge, UK) following the procedures outlined in Weimerskirch et al. (2001). Briefly, both pair members had been captured on the nest in a previous breeding season and had a small transponder (PIT tag) attached to a metal leg band. A loop antenna connected to a data logger was placed around the nest, and whenever the bird returned to or left the nest in subsequent seasons, the data logger recorded its arrival or departure. This provided precise measurements of times spent on and off the nest by each member of a smaller sample of pairs. Where gaps occurred in the visual sighting records, exchanges were assumed to have taken place half way between the detections of the two pair members.
For some years and colonies, monitoring of nest attendance was begun shortly after birds had initiated incubation. We wanted to maximize use of our data but minimize bias associated with using incomplete incubation schedules (e.g. due to having only partial, first incubation shifts by the male). Thus, we used the approach of Gaston et al. (2005) and analysed a sub-sample of shifts, specifically those that were in progress at 10-day intervals preceding the date of hatching (date of hatching for sites where eggs did not hatch was estimated as the median for the year concerned). Analysis of incubation shift duration was based on a maximum of four shifts for each nest from each study region: those in progress 30, 20, 10 and 0 days before hatching.
Data on body condition were also collected at Arctic sites to explore the effects of inter-annual variation in incubation schedules. In each year at Prince Leopold Island, and in 2003 and 2004 at Cape Vera, fulmars were captured using noose poles. At this time, fulmars were weighed (±10 g) and measured (±0.1 g), and gender was ascertained either through morphometrics or dissection (for further details of methods, see Gaston et al. 2005; Mallory and Forbes 2005). We used body mass of fulmars measured during incubation (June or July) to compare mass among years and colonies. Body mass data from the incubation period were not available from Eynhallow.
To analyse incubation parameters of fulmars, we used F ratio tests, ANOVAs (with Tukey–Kramer Multiple Comparisons tests), and Kruskal–Wallis tests (with post hoc Dunn’s Multiple Comparisons tests), depending on whether data could be square-root transformed to approximate normal distributions (Kolmogorov–Smirnov tests; GraphPad Software 1998). Sample coefficients of variation (CoV) and their standard errors were calculated and compared with Levene’s test, according to Sokal and Braumann (1980). All means are presented ±1 SE.
Results
Weather
During the typical incubation period (approximately 6 June to 25 July at Arctic colonies), the mean daily temperature over 5 years (2001–2005) was 2.3 ± 0.2°C (n = 255 days, range −7.3°C to 12°C). In the same years, the position of open water near the colonies differed greatly. In the first week of July (half way through incubation), fulmars at Cape Vera had to fly 188 ± 6 km (CV 7%) over sea ice to reach open water in the North Water Polynya, whereas at Prince Leopold Island, fulmars had to fly 15 ± 70 km (CV 139%). The annual variation in ice position was significantly higher at Prince Leopold Island (F ratio test, F8 = 60.3, P = 0.0008). At Eynhallow in 2004 and 2005, mean daily temperatures were approximately 9°C warmer than at the Arctic sites (10.0−11.9°C, similar to the 30-year average).
Incubation
Pooling data within colonies for all years, typical incubation shift duration differed among sites (KW = 33.9, P < 0.0001), with the mean duration of incubation shifts from the one season at Cape Vera (5.7 ± 0.2 days, median 6 days, n = 152 shifts) being significantly shorter than for three seasons at Prince Leopold Island (7.5 ± 0.2 days, median 8 days, n = 272; Dunn’s Multiple Comparisons Test, P < 0.001) or three seasons at Eynhallow (6.8 ± 0.4 days, median 7 days, n = 69; P < 0.05). However, there was clearly annual variation within sites (Fig. 2). Using colony-years for which we had measurements of ≥50 incubation shifts, mean shift duration differed among the Cape Vera (2005), Prince Leopold Island (2001–2003) and Eynhallow (2004) colonies among years (KW = 143.6, P < 0.0001; Table 1). In 2002, mean incubation shifts undertaken by fulmars at Prince Leopold Island were longer than in any other year (Table 1; Dunn’s Multiple Comparisons test, Ps < 0.001). Mean incubation shift length at Eynhallow in 2004 was similar to that at Cape Vera or at Prince Leopold Island in 2001 (Ps > 0.05), but was longer than was found at Prince Leopold Island in 2003 (P < 0.01). In 2003, 20% of the fulmar incubation shifts at Prince Leopold Island lasted ≤2 days, compared to 4 and 1% of the shifts recorded in 2001 and 2002, respectively. At Cape Vera in 2005 9% of shifts were ≤2 days, and at Eynhallow in 2004, 14% of shifts were ≤2 days.
