Abstract
Physical and biological processes interact to produce pattern in nature. Pattern is scale dependent as processes generating pattern are heterogeneous in time and space. We tested some causes of variation in abundance and distribution of three marginal populations of sublittoral blue mussels, Mytilus edulis, in the non-tidal northeastern Baltic Sea. We studied the role of substrate inclination, perennial algae and siltation along local wave exposure gradients on mussel distribution over a regional salinity gradient. We found marked differences on regional scales (p < 0.001) with lower densities and biomasses of mussels with declining salinity. Along local gradients, mussel densities increased with increasing exposure (p < 0.001) and declining slope and sedimentation (p < 0.01). Site specifically, densities of blue mussels and the perennial red algae, Furcellaria lumbricalis, were positively related, results supported by a colonisation experiment. Also, young post-recruits showed significant relations to adult biomass, wave exposure, algal biomass, bottom slope and sediment cover. Findings showed that the relative importance of the determinants affecting blue mussels at the edge of their range vary with scale and are affected by the density and size structure of mussel populations. The study provides an indication of the types of factors that may be invoked as causes of spatial variation in marginal blue mussel populations and reinforces the need to consider multiple aspects when distributional patterns are assessed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patterns of species distribution and abundance are inherently variable and determined by complex interactions of abiotic and biotic processes (Menge and Branch 2001; Thrush et al. 2005). Elucidating how these processes operate over different scales in determining distribution, abundance and structure of populations lies at the heart of ecology. The relevance of any process is scale dependent; processes important at smaller spatial scales can loose their significance at larger spatial scales. Processes can also interact, or be additive over scales (Benedetti-Cecchi et al. 2000; Thrush et al. 2005). If processes are only sketchily examined, the knowledge of heterogeneity in observed pattern, and the generality or magnitude of the processes, remains unsolved (Stoffels et al. 2003; Thrush et al. 2005). Still, comparatively few studies have examined multi-scale variability, the focus being mainly on single scale processes (Benedetti-Cecchi 2001; Stoffels et al. 2003).
Many independent and interrelated processes are known to account for heterogeneous patterns exhibited by sessile marine communities and the effects of these processes varies with scales, habitat complexity and the degree of environmental stress (e.g., Menge and Branch 2001; Witman and Dayton 2001; McQuaid and Lindsay 2005). How recruitment and settlement processes set the stage for later processes affecting adult abundance are, however, still not entirely known (Morgan 2001). Understanding the processes that determine colonisation processes is therefore, essential for understanding the factors that affect adult abundance and distribution (e.g., Morgan 2001; Underwood and Keough 2001 and references therein).
As typical inhabitants of wave swept shores, mussels live in mechanically stressful environments. Water movement at exposed shores produces severe hydrodynamic forces and as these forces scale with size, wave action may exert selective pressures against large individuals (Westerbom and Jattu 2006; Zardi et al. 2006a). Wave action also influence rocky shore mussel distribution, abundance and size structure of populations by e.g., delivery of food and gases, delivery of propagules, accumulation of sediments and predation effects (Hunt and Scheibling 1996; Robles et al. 2001; Steffani and Branch 2003). Further, wave action alters habitat heterogeneity and affect macrophyte coverage and structure (Kiirikki 1996; McQuaid and Lindsay 2005; Tuya and Haroun 2006). Macrophytes often function as habitat-modifiers, foundation species or facilitators (sensu Bruno and Bertness 2001) creating habitats and spatial refuges from environmental stress (Bégin et al. 2004; Erlandsson and McQuaid 2004; Wieters 2005). Algal facilitators often convert a two-dimensional seascape to a three-dimensional entity and thereby considerably increase available space (Bruno and Bertness 2001) enhancing larval settlement processes (e.g., Bayne 1964; Moreno 1995; McQuaid and Lindsay 2005). The positive effects of these habitat modifiers have not rigorously been emphasised by contemporary ecologists in most systems (Bertness et al. 1999).
We conducted population sampling in this study both along regional and local stress gradients to find out: (1) how regional and local factors are translated to distribution patterns in mussel populations of different size structure and (2) how local factors vary within the larger stress gradient along the distributional margin. This provides a basis for interpreting the distribution patterns of an unstable range margin of these sublittoral blue mussels (Westerbom et al. 2002). Although the ecology of intertidal rocky shore mussel assemblages is studied well, little is still known about the dynamics of sublittoral rocky shore populations (Witman and Dayton 2001). Especially, factors affecting mussel distribution in marginal areas are largely unknown even though studies carried out at the margin are in a key position to advance ecology and evolutionary biology as scientific disciplines (Holt and Keitt 2005).
