Abstract
In this paper we describe a cryopreservation protocol followed by the culture of Symbiodinium sp. isolated from the Caribbean gorgonian Pseudopterogorgia elisabethae as a potential renewable source of the dinoflagellate symbiont. Four different freezing protocols were designed: a controlled cooling device designed to cool at 1°C/min, a three-step protocol (−20°C for 2 h, −70°C for 2 h, liquid nitrogen-LN2), a two-step protocol (−70°C for 2 h, LN2), and a one-step protocol (LN2). All cells were stored in LN2 after cryopreservation. The cryoprotective agents (CPA) used were ethanol (EtOH) and methanol (MeOH) at 10 and 20%, and seawater (FSW) was used as a control. Viability measurements using cell counts showed that all cryopreservation protocols were relatively successful, and no trends were observed regarding freezing protocol or CPA used. After 19 weeks in culture the viability of samples which had high biomass was determined by the fluorescent assay CellTiter Blue™. The most viable cultures were those cryopreserved by a two-step protocol using 20% MeOH or 20% EtOH as a CPA. A genetic examination of the DNA of these samples using Symbiodinium-specific PCR primers confirmed that the composition of the culture had not changed. For the first time, we report that Symbiodinium sp. isolated from a gorgonian can be cryopreserved and subsequently cultured successfully.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cryopreservation, the process of cooling and storing cells, tissues, or organs at very low temperatures to maintain viability has been widely used with many different organisms. To the best of our knowledge, there are only a few reports dealing with cryopreservation protocols for microalga such as dinoflagellates (Anderson 1999; Rhodes et al. 2006; Simione and Daggett 1977), however, no reports comment on the cryopreservation of the symbiotic dinoflagellate Symbiodinium sp. This organism, commonly found within the tissues of many corals, has an important role as a primary producer, greatly contributing to the ecology of the coral reefs. There is also evidence suggesting that Symbiodinium may play a role in secondary metabolite biosynthesis. Free-living dinoflagellates have been identified as the primary source of important secondary metabolites such as shellfish toxins isolated from marine invertebrates (Shimizu 1993). Symbiodinium may play a role in the production of diterpenes in Caribbean gorgonians such as Pseudopterogorgia elisabethae and P. bipinnata (Mydlarz et al. 2003; Boehnlein et al. 2005). For example, P. elisabethae is the source of the pseudopterosins, diterpene glycosides that possess potent anti-inflammatory activity with a unique mechanism of action (Look et al. 1986a, b; Moya 2004).
Although no preservation technique can guarantee total stability of secondary metabolite biosynthesis, cryopreservation has been found to be the best technique for preserving secondary metabolite production in fungi (Ryan et al. 2003) and in microalgae (Hédoin et al. 2006). Multiple cryoprotective agents (CPA) have been tested such as glycerol in fungi and in microalgae (Ryan et al. 2003; Gwo et al. 2005; Poncet and Véron 2003), ethylene and propylene glycol in brown algae and microalgae (Kono et al. 1998; Gwo et al. 2005; Rhodes et al. 2006), methanol (MeOH) for green algae and marine microalgae (Crutchfield et al. 1999; Tzovenis et al. 2004), and dimethyl sulfoxide (DMSO) and glycerol in marine microalgae including some dinoflagellates (Cañavate and Lubian 1997; Rhodes et al. 2006), and a combination of ethylene glycol with polyvinylpyrrolidone and trehalose used with the dinoflagellate Amphidinium carterae (Rhodes et al. 2006) in an effort to preserve biomass and sometimes to preserve the source of secondary metabolite biosynthesis. Unfortunately, a CPA that has been successful with one species does not necessarily translate into a successful CPA when used with another organism. For example, DMSO has been proven to be a versatile CPA among many different species but it was found that MeOH lead to a higher survival rate than DMSO in the chlorophyte Chlamydomonas reinhardtii (Morris et al. 1979). The free-living dinoflagellate Crypthecodinium conhii was cryopreserved by DMSO and glycerol but no alcohols were tested (Simione and Daggett 1977) even though these were successful in other marine microalgae (Tzovenis et al. 2004) and are readily available.