Using only data for Prince Leopold Island and Eynhallow (i.e. those years approximating normal distributions), fulmars exhibited higher variability in incubation shift duration at Eynhallow (CoV 43 ± 5%) and Prince Leopold Island (2003) (48 ± 4%) than at Prince Leopold Island in 2001 (31 ± 3%) or 2002 (30 ± 3%), although these differences were not statistically significant (Levene’s test; F3,319 = 1.97, P = 0.11). The longest incubation shift recorded at both Cape Vera and Eynhallow was 14 days, whereas one shift lasted 18 days at Prince Leopold Island in 2002.
Body mass at Arctic colonies
Body mass of incubating male fulmars differed among colony-years at Cape Vera and Prince Leopold Island (ANOVA, F4,92 = 8.7, P < 0.0001; Fig. 3). Mean mass of males at Prince Leopold Island in 2003 (859 ± 25 g) was significantly heavier than males at that colony in 2001 (714 ± 19 g; Tukey–Kramer, P < 0.001) or 2002 (754 ± 11 g; P < 0.05). Males at Cape Vera in 2004 (812 ± 17 g) were also heavier than Prince Leopold Island males in 2001 (P < 0.01). Among female fulmars, incubation body mass also differed among colony-years (F4,62 = 7.7, P < 0.001). Like males, body mass of females at Prince Leopold Island in 2003 (726 ± 12 g) was higher than females in 2001 (620 ± 20 g; P < 0.01) or in 2002 (634 ± 13 g; P < 0.001). Also, female fulmars at Cape Vera in 2003 were heavier (731 ± 24 g) than Prince Leopold Island females in 2001 or 2002 (Ps < 0.01).
Discussion
The northern fulmar exhibits a flexible incubation rhythm in response to a broad range of climatic and marine environmental conditions. For example, fulmars had a similar range of the length of incubation shifts undertaken, irrespective of whether they bred in the High Arctic (Falk and Møller 1995a; this study), Alaska (Hatch 1990a) or the UK (this study). As predicted, High Arctic fulmars took relatively longer incubation shifts than elsewhere (Table 1), but fulmars breeding at Eynhallow had longer incubation shifts than previously observed in Boreal fulmars breeding at sites in the Pacific (Hatch 1990a).
Incubation in the Arctic
Most incubation shifts by fulmars breeding in the High Arctic (Cape Vera or Prince Leopold Island) lasted ≥5.3 days, which was similar to the average of 6.1 day shifts found by Falk and Møller (1995a) at a High Arctic colony in east Greenland. However, during periods of extensive sea-ice at Prince Leopold Island (2001, 2002), typical shifts increased to >8 days, 51–185% longer than reported for any other northern fulmar colonies (shift duration determined using differing techniques; Table 1). We expected fulmars in the High Arctic to take longer incubation shifts for three reasons: (1) during June and much of July, many marine areas remain ice-covered in this region, so fulmars have to travel farther to find foraging habitat; (2) birds foraging in this region during incubation may experience lower marine productivity compared to birds at more southern colonies (Behrenfeld et al. 2001); and (3) Arctic fulmars cannot rely on fisheries discards, which were believed to have been important food source in Boreal waters (Fisher 1952, but see also Phillips et al. 1999; Thompson 2006). Importantly, marine productivity has a strong influence on seabird reproductive success (Frederiksen et al. 2007). Thus, as suggested by Falk and Møller (1995a), it probably requires more foraging time and effort for High Arctic fulmars to find sufficient food to replenish energy reserves and undertake the next incubation shift (even though they operate in 24-h daylight), compared to Boreal fulmars. However, the 2.3- to 4.3-day difference in mean shift duration between fulmars at Cape Vera and Prince Leopold Island in some years was surprising, and counter to our predictions.