Large-scale processes (10−2 km) within the study area have previously been addressed by Westerbom et al. (2002), showing a distinct pattern of sharply thinning mussel populations along a salinity gradient. This dimension encompasses the entire marginal range of the species in this study system. Westerbom and Jattu (2006) showed that on local scales, at a single locality, stress gradients in wave action strongly affect blue mussel distribution. Here, we examine the relationships between sedimentation, substrate inclination, and abundance of co-occurring perennial red algae, Furcellaria lumbricalis, and blue mussels at three localities spaced along the marginal range. We also test experimentally the role of artificial algae on colonisation success of the mussels. Based on untested observations in Westerbom and Jattu (2006), we predict (1) that perennial macroalgae may have a positive effect on mussel density whereas (2) thin films of sediments on the rocky bottoms affect densities negatively. Finally, as both factors are less abundant on steep bottoms, we predict that the slope of the bottom should affect mussel densities, especially at sites where the bottom complexity is low and sediment accumulation is high.
Materials and methods
Study area
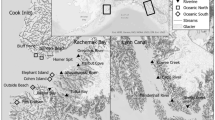
We studied three blue mussel populations in the western Gulf of Finland. Three sites at Hanko, five sites at Tvärminne both situated on the Hanko peninsula (60°N, 23°E) were selected. In addition, three study sites at Söderskär (60°N, 25°E), in the central Gulf of Finland, were included in the study (Fig. 1). The sites were selected based on their exposure to wind and waves so that each area was represented by sheltered, intermediate and exposed sites. The study areas are characterised by high topographic complexity formed by a mosaic of islands, islets, skerries and rocks where ambient physical wave conditions vary considerably over proximate sites (Westerbom and Jattu 2006). The area is characterised by a declining salinity gradient, from west to east, from 6.2 at Hanko to 5.7‰ at Tvärminne and at Söderskär only ca. 5‰ (Finnish Institute of Marine Research—long-term monitoring data carried out in May).
Study area and as a description of the nature of separate areas the Tvärminne area is shown. H is the abbreviation for Hanko, T for Tvärminne and S for Söderskär. At Tvärminne sites are indicated with T and numbers denote the Baardseth index. At Hanko and Söderskär, sites had the exposure 2, 9 and 22. TZS, Tvärminne Zoological Station
Exposure measurements
Wave exposure values were calculated according to the Baardseth (1970) methodology described in Westerbom and Jattu (2006). As the sites in the study are few, these exposure values were not intended to perfectly match the wave conditions at the different study sites, but to give a robust quantitative estimate of the physical conditions prevailing at the 11 sites. It is noteworthy that sheltered and intermediate sites dominate the rocky shore bottoms in the study system, and therefore, as study sites were selected based on their exposure values—not at random—results may underrate the overall importance of processes at the local population level.
Bottom sampling
Mussel samples were collected at depths of 5–8 m, representing both the optimal depth of the mussels (Westerbom et al. 2002; Westerbom and Jattu 2006) and the red alga Furcellaria lumbricalis (hereafter only Furcellaria) (Kiirikki 1996). We collected samples by placing a 20 × 20 cm quadrat at the bottom and using a spatula we thoroughly removed all material within the frame into a bag. Before sampling, both the inclination of the substratum and the sediment load within the frame was estimated. The slope of the sea floor was registered to an accuracy of 5° by means of a float attached to a scale on one side of the sampling gear. The sediment cover was rated using an 1–5 ordinal scale where: 1 = no visible sediment, 2 = a light dust of re-suspended sediments visible only when water is put into motion above the bottom, 3 = some visible sediments covering most of the bottom, 4 = bottoms covered by sediments, a thick dust is re-suspended when the water is flushed above the bottom, 5 = bottoms are completely covered by a multiple layer of particles. Notable is 5 which is a comparative measure and does not indicate a thick absolute layer of sediment. Prior to sampling, the used schedule was calibrated between two SCUBA divers and no differences were seen. Both divers worked side-by-side at each sampling station. Ordinal scales have been commonly used in underwater work where time limits the feasible methods (e.g., Pedersén and Snoeijs 2001; Airoldi 2003).
In laboratory, the samples were roughly sieved through graded sieves (9.5, 4, 2 and 1 mm), algae were separated from the rest of the materials, and mussels in each mesh screen were counted. From each locality and sample type, approximately 100 mussels screen−1 were randomly selected and the maximum shell length was measured with vernier callipers (0.1 mm accuracy) to assess the true size distribution in samples. Biomasses were then estimated (meat weight = shell length2.307 × 10−4.744, shell weight = shell length2.891 10−4.495—for methodology, see Westerbom et al. 2002). The red alga, Furcellaria, was separated from the samples and dried to constant weight at 60°C and weighed to the accuracy of 0.1 mg.
The sampling scheme at each site consisted of 12 random samples taken from substrates with an inclination less than 45° (denoted horizontal samples) and six random samples taken from steep (50–105°) substrates (denoted vertical). Moreover, at Tvärminne, 12 random horizontal samples and six vertical samples at each site (5) were also taken in order to increase data for intra-site comparisons. The mean slope (± SD) of all horizontal samples in the study was 19 ± 11°, whereas the mean slope of all vertical samples was 74 ± 14°. Since a number of surplus samples showed that surface steepness above 90° clearly had a negative effect on mussel numbers, sampling from these walls was minimised.