Freezing and thawing protocols can be as varied as the CPAs used, ranging from plunging cells into liquid nitrogen with uncontrolled thawing to controlled cooling and controlled thawing rates (Bodas et al. 1995; Kono et al. 1998; Ryan et al. 2003). This can be one of the key steps to preserving viable organisms since cell components can be injured during freezing and thawing (Crutchfield et al. 1999). Testing of cell viability (quantitatively and qualitatively) and timing of the testing is also highly variable (Bodas et al. 1995; Kono et al. 1998; Ryan et al. 2003). The physiological state of cryopreserved cells has been tested by a variety of methods such as the production of secondary metabolites by HPLC and TLC (Ryan et al. 2003), microscopic examination of cells with biological stains such as erythrosine (Kono et al. 1998) and Evans blue dye (Crutchfield et al. 1999), colony counts after cell plating (Crutchfield et al. 1999; Lewis et al. 1994), cell counts (Simione and Daggett 1977; Tzovenis et al. 2004), and the ability of preserved cells to divide (Day et al. 1997). Typically the cells are cryopreserved for short periods of time (e.g. 5 days) and examined in culture for short periods of time afterwards (one month; e.g. Tzovenis et al. 2004). Very few studies have examined the long-term cell viability of organisms cryopreserved for extended periods of time. This can be of great significance when access to the source organism is limited (Ryan et al. 2003; Simione and Daggett 1977), and for slow growing cells.
A component that is often missing from the microalgal literature is the genetic identification of cryopreserved organisms after they have been in culture for a period of time since contamination of cultures with physically and physiologically-similar organisms is quite common. Physical identification of microalgae can be misleading and lead to erroneous classification. Additionally, the risk of losing an important species through genetic drift is one of the largest concerns when culturing microalgae (Rhodes et al. 2006). Consequently, an important attribute of any protocol designed to preserve biomass should be long-term phenotypic stability (Hédoin et al. 2006).
We describe a cryopreservation protocol for Symbiodinium sp. isolated from P. elisabethae, which could be used as a culture starter and as a biomass reserve that can be stored and provided on demand. It can also be used as an alternative to maintaining live cultures, which can be very time consuming. This method of cell storage at very low temperatures assures genome stability over extended periods of time, thus preserving a source of Symbiodinium and also establishes a protocol that can be adapted to similar organisms.
Materials and methods
Chemicals and supplies
All HPLC grade-solvents used in this study, thin layer chromatography (TLC) Whatman plates, all plastics, hemacytometer, filters, Trypan Blue dye, and Mr. Frosty™ were obtained from Fisher Scientific (Suwannee, GA, USA). The CellTiter Blue™ assay kit and restriction enzymes were obtained from Promega (Madison, WI, USA). Percoll was obtained from Sigma-Aldrich (St Louis, MO, USA). The DNeasy Plant Mini Kit was obtained from Qiagen (Valencia, CA, USA).
Coral collection and cell isolation
Pseudopterogorgia elisabethae was collected by SCUBA from a depth of ca. 10 m in San Salvador, the Bahamas, May 2004. The coral was immediately assayed for the presence of pseudopterosins by TLC to confirm its identity. P. elisabethae was then homogenated in a blender with 0.22 μm filtered-seawater (FSW). The homogenate was filtered through four layers of cheesecloth and the filtrate was pelleted by centrifugation at 900g. The Symbiodinium sp. pellet was washed with FSW by centrifugation at 900g ten times. The algal symbionts were further purified twice by layering the cell suspension onto a discontinuous Percoll gradient of 20, 40, and 80%. The purified cells were collected at the interface of the 40 and 80% Percoll layers. The cells were then rinsed, centrifuged and re-suspended in FSW. The dinoflagellates were counted by a hemacytometer under light microscopy to determine the cell density (cells/ml).
Cryopreservation
The density of cells was adjusted to a concentration of 1.0 × 106 cells/ml and aliquoted (1 ml) into Nalgene cryopreservation tubes (Fisher Scientific). The cells were pelleted by centrifugation at 900g, the supernatant decanted and 1 ml of the appropriate CPA added to each tube (see Table 1). Each sample was then maintained at 4°C for 10 min and then frozen according to Table 1. After each sample tube was cryopreserved it was placed inside a Cryocane covered with a Cryosheath (Fisher Scientific) and submerged into liquid nitrogen for the duration of the cryopreservation period of 13 weeks. Two aliquots of 2.0 × 107 cells were also stored in liquid nitrogen as DNA vouchers.