Fulmars at Cape Vera during a “typical” ice year faced a similar distance of sea-ice to cross as fulmars at Prince Leopold Island in extensive ice years, which is why we expected fulmars at Cape Vera to have relatively long shifts. However, the duration of their shifts averaged approximately one half day longer (but not statistically different) than shifts at Prince Leopold Island during a year when open water was close to that colony (2003), and incubation body masses in these colony-years were similar. This suggests that routinely traversing 200 km of sea-ice to reach potential feeding sites may not impose an abnormally increased energetic cost for fulmars, unlike sympatric Arctic seabirds (Gaston et al. 2005). Fulmars can fly hundreds of kilometers to feed (Falk and Møller 1995b; Mallory et al. 2008) and 200 km represents only a 4-h flight under calm conditions (Hatch and Nettleship 1998). Such a flight would be even faster and consume less energy with some wind (Furness and Bryant 1996), and wind is almost always present at these colonies (Gaston and Nettleship 1981).
Instead, we interpret these differences in incubation shift duration as local adaptation by fulmars at Cape Vera to predictable conditions, compared to environmental stochasticity at Prince Leopold Island. Annually at Cape Vera, birds can rely on a similar travel distance to open water near the North Water Polynya, a productive, ice-free region which recurs every year due to the physical oceanography of that marine area (Smith and Rigby 1981). Because age and experience play a dominant role in reproductive success (Ollason and Dunnet 1978), experienced, breeding fulmars at Cape Vera could sustain a schedule of relatively long incubation trips and maintain suitable body condition provided they can access distant but predictable foraging areas (Brown and Nettleship 1981; Weimerskirch et al. 1997).
In contrast, sea ice conditions are quite variable at Prince Leopold Island (Gaston et al. 2005), with 70% of the years between 1996 and 2005 having open water immediately beside the colony in mid-incubation. However, in the 3 years when the sea-ice was late and extended east through Lancaster Sound, approximately 13,000 km2 of ice-free ocean were unavailable for foraging compared to years when the ice edge was at the island. In 3 years when the sea-ice was 150 km west of the island, an additional 10,000 km2 of foraging habitat was available. Perhaps more importantly, Lancaster Sound is undoubtedly more productive in years when ice breaks up earlier, allowing sea surface temperatures to warm and light to penetrate, stimulating primary production (Welch et al. 1992). That incubation shifts were longer in extensive ice years suggests that at least some fulmar pairs had the behavioural flexibility and body reserves to withstand these conditions, although there appeared to be a cost to the parents in terms of reduced body condition (Fig. 3). Nonetheless, Gaston et al. (2005) noted higher egg neglect during incubation in 2001 and 2002, suggesting some birds had to abandon nests during an incubation shift before their mate returned. This was rarely observed in 2 years at Cape Vera, but has been observed commonly amongst Boreal fulmars in recent breeding seasons on Eynhallow (PM Thompson, unpubl. data).
Gaston et al. (2005) also showed that the reduced reproductive success in 2001 and 2002 came principally through reduced fledging success. However, late or extensive sea ice might not always result in poor reproductive success for fulmars. In 2001, the reduced success was attributable principally to late season snowstorms that killed chicks (Gaston et al. 2005), which can happen in any year. In 2001, thick-billed murre (Uria lomvia) reproductive parameters were below normal, but not nearly as poor as in 2002, when they were the poorest ever recorded at this colony. Given that High Arctic fulmars can adjust incubation schedules to accommodate late ice years, rather than simply abandoning reproduction as would be expected if the chances of breeding successfully were predictably low (Trivers 1972; Chaurand and Weimerskirch 1994), we suspect that marine production was dramatically low in 2002, perhaps because of two consecutive, late ice years, resulting in marginal feeding conditions (e.g. Reid and Croxall 2001).
In addition to individual variation in incubation behaviour (Hatch 1990b), we suggest that fulmars breeding at Prince Leopold Island are predisposed to exploiting nearby feeding areas in typical, open water years, whereas most fulmars at Cape Vera are forced to make long foraging trips for most of the breeding season. At Cape Vera, surveys and satellite tracking indicate that few breeding fulmars forage in the nearby polynya, presumably because it does not provide suitable quantity or quality of food resources, and instead they fly >200 km to the North Water Polynya (Mallory et al. 2008). Thus, annual environmental conditions at Cape Vera constrain the behavioural options available to fulmars for incubation scheduling, whereas at Prince Leopold Island, more opportunities exist for expression of individual variation in incubation schedules in ice-free years, but these fulmars are severely constrained during extensive ice years.