Colonisation experiment
Colonisation of mussels and the effects of habitat complexity were surveyed using artificial substrata imitating Furcellaria. We used three sets of bricks with each 10 replicates depth−1 [ordinary (cm) 25L × 12W × 6H tiles]. (1) Plain bricks were used to imitate empty space (hereafter denoted E for empty), (2) bricks with pre-attached mussels (>10 mm) to imitate adult bed structure with natural coverage (hereafter denoted M for mussel) and (3) bricks with artificial algae to survey how algal structures possibly influence the establishment of mussel beds. In this latter case, we separate between mussels colonising to the brick itself (henceforth AB-Artificial brick) and those to the artificial algae (henceforth AR-Artificial rope). In cases where we include data from both the brick and the rope we use the abbreviation ABR. We constructed artificial algae by opening and unpicking polyester ropes (∅ 8 mm) and forming a turf by separating the thin filaments (see Fig. 2). Three holes were drilled through the bricks and the ropes were inserted through the holes. Finally, the ropes were cut to 10 cm length and secured to the brick. Bricks with attached mussels were constructed by allowing large mussels (> 10 mm) attach to the bricks in a flow through water basin for 3 weeks. In late May 2001 (well before the settlement of mussels), the bricks were put out by SCUBA at 5 and 12 m depth at a sheltered site (Baardseth index = 1) where pelagic mussel larvae are abundant but few recruit successfully (Westerbom and Jattu 2006). In summer 2003, bricks were retrieved, ropes were cut; mussels were scraped off the bricks from the top horizontal surface only and were counted and measured. The experiment was allowed to run for 2 years, since visual inspections in summer 2002 indicated very low colonisation of mussels on the bricks. When interpreting the results from the colonisation experiment, two aspects should be considered. (1) Surface texture always differs between artificial filaments and natural algae. However, marine invertebrates have often been shown to respond to artificial algae in similar ways as to natural substrates (e.g., Moreno 1995; Griffiths et al. 2006; Irving et al. 2007). (2) Since the bricks were set on a natural bottom, mussels may have crawled up on the bricks. However, bricks were set on a sheltered bottom, where natural mussels have a very low coverage (Westerbom and Jattu 2006). Crawling behaviour will, therefore, not account for the large difference between the bricks, even though some crawling (in both directions) among mussels has taken place. When testing the results in the colonisation experiment, only the smallest mussels (<10 mm) were used, as we could not, with full certainty, distinguish between colonised and pre-attached mussels. Pre-attached mussels have usually been marked in similar experiments. Given that the mussels in the Baltic are very small, and given the high number of pre-attached mussels in the experiment, marking of all attached mussels would have been highly labour intensive.
Data analysis
Parametric ANOVAs were conducted if possible. To meet the assumptions of normality (Wilk Shapiro) and variance homogeneity (Levene’s test), transformations (square root and log + 1) were undertaken if necessary. Significant results were followed by Tukey (p < 0.05) tests. Values are given as means ± SE. In statistical calculations, comparing large and intermediate areas and facilitation effects, only random horizontal samples are used. To avoid unbalanced samples between areas, we only included the first 12 horizontal samples from Tvärminne and excluded the moderately exposed and moderately sheltered sites in inter-area comparisons. Investigating local slope effects, all samples were included. To test differences in cumulative frequency distributions between areas, Kolmogorov–Smirnov tests were conducted.
Non-parametric ranked ANOVA (Scheirer–Ray–Hare test) was used to test the effects of depth and treatment in the colonisation experiment. This test was used as variances were unequal between treatments and depths even after data transformations (Levene’s test). After this initial test, we performed 1-way parametric or non-parametric tests to evaluate the sources of variation. Significant results were then tested with Tukey’s test (p < 0.05). Backward stepwise multiple regressions were used to investigate if there existed relationships between the dependent variable mussel numbers and independent variables: salinity (inter-area), wave exposure (intra-area), slope, sediment cover, and macroalgal biomass (intra-site). As the sediment data were on ordinal scales, and as it could not as such be included in the multiple regressions, we constructed dummy variables of the sediment data. Sediment cover 1 and 2 were combined to group 0 and sediment cover 3–5 was recoded to group 1. Since there were problems with multicollinearity including salinity in the model (collinearity statistics >30), also salinity (area) had to be recoded. Therefore, Hanko and Tvärminne, which are geographically close, were combined to form one dummy (area) group. We also tested the factors affecting the distribution of the smallest mussels (1.5–3.4 mm), operationally termed recruits, in the habitat. As there were no problems with multicollinearity (collinearity statistics = 8), in multiple regression including recruits as the dependent variable, only data on sediments had to be binary. All analyses were done using the statistical package SPSS.