Symbiodinium cell culture
After 13 weeks the cells were removed from the liquid nitrogen and the tubes quickly defrosted by submersion in water at room temperature. The cells were pelleted at 900g and the CPA removed. The cells were washed with FSW twice more before being inoculated into 4 ml of ASP-8A Symbiodinium (zooxanthellae) culture medium with an antibiotic treatment at 1% v/v (Provasoli et al. 1957). The cultures were then maintained in a Precision incubator (Fisher Scientific) at 26°C on a 14:10 light:dark cycle for a period of 19 weeks.
Cell viability
Samples were counted with a hemacytometer during weeks 1, 8, 15, and 19. The percent survival rate based on the initial cell count was determined by calculating the percent of live cells compared to the initial cell count, or cell number, of 1 × 106 cells. Live cells were identified by using the Trypan Blue dye exclusion assay and examination by light microscopy.
Trypan Blue dye exclusion assay can overestimate the amount of viable cells (Altman et al. 1993). Thus, cell viability was also assessed at the end of the 19-week period by using samples with sufficient cell density for the CellTiter Blue viability assay. This fluorescent assay is based on the ability of living cells to convert a redox dye (resazurin) into a fluorescent end product (resorufin). Non-viable cells do not have the metabolic capacity to produce resorufin and do not generate a fluorescent signal. Three cell concentrations of the selected cryopreserved sample sets were incubated with CellTiter Blue: 2.5 × 104, 1.25 × 104 and 0.625 × 104. The fluorescence excitation was set at 560 nm and the emission was monitored at 590 nm after 4 and 22 h of incubation. Fluorescence was monitored by a Spectramax Plus™ microplate reader (Molecular Devices, Sunnyvale, CA, USA). Statistical tests were performed using the software package SigmaStat 3.1 (Systat Software Inc., Point Richmond, CA, USA).
Chemical analysis
TLC was performed using pre-coated silica plates with hexanes:ethyl acetate (50:50) as a mobile phase. The plates were developed with a 10% sulfuric acid in MeOH and charred in an oven.
DNA analysis
The DNA from genetic vouchers and cryopreserved samples after culturing was isolated using a DNeasy Plant mini kit. The small subunit of ribosomal genomic DNA (SS-rDNA) was PCR-amplified by using the universal primer ss5 with the Symbiodinium-specific primer ss3Z according to established procedures (Rowan and Powers 1991a, b). The SS-rDNA amplicon was digested with the restriction enzymes TaqI and DpnII and visualized by gel electrophoresis on a 2.5% agarose gel for restriction fragment length polymorphism (RFLP) analysis as previously described (Boehnlein et al. 2005).
The internal transcribed spacer region (ITS-rDNA) was PCR-amplified using the Symbiodinium-specific primers ZITSUPM13 and ZITSDNM13 designed to amplify between the conserved regions of the 3′ and 5′ ends of Symbiodinium 18S and 28S-rDNA genes (Santos et al. 2001). PCR amplicons were visualized by gel electrophoresis on a 1.2% agarose gel stained with ethidium bromide. The 750-bp amplicon was sequenced by the Florida State University Biology DNA Sequencing Facility. Sequences were viewed and edited using ChromasLite2000 (http://www.technelysium.com.au/chromas.html) and EditSeq (Lasergene, DNASTAR, Madison, WI, USA), and aligned using Biology Workbench (http://www.workbench.sdsc.edu). Sequences were also compared to those in databases using the Basic Local Alignment Search Tool (BLAST) algorithm to identify known sequences with a high degree of similarity. Homology comparisons to other published Symbiodinium ITS sequences were performed using MegAlign (Lasergene). The Symbiodinium ITS gene sequence was submitted to GenBank and assigned the accession number DQ238587.
Results
Our main goal for this project was to design a simple cryopreservation protocol for Symbiodinium sp. that could be performed in the field with limited resources to cryopreserve the organism for extended periods of time, and to assess the long-term cell viability by survival rates based on cell counts and cell viability assays. We examined alcohol based CPAs at various concentrations and cooling rates, as well as the viability of the cells over an extended period of time, as most cryopreservation studies only examine short time periods. A second goal was to confirm the genetic identity of cryopreserved and cultured Symbiodinium sp. by using the polymerase chain reaction (PCR) and primers designed to specifically amplify the Symbiodinium ribosomal DNA regions, RFLP analysis and gene sequence analysis.