Incubation at the Boreal colony
There are no comparable studies of fulmar incubation patterns at other Boreal colonies within the Atlantic. Instead, previous studies of nest attendance have focused on the chick rearing period, when foraging trips tend to be shorter, and are likely to be shaped by the chick’s energetic requirements (e.g. Weimerskirch et al. 2001). Other work has been limited to just a portion of the incubation period (e.g. Dunnet et al. 1963; Ojowski et al. 2001) or to observations of a few pairs in a single season (Williamson 1952; Mougin 1967). The most extensive data on attendance throughout incubation by Boreal fulmars comes from Hatch’s (1990a) study at a Pacific colony in Alaska. There, shifts averaged 4.6 days, and only in one of 5 years was the annual mean shift duration >5.0 days (Hatch 1990a). In our study, fulmars exhibited annual average incubation shifts of 5.0–8.8 days at Eynhallow, albeit on smaller datasets. Although we used slightly different techniques for calculating average shifts, our sub-sample approach meant that our averages excluded the long, first shift by the male (Hatch 1990a), and therefore our averages may be biased somewhat low. Nonetheless, average shifts on Eynhallow were markedly longer than reported by Hatch (1990a) or any other Boreal colonies, and more similar to incubation at Arctic colonies (above).
Although the small sample sizes from Eynhallow in 2003 and 2005 provided limited power for detecting inter-annual variation in average incubation shifts, available data indicate that these longer shifts were typical through most of our study period. Our observations coincide with a period when seabird reproduction at many sites around the UK was poor (Proffitt 2004; Mavor et al. 2006), an effect attributed to reduced food supplies probably induced by a combination of changing climate and fishery effects on the marine ecosystem (Frederiksen et al. 2004, 2006). In fact, 2004 saw the lowest reproductive success recorded on Eynhallow during >50 years of study. Occasional, long incubation shifts seem to be a regular feature of some fulmars breeding in the Boreal zone (Mougin 1967; Hatch 1990a; this paper). However, we suggest that the relatively long, average shifts observed recently at Eynhallow may represent a behavioural adjustment by the fulmars to altered or reduced food supplies, not unlike the adjustments made by fulmars breeding at Prince Leopold Island in 2001 and 2002. On Prince Leopold, this strategy was associated with reduced body condition. Further work is now required to assess whether Boreal fulmars also incur a similar cost from these long incubation shifts through changes in body condition or future reproductive or survival prospects.
Colony size
Lewis et al. (2001) found that mean foraging trip duration by northern gannets (Morus bassanus) was longer at larger colonies, probably due to density-dependent effects of competition for depleted food resources at these colonies. While fulmar colony size can vary greatly (Table 1), fulmars generally fly vast distances on incubation recesses, with trip duration measured in days, and thus are probably less susceptible to the effects of density-dependence that can influence foraging trip duration in other seabirds (Birt et al. 1987; Lewis et al. 2001; Gaston et al. 2007). In fact, using the 2003 data for Prince Leopold Island (i.e. a normal year), there was no significant relationship between mean incubation shift duration and fulmar colony size (square root transformed) where incubation rhythms have been measured (Table 1; r7 = 0.03, P = 0.94). This suggests that regional or annual variation in marine food supplies probably have an overriding influence on incubation shift duration in fulmars, as suggested by Hatch (1990a), and as found by Lewis et al. (2006) in cape gannets (M. capensis).
Central place foraging
One question of interest is why foraging trips are not longer in all years, given that some fulmar pairs are capable of reproducing successfully by adjusting foraging trips for longer durations? Minimizing travel time should be advantageous for central place foragers (Orians and Pearson 1979). We suspect that in more productive years, fulmars can replenish energetic stores in trips of shorter duration, and probably reach a threshold mass enabling them to persist through a normal or even abnormally long, upcoming incubation shift. As well, efficient flight is critical for effective foraging in this species (Furness and Bryant 1996), so it also may be advantageous for breeding fulmars to maintain relatively lower body mass to improve flight efficiency (Rayner 1999). Moreover, pairs undoubtedly derive benefits by establishing a synchronized, sustainable rhythm for both mates (Hatch 1990b), which also helps reinforce pair bonding, and which should be more attainable with shorter incubation shifts. This aspect may be critical, as age and breeding experience are the dominant factors influencing annual reproductive success in fulmars (Ollason and Dunnet 1978; Hatch 1990b).