Results
Patterns along the large-scale salinity gradient
There was a distinct reduction in abundance (Kruskal–Wallis, H 2 = 24.2, p < 0.001, Tukey post-hoc tests showing that only Söderskär differed from the two other areas) and an increased dominance of small mussels, (Kolmogorov–Smirnov test; D = 0.05, n Hanko = 32 784, n Tvärminne = 34 129, p < 0.001 and D = 0.28, n Tvärminne = 34 129, n Söderskär = 9 900, p < 0.001) when moving from west to east (Fig. 3). This decline in mussel size was especially evident at the easternmost area where all sampled mussels were extremely small (medians with quartiles (mm) for the areas: Hanko = 5(3, 8), Tvärminne = 4(3, 7), Söderskär 3(3, 5)). Biomasses (kg m−2 ± SE) were higher at Hanko (0.731 ± 0.054) than at Tvärminne (0.555 ± 0.043) and at Tvärminne markedly higher than at Söderskär (0.03 ± 0.003, Kruskal–Wallis, H 2 = 57.8, p < 0.001). Biomass (g m−2) of Furcellaria differed between areas (Kruskal–Wallis, H 2 = 20.56, p < 0.001) but the pattern was reversed relative to mussels, being significantly higher at Söderskär (24.0 ± 2.2) than at the two western areas Tvärminne (16.3 ± 2.0) and Hanko (14.6 ± 3.7) where no inter-area differences were seen (Fig. 4).
Mytilus edulis. Mean abundance (open circle ± SE) and mean biomass (bar ± SE) of mussels at different areas and sites. Median degree of sedimentation cover shown with black dots. Non-linear regressions are fitted on the data on mussel density. Note that the scale differs between Söderskär and the two other areas
Patterns along exposure gradients
A spatial trend for lower abundance of mussels at the innermost sites and increasing densities with increasing exposure was evident (Fig. 3). At Tvärminne, the density of mussels was strongly related to wave exposure (Kruskal–Wallis H 4 = 92.7, p < 0.001) and only the closest neighbouring sites (e.g., T6/T8 and T8/T11—see Fig. 1) showed non-significant differences (Tukey post-hoc test p < 0.05). Biomasses were lowest at the innermost site (Kruskal–Wallis H 4 = 93.0, p < 0.001) and highest at sites of moderately high exposure while remaining sites did not differ with regard to biomass (Tukey post-hoc test). At Hanko, densities increased with exposure (ANOVA, F 2, 33 = 35.68, p < 0.001, log transformed data) while at Söderskär only the inner site differed from the two exposed sites, having significantly less mussels (Kruskal–Wallis, H 2 = 23.1, p < 0.001). At Söderskär, biomasses were higher at the two outer sites (Kruskal–Wallis, H 2 = 16.8, p < 0.001) whereas the sites at Hanko did not differ with regard to biomass (ANOVA F 2, 33 = 1.3, p = 0.28).
Biomass of Furcellaria correlated positively with wave exposure at Hanko (Spearman’s rho r s = 0.46, n = 36, p < 0.01) and Tvärminne (Spearman’s rho r s = 0.44, n = 120, p < 0.01) but not at Söderskär (Spearman’s rho r s = −0.28, n = 36, p = 0.1). Exposure and sediment cover were inversely correlated at Hanko and Tvärminne (Spearman’s rho r s = −0.68, n = 36 respectively Spearman’s rho r s = −0.84, n = 120, p < 0.001) and at Söderskär barely insignificant (Spearman’s rho r s = −0.31, n = 36, p = 0.06, Fig. 3). Sedimentation and mussel numbers (Fig. 5) correlated negatively (p < 0.001) at all areas (Hanko Spearman’s rho r s = −0.62, Tvärminne Spearman’s rho r s = −0.74, Söderskär Spearman’s rho r s = −0.59).
Small scale variability—effects of slope
Generally, mussel numbers decreased with increasing inclination of the substratum, but slope only marginally explained the variation (r 2 = 0.03, n = 288, p = 0.05). At site-specific scales, increases and decreases on steep surfaces were shown (Fig. 6). At all areas the most exposed sites showed significantly higher abundances of mussels on horizontal bottoms relative to vertical (t tests), whereas at the sheltered sites the pattern was less clear with both higher or lower numbers of mussels in relation to bottom slope (Fig. 6). At intermediate sites, horizontal bottoms showed higher densities of mussels, but results were significant only at Söderskär. Recruit density was significantly higher on horizontal bottoms on all other sites than the sheltered sites at Hanko and Tvärminne and at the moderately sheltered site at Tvärminne. Biomasses were significantly (t test) lower on vertical slopes only at the outermost site at Hanko, and were significantly higher or showed non-significant patterns on all other sites (Fig. 7). This general trend of significantly or non-significantly lower abundances on steep surfaces but concomitantly non-significantly or significantly higher biomasses on vertical surfaces shows that mussels on steep surfaces are on average larger. Algal biomass correlated negatively with slope (Hanko Spearman’s rho r s = −0.5, n = 54, p < 0.001, Tvärminne Spearman’s rho r s = −0.46, n = 180, p < 0.001, Söderskär Spearman’s rho r s = −0.71, n = 54, p < 0.001, Fig. 8). Sedimentation and substrate inclination were inversely correlated at the sheltered and intermediate sites (sheltered, Spearman’s rho r s = −0.42, n = 72, p < 0.001, intermediate, Spearman’s rho r s = −0.46, n = 72, p < 0.001) whereas at the outermost sites no significant correlations were seen (Spearman’s rho r s = −0.18, n = 72, p = 0.14, Fig. 9).