Cell viability by cell counts and Trypan Blue™ exclusion assay
A general examination of the cell survival rate demonstrated that cell numbers declined through the first 8 weeks and stabilized towards the end of the study (Table 1). For every week where cell viability was tested, the survival rates for each protocol (Table 1) were statistically analyzed against the other protocols to determine which protocol provided a higher survival rate. For this purpose, the survival rates for each CPA within the same protocol were averaged into a single number (n = 25) and statistically analyzed by one-way ANOVA. No statistically significant differences were found between the different protocols within the same week. The results were the same for each one of the four time periods tested.
The suitability of each CPA within a cryopreservation protocol (Table 1) was also tested for significant statistical differences by one-way ANOVA (Holm–Sidak test). In the majority of the cases no statistically significant differences were observed with regards to survival rate. There were two exceptions where statistical differences were noted, both during week 19. In the 3-step protocol the survival rate for CPA EtOH 20% (4.3 ± 0.7) was statistically significantly higher than the rate obtained for the CPA MeOH 10% (0.2 ± 0.2). Interestingly, there were no other statistically significant differences in survival rate of samples in the 3-step protocol even though an initial visual inspection of the rates may suggest that there are other differences. An examination of survival rates for the 2-step protocol, indicated that the CPA MeOH 20% (5.1 ± 0.8) was significantly higher than that from MeOH 10% (1.5 ± 0.9). There was no statistically significant difference between the EtOH 10%, EtOH 20% and FSW control. Thus, compared to all the survival rates obtained for all the protocols during week 19, two samples (3-step 20% EtOH, and 2-step 20% MeOH) had the highest survival rates of all the samples.
An interesting observation is the rapid survival rate decline during weeks 1–15 followed by a plateau in cell number during weeks 15–19. When these survival rates where statistically analyzed for significant differences (one-way ANOVA) for each single treatment during weeks 1, 8, 15, and 19, some interesting trends were noted. For the most part survival rates during week 1 were found to be statistically different from those of weeks 8, 15, and 19, however the rates for these last three weeks were seldom statistically different. Notable exceptions can be observed in Table 1. During the cryopreservation protocol Mr. Frosty with EtOH 20% and MeOH 10% and the 2-Step protocol with EtOH 10%, week 1 is statistically different from weeks 15 and 19 but not from week 8. Another interesting observation was made for Mr. Frosty MeOH 20% and 3-Step EtOH 20% where statistically significant differences were not noted. A final exception was the 1-Step MeOH 20% where weeks 1 and 8 were statistically different from each other and also from weeks 15 and 19.
Cell viability by fluorescent CellTiter Blue™ assay
At the end of the 19-week period, cell viability was assessed using the fluorescent-bioassay assay CellTiter Blue. Because a minimum cell density was required for the assay, only samples with the highest survival rates were examined (Table 2). This test was not done throughout the study because of limited sample quantities, as the assay renders the cells unsuitable for further experimentation. The results were statistically analyzed by one-way ANOVA with a confidence interval of 95%. In contrast to the previous results, very noticeable differences regarding cell viability were observed and determined to be statistically significant. Symbiodinium cells cryopreserved in a 2-step protocol using 20% MeOH or EtOH as a CPA displayed dramatically higher cell viability than those preserved using Mr. Frosty™ 20% MeOH or a 3-step protocol using 20% MeOH or EtOH (P < 0.05; Table 2). It is noteworthy that the all the samples chosen for the viability assay were cryopreserved with either 20% MeOH or 20% EtOH as a CPA and that all displayed significant viability, albeit at different levels.
Genetic examination
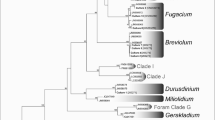
PCR amplification using SS-rDNA universal Symbiodinium primers yielded a 1,650 bp product for both the DNA voucher samples and the cryopreserved samples that were in culture at week 19. The RFLP patterns from TaqI and DpnII of the cryopreserved samples from culture and the DNA vouchers demonstrated the presence of Symbiodinium sp. clade B in both instances by comparison to published RFLP patterns (Fig. 1; Toller et al. 2001). The samples were further analyzed by the PCR amplification of the ITS region using Symbiodinium-specific primers. The genetic sequences, 750 bp, were analyzed through BLAST and both confirmed to be 100% homologous to published Symbiodinium sp. clade B sequences when aligned with other published Symbiodinium clade B sequences in the NCBI database.