Conclusions
Our study, and the previous work by Falk and Møller (1995a) suggest that the typical incubation behaviour of High Arctic northern fulmars differs from that of fulmars nesting in the southern part of the breeding range (Williamson 1952; Mougin 1967; Hatch 1990a). These differences are thought to accommodate lower marine productivity early in the breeding season, and the physical impediments posed by sea-ice. However, in both the Boreal and High Arctic oceanographic zones, fulmars are capable of adjusting their incubation schedules to meet variation in environmental conditions. During years of food scarcity, some pairs can lengthen incubation shifts to accommodate the increased time required to find sufficient food, a strategy accompanied by a reduction in body condition. Given that Arctic marine ecosystems are experiencing rapid changes due to global warming (ACIA 2005), including earlier breakup of sea-ice and increased sea surface temperatures, Arctic fulmars should find future marine conditions more favourable for foraging during incubation, and may respond by adopting incubation rhythms with shorter shifts, more typical of fulmars at southern colonies. In contrast, breeding fulmars around the UK and other North Sea coasts may in the future need to compensate for decreasing food supplies by adjusting their incubation rhythms towards a more Arctic pattern.
References
ACIA (2005) Arctic climate impact assessment. Cambridge University Press, Cambridge
Aebischer NJ, Coulson JC, Colebrook JM (1990) Parallel long-term trends across four marine trophic levels and weather. Nature 347:753–755
Behrenfeld MJ, Randerson JT, McClain CR, Feldman GC, Los SO, Tucker CJ, Falkowski PG, Field CB, Frouin R, Esaias WE, Kolber DD, Pollack NH (2001) Biospheric primary production during an ENSO transition. Science 291:2594–2597
Birt VL, Birt TP, Goulet D, Cairns DK, Montevecchi WA (1987) Ashmole’s halo: direct evidence for prey depletion by a seabird. Mar Ecol Progr Ser 40:205–208
Brown RGB, Nettleship DN (1981) The biological significance of polynyas to arctic colonial seabirds. In: Stirling I, Cleator H (eds) Polynyas in the Canadian Arctic. Can Wildl Serv Occas Pap No 45:56–66
Cairns DK (1987) Seabirds as indicators of marine food supplies. Biol Oceanogr 5:261–271
Chastel O, Weimerskirch H, Jouventin P (1995) Influence of body condition on reproductive decision and reproductive success in the blue petrel. Auk 112:964–972
Chaurand T, Weimerskirch H (1994) Incubation routine, body mass regulation and egg neglect in the blue petrel Halobaena caerulea. Ibis 136:285–290
Dunnet GM (1991) Population studies of the Fulmar on Eynhallow, Orkney Islands. Ibis 133(Suppl 1):24–27
Dunnet GM, Anderson A, Cormack RM (1963) A study of survival of adult fulmars with observations on the pre-laying exodus. Br Birds 56:2–18
Falk K, Møller S (1995a) Breeding ecology of the fulmar Fulmarus glacialis and the kittiwake Rissa tridactyla in high-arctic northeastern Greenland, 1993. Ibis 139:270–281
Falk K, Møller S (1995b) Satellite tracking of high-arctic northern fulmars. Polar Biol 15:495–502
Fisher J (1952) The fulmar. Collins, London
Frederiksen M, Wanless S, Harris MP, Rothery P, Wilson LJ (2004) The role of industrial fisheries and oceanographic change in the decline of North Sea black-legged kititwakes. J Appl Ecol 41:1129–1139
Frederiksen M, Edwards M, Richardson AJ, Halliday NC, Wanless S (2006) From plankton to top predators: bottom-up control of a marine food web across four trophic levels. J Anim Ecol 75:1259–1268
Frederiksen M, Mavor R, Wanless S (2007) Seabirds as environmental indicators: the advantages of combining datasets. Mar Ecol Prog Ser 352:205–211
Furness RW, Bryant DM (1996) Effect of wind on field metabolic rates of breeding northern fulmars. Ecology 77:1181–1188
Furness RW, Camphuysen CJ (1997) Seabirds as monitors of the marine environment. ICES J Mar Sci 54:726–737