Mussel-algal relations
At six out of 11 sites, mussel numbers and biomass of Furcellaria were positively related (Table 1). These relations were strongest at localities where the biomass of the alga was high and the biomass of mussels was low (with the intermediate site at Hanko as the only exception). Only at the outermost site at Tvärminne was algal biomass high but lacked a significant correspondence to mussel numbers. Conversely, only at the innermost site at Tvärminne were mussels positively associated to low algal biomass.
Colonisation experiment
The colonisation experiment showed that mussels were more abundant on ABR-bricks (artificial Furcellaria) than on E-bricks (empty) and M-bricks (mussel) (Sheirer–Ray–Hare test H 2 = 42.8, p < 0.001, Fig. 10) and this accounted for both small (size class 1) (H 2 = 38.4, p < 0.001) and medium sized mussels (size class 2) (Scheirer–Ray–Hare test H 2 = 43.24, p < 0.001). Mussels were also more abundant at 12 m depth than at 5 m (H 1 = 7.9, p < 0.01), but there were no interactions between depth and treatment (H 2 = 1.2, ns). E- and M-bricks did not differ regarding small mussels but differed significantly regarding medium sized mussels, with more mussels on M-bricks. Since the overwhelming dominance of mussels on ABR-bricks originated from the ropes, we were also interested in whether there were differences in mussel abundances on bricks only (AB). Indeed, AB-bricks also supported significantly more mussels than E- and M-bricks (Sheirer–Ray–Hare, H 2 = 27.7, p < 0.001) and again M-bricks had more mussels than E-bricks. The smallest mussels were more abundant on AB-bricks (Sheirer–Ray–Hare, H 2 = 6.78, p < 0.05), whereas no differences could be seen between M- and E-bricks. Medium sized mussels were more abundant on AB-bricks than on E- and M-bricks, and were more abundant on M-bricks than on E-bricks (Sheirer–Ray–Hare, H 1 = 31.69, p < 0.001). Large mussels (not shown in the figure) were significantly more abundant on M bricks than on ABR- and E-bricks, where no differences could be seen (Sheirer–Ray–Hare, H 2 = 45.2, p < 0.001).
Mytilus edulis. Abundance of different size categories of mussels on different treatments and depths. AR and AB are different parts of the same brick (ABR). AR denotes mussels attached to the rope and AB refers to mussels attached only to the brick. M stand for bricks with pre-attached large mussels and E is the abbreviation for empty. Each treatment was set out in 10 replicates at 5 and 12 m depth. Note the difference in the scale. The figure only shows significant differences (p < 0.001) between treatments ABR, E and M
Multiple regression models
A multiple backward regression model largely confirmed the results of simple regressions and ANOVAs. Multiple regressions explained 60% of the overall variation of total abundance of mussels in the area with salinity (area) and exposure as the most relevant factors followed by algal biomass, sediment cover and slope (Table 2). Recruits were positively related to adult biomass, wave exposure, algal biomass and negatively affected by slope and sediment cover, with a combined r 2 of 0.55 (Table 3). Computing the analysis on horizontal values only resulted in a much stronger model explaining 77% of the variation in recruit numbers with adult biomass, exposure, algae as the positive predictors, and sediment cover as the sole negative predictor.
Discussion
There are several general environmental and biological factors that influence the distribution and abundance of blue mussels in the area, but the relative importance of the determinants varies with scale. On a regional scale (10−2 km) salinity is the major factor affecting size structure and distribution of blue mussels (Westerbom et al. 2002). On local scales (10−3–10−4 m), wave exposure, and its attendant effects (e.g., delivery of food and gases, delivery of propagules, predation effects, accumulation of sediments), is the main determinant affecting blue mussels. At further smaller spatial scales (1–10 m) inclination of the substratum, occurrence of perennial algae, sediment cover, depth (Kautsky and van der Maarel 1990; Westerbom et al. 2002; Westerbom and Jattu 2006) and small scale surface texture (Qvarfordt et al. 2006) partially determines distributions of local mussel populations. The multiple regression model in this study suggested that large-scale salinity gradients and intermediate-scale wave-exposure gradients are good predictors for adult mussel distribution whereas adult biomass and exposure are the most important factors affecting the distribution of small post-recruits in the studied area.
Effects of large-scale salinity gradients
The paramount process affecting blue mussel distribution in the study area is a gradient of declining seawater salinity that eventually cuts off the range of the mussel with declining numbers of mussels, sporadic recruitment and declining growth rates in a west-eastward direction (Westerbom et al. 2002). This primary gradient produces a gradient of declining mussel sizes from east to west and influences how mussels respond to intermediate and small scale processes, partly determining the magnitude and importance of processes at smaller spatial scales.