A Agarose gel electrophoresis for A: SSrDNA PCR showing expecting amplicon size at 1.65 k-bp, 1.2% agarose stain with ethidium bromide, lanes 1 1 k-bp DNA plus ladder, 2 negative control, 3 voucher DNA, 4 week 19 Symbiodinium in culture; B RFLP analysis of SSrDNA, 2.5% agarose stain with ethidium bromide, lanes 1,4,7 1 k-bp DNA plus ladder, 2 TaqI pattern for voucher, 3 TaqI pattern for week 19 Symbiodinium in culture, 5 DpnII pattern for voucher, 3 DpnII pattern for week 19 Symbiodinium in culture; C ITS PCR showing expected amplicon of 0.75 k-bp, 1.2% agarose stained with ethidium bromide, lanes 1 1 k-bp DNA plus ladder, 2 voucher DNA, 3 and 4 week 19 Symbiodinium in culture (no negative control shown)
Discussion
This study demonstrated that Symbiodinium sp. from the gorgonian P. elisabethae can be cryopreserved for relatively long periods of time (3 months) and successfully re-inoculated into culture medium. The CPAs chosen for this study included MeOH and EtOH, while using FSW as a control. While monohydric alcohols such as MeOH and EtOH have been shown to be successful CPAs, some have suggested that their perceived toxicity might be problematic and therefore ineffective as CPAs (Lewis et al. 1994). Other CPAs such as glycerol have been examined in preserving unicellular organisms and have demonstrated a causative effect of toxicity within cells or swelling, membrane perforation and/or vesticulation of the endomembrane systems (Fields et al. 1997). It has also been reported that DMSO and MeOH are two of the most successful CPAs used in cryopreservation studies. This study focused on the use of the permeating CPAs MeOH and EtOH because they have been recently shown to be as effective as DMSO (Rhodes et al. 2006) but exhibit lower occurrences of toxicity (Hubálek 2003). As CPAs are known to be cytotoxic above certain levels, we used two concentrations of both MeOH and EtOH (Kono et al. 1998; Mortain-Bertrand et al. 1996).
Our results for survival rates determined by cell counts and Trypan Blue exclusion assay indicated that although some treatment sets appear to be more viable than others after the cryopreservation and culturing process, there were no statistically significant differences between the four different protocols tested or with the majority of the different CPAs tested (within an individual protocol). Importantly, statistically significant differences were observed during week 19 for the different CPAs in the 2- and 3-Step protocols, which may suggest that a higher concentration of EtOH or MeOH (20%) is more beneficial as a CPA rather than lower alcohol concentrations or FSW. It appears that the lower concentration of MeOH and EtOH (10%) had no statistically significant effect as CPAs since the survival rates were similar to those for the control samples in FSW. MeOH (10%) was the only CPA successful in the cryopreservation of the aquatic microalga Euglena, although it was ineffective in the cryopreservation of marine diatoms (Hubálek 2003). This same concentration of MeOH was found to be successful in cryopreserving the microalgae Chaetoceros calcitrans and Nitzcshia ovalis, although lower concentrations were tested ranging from 0.5 to 5% (Rhodes et al. 2006).
While some cell death could be attributed to changes in temperature and light:dark cycles, this phenomenon has been frequently observed in our lab in Symbiodinium cultures that have not been cryopreserved. A slow initial growth period after the cells are inoculated into growth medium has been observed. Once this slow growth period is over a sharp increase in cell growth is also observed regularly, suggesting that there is an acclimation period to culture conditions. This repeated phenomenon was seen in multiple cell cultures of Symbiodinium sp. from the genus Pseudopterogorgia, under various physical conditions and in various media (Newberger 2006).
While no obvious trends can be observed from the majority of the data obtained through cell counting, the cell viability data obtained from the fluorescent assay CellTiter Blue® provided quantitative assessment of which protocols resulted in the most viable cell cultures. The limitations of a minimum cell density required for this assay did not allow us to perform it with every sample. For this reason, the samples tested were the sets cryopreserved with both 20% MeOH and 20% EtOH for the 2- and 3-Step protocols and the 20% MeOH of Mr. Frosty™. The results from this assay suggest that a 2-step cryopreservation protocol is more successful at maintaining the overall viability of the cells than a 3-step protocol or the use of Mr. Frosty™. In agreement with our data, two-step cooling protocols have been used successfully with other systems (Day et al. 1997). It has also been noted that at slower cooling rates ice crystals develop and grow wildly within the cells which can cause membrane perforations (Fields et al. 1997). Most likely, during the 3-step protocol and while using Mr. Frosty™ the slower rate of cooling might have lead to excessive cell dehydration, making these less efficient methods for cryopreservation and recovery of cells post-treatment. This might explain why the slower cooling protocols Mr. Frosty™ and the 3-Step Protocol were not as successful as the 2-Step protocol. None of the cells that were directly cooled in LN2 (1-step protocol) displayed high cell numbers at the end of the trial, indicating that this method was the least efficient at cryopreserving Symbiodinium. Gwo et al. (2005) have also reported widespread cell death associated the direct freezing of the microalga Nannochloropsis oculata in liquid nitrogen. This corresponds with other reports that faster cooling rates provide insufficient time for cell dehydration and thus the probability of intracellular ice nucleation increases (Morris 1981) which may cause damage to the plasma membrane and the organelles. While 2-Step protocols have historically been unsuccessful with Euglena and many other microalgae (Day et al. 2000), our 2-Step protocol was the most successful for cryopreserving our Symbiodinium sp. isolated from a gorgonian.