Gaston AJ, Nettleship DN (1981) The thick-billed murres of Prince Leopold Island. Can Wildl Serv Monogr 6
Gaston AJ, Gilchrist HG, Mallory ML (2005) Variation in ice conditions has strong effects on the breeding of marine birds at Prince Leopold Island, Nunavut. Ecography 28:331–344
Gaston AJ, Mallory ML, Gilchrist HG, O’Donovan K (2006) Status, trends and attendance patterns of the northern fulmar Fulmarus glacialis in Nunavut, Canada. Arctic 59:165–178
Gaston AJ, Ydenberg RC, Smith GEJ (2007) Ashmole’s halo and population regulation in seabirds. Mar Ornithol 35:119–126
Inc GraphPad Software (1998) GraphPad Instat Ver. 3.00. GraphPad Software, San Diego
Hatch SA (1990a) Incubation rhythm in the fulmar Fulmarus glacialis: annual variation and sex roles. Ibis 132:515–524
Hatch SA (1990b) Individual variation in behavior and breeding success of Northern Fulmars. Auk 107:750–755
Hatch SA, Nettleship DN (1998) Northern Fulmar (Fulmarus glacialis). In: Poole A, Gill F (eds) The birds of North America: 361. The Birds of North America, Philadelphia
Johnstone RM, Davies LS (1990) Incubation routines and foraging trip regulation in the grey-faced petrel Pterodroma macroptera. Ibis 132:14–20
Lewis S, Sherratt TN, Hamer KC, Wanless S (2001) Evidence of intra-specific competition for food in a pelagic seabird. Nature 412:816–819
Lewis S, Schreiber EA, Daunt F, Schenk GA, Wanless S, Hamer KC (2004) Flexible foraging patterns under different time constraints in tropical boobies. Anim Behav 68:1331–1337
Lewis S, Gremillet D, Daunt F, Ryan PG, Crawford RJM, Wanless S (2006) Using behavioral and state variables to identify proximate causes of population change in a seabird. Oecologia 147:606–614
Mallory ML, Forbes MR (2005) Sex discrimination and measurement bias in northern fulmars (Fulmarus glacialis) from the Canadian arctic. Ardea 93:25–36
Mallory ML, Forbes MR (2007) Does sea-ice constrain the breeding schedules of high arctic northern fulmars? Condor 109:895–907
Mallory ML, Akearok JA, Edwards DB, O’Donovan K, Gilbert CD (2008) Autumn migration and wintering of northern fulmars (Fulmarus glaicalis) from the Canadian high Arctic. Polar Biol. doi:10.1007/s00300-008-0417-0
Mavor RA, Parsons M, Heubeck M, Schmitt S (2006) Seabird numbers and breeding success in Britain and Ireland, 2005. UK Nature Conservation No. 30
Mitchell PI, Newton SF, Ratcliffe N, Dunn TE (2004) Seabird populations of Britain and Ireland. T & AD Poyser, London
Mougin J-L (1967) Etude écologique des dues espèces de fulmar: le fulmar Atlantique, Fulmarus glacialis, et le Fulmar Antarctique, Fulmarus glacialoides. Oiseau 37:57–103
Ojowski U, Eidtmann C, Furness RW, Garthe S (2001) Diet and nest attendance of incubating and chick-rearing northern fulmars (Fulmarus glacialis) in Shetland. Mar Biol 139:1193–1200
Ollason JC, Dunnet GM (1978) Age, experience and other factors affecting the breeding success of the fulmar Fulmarus glacialis in Orkney. J Anim Ecol 47:961–976
Orians GH, Pearson NE (1979) On the theory of central place foraging. In: Horn DJ, Mitchell RD, Stairs GR (eds) Analysis of ecological systems. Ohio State University Press, Columbus, pp 155–177
Pauly D, Maclean J (2003) In a perfect ocean: the state of fisheries and ecosystems in the North Atlantic Ocean. Island Press, Washington
Phillips RA, Petersen MK, Lilliendahl K, Solmundsson J, Hamer KC, Camphuysen K, Zonfrillo B (1999) Diet of the northern fulmar Fulmarus glacialis: reliance on commercial fisheries? Mar Biol 135:159–170
Proffitt F (2004) Reproductive failure threatens bird colonies on North Sea coast. Science 304:1090
Rayner JMV (1999) Estimating power curves of flying vertebrates. J Exp Biol 202:3449–3461
Reid K, Croxall JP (2001) Environmental response of upper trophic level predators reveals a system change in an Antarctic marine ecosystem. Proc R Soc Lond B 268:377–384