Effects of local wave exposure gradients
Here, we showed that densities of mussels increased towards increasing exposure while biomasses reached peak values at intermediate sites. We also showed that densities and biomasses declined along exposure gradients to a lesser extent at Söderskär than at the two other areas. This pattern of higher densities, but lower biomasses, at the most exposed sites at Hanko and Tvärminne is explained by considerably smaller mussels at sites experiencing strong wave action. At Söderskär however, mussels seldom reach sizes that are negatively affected by beating waves (Westerbom et al. 2002) likely explaining the considerably lower decline in mussel biomass towards increasing stress (see also Westerbom and Jattu 2006).
Sediment accumulation is linked to wave exposure, and where wave action is intense, ephemeral sediments are not accumulated (Kiirikki 1996). Rocky shore mussels have shown inverse relationships between increased sedimentation and propagule recruitment (Hunt and Scheibling 1997) and even short exposures to sediments may substantially increase their mortality (Phillips and Shima 2006). Sedimentation affects mussel recruitment both primarily by preventing mussel colonisation to bare bottoms and post-recruitment survival, and secondarily by preventing establishment of algal stands facilitating mussel colonisation. The role of ephemeral films of sediments may be especially detrimental at landward areas and areas in early stages of succession where adult bed structure is absent and sediments abundant. This is confirmed by the colonisation experiment and multiple regressions, showing that successful establishment of mussels are higher in patches with existing bed structure than on bare bottoms lacking physical complexity.
Effects of substrate inclination
A worldwide phenomenon is the dominance of macroalgae on horizontal and gently sloping bottoms whereas sessile invertebrates typically dominate steep bottoms and vertical walls (Witman and Dayton 2001). Higher numbers of mussels on vertical walls relative to horizontal bottoms at some sheltered sites may have originated from negative sedimentation effects on mussel recruits and/or early post-recruits. If so, recruiting mussels should respond differently to habitats with complex structures formed by mussel beds than to habitats lacking adult structure and should respond differently along sedimentation gradients—as it did in the study. Where sediments were low, vertical surfaces did not support more mussels than horizontal bottoms, but where sediments were high and the complexity of the bottom was low, densities were higher on the steep surfaces relative to the horizontal bottoms (Fig. 6). Horizontal and vertical bottoms also differed regarding mussel size structure (relating density to biomass) suggesting (1) different recruitment history with older mussels on steep surfaces, or (2) better feeding conditions on steep bottoms enhancing faster growth. At the exposed localities, however, where delivery of particles is high, horizontal bottoms supported significantly more mussels than vertical slopes at both Hanko and Tvärminne (see also Qvarfordt et al. 2006). At Söderskär, mussels probably are too small to be markedly affected by pounding waves, even at the outermost site, which explains the higher biomass on vertical surfaces at the outermost site (Westerbom and Jattu 2006).
Furcellaria relationships
Algal turfs typically modify rocky shores by increasing complexity with effects on abundance and diversity of associated invertebrates (Bégin et al. 2004). Results of this study support earlier findings; algae had a positive effect on mussel densities. However, in opposite to the findings made by e.g., Bayne (1964), the proximity of mussels to the algae were not limited to early plantigrades, but also adult and large mussels (Fig. 11) were closely associated with the algae (see also Davis and Moreno 1995).
Mytilus edulis and Furcellaria lumbricalis. A typical image at moderately sheltered localities in the western Gulf of Finland where mussels are attached to Furcellaria lumbricalis, whereas the surrounding bottom is largely free from larger mussels. Other algae are Fucus vesiculosis, Cladophora rupestris and mostly epiphytic Ceramium tenuicorne. Photograph taken at 5 m depth. © Mats Westerbom
Positive interactions were strongest at sites of moderate to intermediate exposure where temporally low water movements allow fine sediments to accumulate on the rocky surfaces. Sedimentation effects and positive interactions between the algae and mussels may be connected, as algae may increase recruitment success at sites with low complexity but moderate sediment cover. At further sheltered sites, sediments together with lower water transparency limit algal settlement and growth (Kiirikki 1996; Pedersén and Snoeijs 2001), possibly also affecting colonisation success of mussels. This interpretation is substantiated by the colonisation experiment where it was shown that filamentous robust structures increase blue mussel colonisation to the rocky bottom in the proximity of the structure. Also, the tendency of recruits to be higher on horizontal surfaces relative to vertical (Fig. 6), and the higher predictability of the multiple regression including only horizontal samples, may reflect the importance of algal structures, as these are few or lacking from steep bottoms (Fig. 8). Complex structures seem to facilitate mussel colonisation whereas isolated smooth rocky surfaces seem to be poor surfaces for mussels to colonise (Navarrete and Castilla 1990; McCook and Chapman 1991).