The differences in cell viability observed by fluorescence (Table 2) could not be directly correlated with those observed by cell survival rate using the dye exclusion assays (Table 1). The differences in sensitivity of these two tests could explain these differences. The volumes used for the dye exclusion assay (10 μl) are tenfold less than those used for the CellTiter Blue assay (100 μl) and at such small cell concentrations the assay with the higher volume would provide a more accurate estimate of cell viability. Alternatively, the enzymatic machinery of the cells cryopreserved using the 2-Step protocol could have been better preserved than those which were subjected to the 3-Step protocol or Mr. Frosty™, which might explain the higher enzymatic activity displayed by these, even though all had similar survival rates, as shown in Table 1.
This is one of the few reports that confirmed the genetic identity of the cryopreserved organisms pre- and post-cryopreservation. By means of PCR with primers that specifically amplify DNA from Symbiodinium we were able to conclude that the organism which was isolated from P. elisabethae at the beginning of this experiment was the same organism that was present at the end of week 19. Microorganisms can be misidentified through microscopy and other taxonomic observations, and thus the use of PCR provides a higher level of confidence in assessing the identity of cells in culture. The results from this experiment show that cryopreservation is a viable method of preserving the genetic identity of cultured organisms and a way of avoiding genetic drift which endangers the genetic stability of cultures maintained for long periods of time (Gwo et al. 2005; Hédoin et al. 2006; Poncet and Véron 2003; Rhodes et al. 2006).
To the best of our knowledge this is the first report of a successful cryopreservation study involving Symbiodinium sp. isolated from a gorgonian. From this data it appears that Symbiodinium sp. from P. elisabethae to a certain extent has an inherent cryoprotective mechanism that allows it to avoid injury during cryopreservation. This is evident as even the lack of a CPA resulted in at least a minimal level of viable cells in culture. It has been observed by our group that Symbiodinium sp. isolated from P. elisabethae produce a high amount of “mucus”, possibly a polysaccharide. This might act as a natural CPA thus preventing injury to cryopreserved cells. This hypothesis is supported by Morris and Clarke (1978) who showed that Chlorella cells demonstrated an increased resistance to freezing and thawing damage as they accumulated lipids, showing that compounds naturally present in cells can act as cryopreservatives. Data from Kišidayová and associates (2005) further supported the idea of fatty acids and cryoresistance, by improving the membrane integrity of their ciliates by adding myo-inositol and linoleic acid supplements. Overall, these results correspond with a report by Tzovenis et al. (2004) who found that when assessing cell viability in other microalgae which were cryopreserved with similar CPAs, the microalgae often rely on their own cryoprotective mechanisms. The variety of CPAs tested so far by our group and other researchers and the ability of these cultures to be regenerated are in agreement with this statement and suggest that it is unlikely that a universal protocol could be designed for the cryopreservation of organisms. However, cell counts alone do not display the complete physiological state of the cells. By use of more sensitive fluorescent bioassays, it was concluded that the 2-step protocols with either MeOH (20%) or EtOH (20%) as CPAs are the by far the most effective at allowing cryopreserved cultures to regain their normal physiological state indicating that the most important events take place during the freezing process, which was also observed by Tzovenis and co-workers (2004). Future directions for this work include testing other commonly used CPAs such as glycerol and DMSO with other symbiotic isolates from Caribbean gorgonians.