Salomonsen F (1965) The geographical variation of the fulmar (Fulmarus glacialis) and the zones of marine environment in the North Atlantic. Auk 82:327–355
Schreiber EA (2002) Climate and weather effects on seabirds. In: Schreiber EA, Burger J (eds) Biology of marine birds. CRC Press, New York, pp 179–215
Shaw DN, Holt CA, Maggs HE, de Palacio D (2002) Fair Isle seabird studies. JNCC Report 332. Joint Nature Conservation Committee, Aberdeen
Smith M, Rigby B (1981) Distribution of polynyas in the Canadian Arctic. In: Stirling I, Cleator H (eds) Polynyas in the Canadian Arctic. Can Wildl Serv Occas Pap 45:7–28
Sokal RR, Braumann CA (1980) Significance tests for coefficients of variation and variability profiles. Syst Zool 29:50–66
Thompson PM (2006) Identifying drivers of change; did fisheries play a role in the spread of North Atlantic fulmars? In: Boyd IL, Wanless S, Camphuysen CJ (eds) Top predators in marine ecosystems: their role in monitoring and management. Cambridge University Press, Cambridge, pp 143–156
Thompson PM, Ollason JC (2001) Lagged effects of ocean climate change on fulmar population dynamics. Nature 413:417–420
Trivers RL (1972) Parental investment and sexual selection. In: Campbell B (ed) Sexual selection and the descent of man 1871–1971. Aldine Press, Chicago, pp 136–179
Warham J (1990) The petrels—their ecology and breeding systems. Academic Press, London
Weimerskirch H (1995) Regulation of foraging trips and incubation routine in male and female wandering albatrosses. Oecologia 102:37–43
Weimerskirch H (1998) How can a pelagic seabird provision its chick when relying on a distant food source? Cyclic attendance at the colony, foraging decision, and body condition in sooty shearwaters. J Anim Ecol 67:99–109
Weimerskirch H, Mougey T, Hindermeyer X (1997) Foraging and provisioning strategies of black-browed albatrosses in relation to the requirements of the chick: natural variation and experimental study. Behav Ecol 8:635–643
Weimerskirch H, Chastel O, Cherel Y, Henden J-A, Tveraa T (2001) Nest attendance and foraging movements of northern fulmars rearing chicks at Bjørnøya, Barents Sea. Polar Biol 24:83–88
Welch HE, Bergmann MA, Siferd TD (1992) Energy flow through the marine ecosystem of the Lancaster Sound region, Arctic Canada. Arctic 45:343–357
Williamson K (1952) The incubation rhythm of the fulmar. Scot Nat 64:138–147
Wilson RP, Scolaro JA, Gremillet D, Kierspel MAM, Laurenti S, Upton J, Gallelli H, Quintana F, Frere E, Muller G, Straten MT, Zimmer I (2005) How do magellanic penguins cope with variability in their access to prey? Ecol Monogr 75:379–401
Acknowledgments
We thank the many students and colleagues who made the Cape Vera, Prince Leopold Island and Eynhallow projects possible from 2000 to 2005. Canadian work was conducted in accordance with Canadian Council on Animal Care guidelines and under the following permits: research (NUN-SCI-03-02, WL000190, WL000714), animal care (2003PNR017, 2004PNR021, 2005PNR021), and land use (59A/7-2-2). The Canadian work would not have been possible without the financial and logistic support provided by Environment Canada (NCD and NEI), Natural Resources Canada (PCSP), the Nunavut Wildlife Management Board, and Carleton University. Scottish data collection was funded by the Royal Society and Talisman Energy (UK) Ltd. Sue Lewis was supported by a Leverhulme Fellowship and Barbara Cheney was partially supported by a Nuffield Bursary. We thank Orkney Island’s Council for access to Eynhallow.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. A. Poulet.
Rights and permissions
About this article
Cite this article
Mallory, M.L., Gaston, A.J., Forbes, M.R. et al. Flexible incubation rhythm in northern fulmars: a comparison between oceanographic zones. Mar Biol 154, 1031–1040 (2008). https://doi.org/10.1007/s00227-008-0994-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-008-0994-z