Results suggest that the importance of robust algal structures is higher in areas where large-scale disturbance is frequent and mussel beds are few or ephemeral, but levels off towards areas with stable mussel beds. As shown in the colonisation experiment, when complex structures are lacking, successful recruitment to mussel patches is significantly higher than comparative recruitment to empty space. However, our results are not admittedly clear-cut since positive mussel-algal relations were generally not observed at sites with high mussel biomass. Plausible reasons may be that (1) dense mussel beds with large mussels outcompete the algae and (2) at some point at exposed sites, waves easily remove large mussels from the algae but mussels are more firmly attached to the bottom when they live in dense mussel beds. If so, mussels should also be associated with algae at the most exposed sites at Söderskär due to their overall small size, as they did. Based on field observations and experiments, we hypothesise that Furcellaria may be beneficial for mussels for several reasons. (1) At sites with high loads of sediments and low abundance of conspecifics, perennial macroalgae can function as attachment structures facilitating colonisation of both recruits and adult mussels. (2) Survival of recruits and post-recruits may be higher on Furcellaria due to reduced risk of gill damage caused by scouring sediments (Cheung and Shin 2005; Zardi et al. 2006b) or due to better feeding conditions when lifted up from the bottom and (3) Furcellaria may also reduce intraspecific competition effects by elevating attached mussels from the mussel bed and may reduce predation pressure, if mussels can hide among algal structures. At Söderskär where predation pressure is extremely intense (Westerbom et al. 2002, 2006), and mussels sufficiently small to find refuges among the algae, this may be especially important alleviating predation pressure, environmental stress and consequently increasing population size (for more discussion on predation effects in this system: see Westerbom and Jattu 2006; Westerbom et al. 2006).
In summary, our results emphasise the importance of incorporating processes relevant at different spatial scales when distribution pattern of rocky shore organisms are described. Here, we showed that processes operating at small scales are embedded in large-scale pattern. Mussel size structure influenced the strength of the relevant processes; whereas wave exposure had the strongest effect on mussel distribution in comparatively dense populations, the effects were less obvious among sparse populations comprising small individuals. Conversely, and in line with theory (e.g., Bruno and Bertness 2001), biogenic structures appeared to show an opposite pattern, being more important towards the edge margin where abiotic and biotic stress is most intense (Westerbom et al. 2002, 2006). Traditionally, biotic interactions have been regarded as having little importance in affecting distribution patterns in the Baltic proper (Kautsky and van der Maarel 1990). Towards the edge, however, the importance of biotic interactions may increase and to understand the functioning of marginal populations, research should therefore, strive for multi-scale approaches in order to link ecosystem patterns with ecosystem processes.
References
Airoldi L (2003) The effects of sedimentation on rocky coast assemblages. Oceanogr Mar Biol 41:161–236
Baardseth E (1970) A square-scanning, two stage sampling method of estimating seaweed quantities. Rep Norw Inst Seaweed Res 33:1–41
Bayne BL (1964) Primary and secondary settlement in Mytilus edulis L. (Mollusca). J Anim Ecol 33:513–523
Bégin C, Johnson L, Himmelman JH (2004) Macroalgal canopies: distribution and diversity of associated invertebrates and effects on the recruitment and growth of mussels. Mar Ecol Prog Ser 271:121–132
Benedetti-Cecchi L (2001) Variability in abundance of algae and invertebrates at different spatial scales on rocky sea shores. Mar Ecol Prog Ser 215:79–92
Benedetti-Cecchi L, Bulleri F, Cinelli F (2000) The interplay of physical and biological factors in maintaining mid-shore and low-shore assemblages on rocky coasts in the north-west Mediterranean. Oecologia 123:406–417
Bertness MD, Leonard GH, Levine JM, Schmidt PR, Ingraham AO (1999) Testing the relative contribution of positive and negative interactions in rocky intertidal communities. Ecology 80:2711–2726
Bruno JF, Bertness MD (2001) Habitat modification and facilitation in benthic marine communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland, pp 201–218
Cheung SG, Shin PKS (2005) Size effects of suspended particles on gill damage in green-lipped mussel Perna viridis. Mar Pollut Bull 51:801–810
Davis AR, Moreno CA (1995) Selection of substrata by juvenile Choromytilus chorus (Mytilidae): are chemical cues important. J Exp Mar Biol Ecol 191:167–180
Erlandsson J, McQuaid CD (2004) Spatial structure of recruitment in the mussel Perna perna at local scales: effects of adults, algae and recruit size. Mar Ecol Prog Ser 267:173–185
Griffiths SP, Davis AR, West RJ (2006) Role of habitat complexity in structuring temperate rockpool ichthyofaunas. Mar Ecol Prog Ser 313:227–239
Holt RD, Keitt TH (2005) Species’ borders: a unifying theme in ecology. Oikos 108:3–6
Hunt HL, Scheibling RE (1996) Physical and biological factors influencing mussel (Mytilus trossulus, M edulis) settlement on a wave-exposed rocky shore. Mar Ecol Prog Ser 142:135–145