Abbreviations
- CPA:
-
Cryopreservative agent
- EtOH:
-
Ethanol
- MeOH:
-
Methanol
- LN2 :
-
Liquid nitrogen
- SW:
-
Seawater
- FSW:
-
Filtered seawater
References
Altman SA, Randers L, Rao G (1993) Comparison of trypan blue dye exclusion and fluorometric assays for mammalian cell viability determinations. Biotechnol Progr 9:671–674
Anderson RA (1999) National Center for Culture of Marine Phytoplankton’s (CCMP) role in isolating and maintaining cultures of Pfiesteria and Pfiesteria-like organisms and the procedure for cryopreserving PLO species. In: Litaker W, Scholin C, Vasta GR (eds) Molecular approaches for the identification and environmental detection of Pfiesteria piscicida and Pfiesteria-like dinoflagellates. http://www.whoi.edu/redtide/pfiesteria/molecular/Molecular_Workshop_Report.pdf:, pp 14–16
Bodas K, Brennig C, Diller KR, Brand J (1995) Cryopreservation of blue-green and eukaryotic algae in the culture collection at the University of Texas at Austin. Cryo Lett 16:267–274
Boehnlein JM, Santiago-Vázquez LZ, Kerr RG (2005) Diterpene biosynthesis by the dinoflagellate symbiont of the Caribbean gorgonian Pseudopterogorgia bipinnata. Mar Ecol Prog Ser 303:105–111
Cañavate P, Lubian LM (1997) Effects of slow and rapid warming on the cryopreservation of marine microalgae. Cryobiology 35:143–149
Crutchfield AIM, Diller KR, Brand J (1999) Cryopreservation of Chlamydomonas reinhardtii (Chlorophyta). Eur J Phycol 34:42–52
Day JG, Watanabe MM, Morris GJ, Fleck RA, McLellan MR (1997) Long-term viability of preserved eukaryotic algae. J Appl Phycol 9:121–127
Day JG, Fleck RA, Benson EE (2000) Cryopreservation-recalcitrance in microalgae: novel approaches to identify and avoid cryo-injury. J Appl Phycol 12:369–377
Fields SD, Strout GW, Russell SD (1997) Spray-freezing freeze substitution (SFFS) of cell suspensions for improved preservation of ultrastructure. Microsc Res Tech 38:315–328
Gwo J-C, Chiu J-Y, Chou C-C, Cheng H-Y (2005) Cryopreservation of a marine microalga, Nannochloropsis oculata (Eustigmatophyceae). Cryobiology 50:338–343
Hédoin H, Pearson J, Day JG, Philip D, Young AJ, Hall TJ (2006) Porphyridium cruentum A-408 and Planktothrix A-404 retain their capacity to produce biotechnologically exploitable metabolites after cryopreservation. J Appl Phycol 18:1–7
Hubalek Z (2003) Protectants used in the cryopreservation of microorganisms. Cryobiology 46:205–229
Kišidayová S, Váradyová Z, Michalowski C, Newbold J (2005) Regeneration of cryoresistance of in vitro rumen ciliate cultures. Cryobiology 51:76–84
Kono S, Kuwano K, Saga N (1998) Cryopreservation of Eisenia bicyclis (Laminaria, Phaeophyta) in liquid nitrogen. J Mar Biotecnol 6:220–223
Lewis JG, Learmonth RP, Watson K (1994) Cryoprotection of yeast by alcohols during rapid freezing. Cryobiology 31(2):193–198
Look SA, Fenical W, Jacobs RS, Clardy J (1986a) The pseudopterosins: anti-inflammatory and analgesic natural products from the sea whip Pseudopterogorgia elisabethae. Proc Nat Acad Sci USA 83:6238−6240
Look SA, Fenical W, Matsumoto GK, Clardy J (1986b) The pseudopterosins: a new class of anti-inflammatory and analgesic diterpene pentosides from the marine sea whip Pseudopterogorgia elisabethae (Octocorallia). J Org Chem 51:5140–5145
Morris GJ (1981) Cryopreservation. An introduction to cryopreservation in culture collections. Institute of Terrestrial Ecology. Culture Center for Algae and Protozoa, Cambridge
Morris GJ, Clarke A (1978) The cryopreservation of chlorella 4. Accumulation of lipids as a protective factor. Arch Microbiol 119:153–156
Morris GJ, Coulson GE, Clarke A (1979) The cryopreservation of Chlamydomonas. Cryobiology 16:401–410
Mortain-Bertrand A, Etchart F, deBoucaud M-T (1996) A method for the cryocconservation of Dualiella salina (Chlorophyceae): effect of glycerol and cold adaptation. J Phycol 32:346–352
Moya CE (2004) Tetrahymena thermophila used as a pharmacological model to study the cellular mechanism of action of Pseudopterosin A. Ph.D. thesis, University of California, Santa Barbara