Hunt HL, Scheibling RE (1997) Role of post-settlement mortality in recruitment of benthic marine invertebrates. Mar Ecol Prog Ser 155:269–301
Irving AD, Tanner JE, McDonald BK (2007) Priority effects of faunal assemblages within artificial seagrass. J Exp Mar Biol Ecol 340:40–49
Kautsky H, van der Maarel E (1990) Multivariate approaches to the variation in phytobenthic communities and environmental vectors in the Baltic Sea. Mar Ecol Prog Ser 60:169–184
Kiirikki M (1996) Mechanisms affecting macroalgal zonation in the northern Baltic Sea. Eur J Phycol 31:225–232
McCook LJ, Chapman ARO (1991) Community succession following massive ice-scour on an exposed rocky shore: effects of Fucus canopy algae and of mussels during late succession. J Exp Mar Biol Ecol 154:137–169
McQuaid CD, Lindsay TL (2005) Interacting effects of wave exposure, tidal height and substratum on spatial variation in densities of mussel Perna perna plantigrades. Mar Ecol Prog Ser 301:173–184
Menge BA, Branch GM (2001) Rocky intertidal communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland
Moreno CA (1995) Macroalgae as a refuge from predation for recruits of the mussel Choromytilus chorus (Molina, 1782) in Southern Chile. J Exp Mar Biol Ecol 191:181–193
Morgan SG (2001) The larval ecology of marine communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland
Navarrete SA, Castilla JC (1990) Barnacle walls as mediators of intertidal mussel recruitment: effects of patch size on the utilization of space. Mar Ecol Prog Ser 68:113–119
Pedersén M, Snoeijs P (2001) Patterns of macroalgal diversity, community composition and long-term changes along the Swedish west coast. Hydrobiologia 459:83–102
Phillips NE, Shima JS (2006) Differential effects of suspended sediments on larval survival and settlement of New Zealand urchins Evechinus chloroticus and abalone Haliotis iris. Mar Ecol Prog Ser 314:149–158
Qvarfordt S, Kautsky H, Malm T (2006) Development of fouling communities on vertical structures in the Baltic Sea. Estuar Coast Shelf Sci 67:618–628
Robles CD, Alvarado MA, Desharnais RA (2001) The shifting balance of littoral predator-prey interaction in regimes of hydrodynamic stress. Oecologia 128:142–152
Steffani CN, Branch GM (2003) Growth rate, condition, and shell shape of Mytilus galloprovincialis: responses to wave exposure. Mar Ecol Prog Ser 246:197–209
Stoffels RJ, Closs GP, Burns CW (2003) Multiple scales and the relationships between density and spatial aggregation in littoral communities. Oikos 103:81–92
Thrush SF, Hewitt JE, Herman PMJ, Ysebaert T (2005) Multi-scale analysis of species-environment relationships. Mar Ecol Prog Ser 302:13–26
Tuya F, Haroun RJ (2006) Spatial patterns and response to wave exposure of shallow water algal assemblages across the Canarian Archipelago: a multi-scaled approach. Mar Ecol Prog Ser 311:15–28
Underwood AJ, Keough MJ (2001). Supply-side ecology. The nature and consequences of variation in recruitment of intertidal organisms. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland
Westerbom M, Jattu S (2006) Effects of wave exposure on the sublittoral distribution of blue mussels (Mytilus edulis) in a heterogeneous archipelago. Mar Ecol Prog Ser 306:191–200
Westerbom M, Kilpi M, Mustonen O (2002) Blue mussels, Mytilus edulis, at the edge of the range: population structure, growth and biomass along a salinity gradient in the northeastern Baltic Sea. Mar Biol 140:991–999
Westerbom M, Lappalainen A, Mustonen O (2006) Invariant size selection of blue mussels by roach despite variable prey size distributions? Mar Ecol Prog Ser 328:161–170
Wieters EA (2005) Upwelling control of positive interactions over mesoscales: a new link between bottom-up and top-down processes on rocky shores. Mar Ecol Prog Ser 301:43–54
Witman JD, Dayton PK (2001) Rocky subtidal communities. In: Bertness MD, Gaines SD, Hay ME (eds) Marine community ecology. Sinauer Associates, Sunderland
Zardi GI, Nicastro KR, McQuaid CD, Rius M, Porri F (2006a) Hydrodynamic stress and habitat partitioning between indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels: constraints of an evolutionary strategy. Mar Biol 150:79–88
Zardi GI, Nicastro KR, Porri F, McQuaid CD (2006b) Sand stress as a non-determinant of habitat segregation of indigenous (Perna perna) and invasive (Mytilus galloprovincialis) mussels in South Africa. Mar Biol 148:1031–1038
Acknowledgements
We appreciate the constructive comments of Prof. N. Kautsky on the draft of the manuscript. We are very grateful to M. Hario at the Finnish Game and Fisheries Research Institute for helping in the field at Söderskär and to the Finnish Institute of Marine Research for providing data on offshore salinity conditions. Constructive and most valuable comments were provided by two anonymous referees on an earlier version of the manuscript. The study was supported by the EU-project (AC 340165) and grants from Svenska kulturfonden (MW), Oscar Öflunds stiftelse (MW), Waldemar von Frenckells stiftelse (MW) and Walter och Andrée de Nottbecks stiftelse (MW; OM). The study complies with current laws in Finland.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M. Kühl.
Rights and permissions
About this article
Cite this article
Westerbom, M., Mustonen, O. & Kilpi, M. Distribution of a marginal population of Mytilus edulis: responses to biotic and abiotic processes at different spatial scales. Mar Biol 153, 1153–1164 (2008). https://doi.org/10.1007/s00227-007-0886-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0886-7