Mydlarz LD, Jacobs RS, Boehnlein J, Kerr RG (2003) Pseudopterosin biosynthesis in Symbiodinium sp., the dinoflagellate symbiont of Pseudopterogorgia elisabethae. Chem Biol 10:1–20
Newberger NC (2006) Bioactive terpene production associated with Caribbean gorgonians from the genera Pseudopterogorgia and Eunicea: discovery of a sustainable production method. Ph.D. thesis, Florida Atlantic University, Boca Raton
Poncet J-M, Véron B (2003) Cryopreservation of the unicellular marine alga, Nannochloropsis oculata. Biotechnol Let 25:2017–2022
Provasoli L, McLaughlin JJA, Droop MR (1957) The development of artificial media for marine algae. Arch Mikrobiol 25:392–428
Rhodes L, Smith J, Tervit R, Roberts R, Adamson J, Adams S, Decker M (2006) Cryopreservation of economically valuable marine micro-algae in the classes Bacillariophyceae, Chlorophyceae, Cyanophyceae, Dinophyceae, Haptophyceae, Prasinophyceae, and Rhodophyceae. Cryobiology 52:152–156
Rowan R, Powers DA (1991a) A molecular genetic classification of zooxanthellae and the evolution of animal–algal symbioses. Science 251:1348–1351
Rowan R, Powers DA (1991b) Molecular genetic identification of symbiotic dinoflagellates (zooxanthellae). Mar Ecol Prog Ser 71:65–73
Ryan MJ, Smith D, Bridge PD, Jeffries P (2003) The relationship between fungal preservation method and secondary metabolite production in Metarhizium anisopliae and Fusarium oxysporum. World J Microb Biot 19:839–844
Santos SR, Taylor DJ, Coffroth MA (2001) Genetic comparison of freshly isolated versus cultured symbiotic dinoflagellates: implications for extrapolating to the intact symbiosis. J Phycol 37:900–912
Shimizu Y (1993) Dinoflagellates as sources of bioactive molecules. In: Attaway D, Zaborsky OR (eds) Marine biotechnology, pharmaceutical and bioactive natural products, vol 1. Plenum Press, New York, pp 391–410
Simione FP Jr, Daggett P-M (1977) Recovery of a marine dinoflagellate following controlled and uncontrolled freezing. Cryobiology 14:362–366
Toller WW, Rowan R, Knowlton N (2001) Zooxanthellae of the Montastraea annularis species complex: patterns of distribution of four taxa of Symbiodinium on different reefs across depths. Biol Bull 201:348–359
Tzovenis I, Triantaphyllidis G, Naihong X, Chatzinikolaou E, Papadopoulou K, Xouri G, Tafas T (2004) Cryopreservation of marine microalgae and potential toxicity of cryoprotectants to the primary steps of aquaculture food chain. Aquaculture 230:457–473
Acknowledgments
We are grateful to Dr. L. K. Ranzer for her assistance with cell isolation and the cryopreservation of Symbiodinium cells aboard the R/V Suncoaster. We would like to acknowledge funding from the Center of Excellence in Biomedical and Marine Biotechnology, the National Science Foundation grant MCB-0119011, the Florida Sea grant number R/LP-MB-14, the Florida Institute of Oceanography for time on the R/V Suncoaster and the Canada Research Chair program. This material is based upon work supported by the National Science Foundation under a grant awarded in 2003 to Lory Z. Santiago-Vázquez (award #0310283). Any opinions, findings, and conclusions or recommendations expressed in this publication are those of the authors and do not necessarily reflect the views of the National Science Foundation. We acknowledge the government of the Bahamas for issuing a scientific collecting permit and allowing us to work in their territorial waters. The experiments comply with the current laws of the countries where the experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.W. Sammarco.
Lory Z. Santiago-Vázquez and Nealie C. Newberger contributed equally to this publication.
Rights and permissions
About this article
Cite this article
Santiago-Vázquez, L.Z., Newberger, N.C. & Kerr, R.G. Cryopreservation of the dinoflagellate symbiont of the octocoral Pseudopterogorgia elisabethae . Mar Biol 152, 549–556 (2007). https://doi.org/10.1007/s00227-007-0704-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0704-2