Abstract
We examined physiological stress responses in the edible crab, Cancer pagurus, subjected to the commercial fishery practice of manual de-clawing. We measured haemolymph glucose and lactate, plus muscular glycogen and glycogen mobilisation, in three experiments where the crabs had one claw removed. In the first, crabs showed physiological stress responses when ‘de-clawed’ as compared to ‘handled only’ over the short term of 1–10 min. In the second, de-clawing and the presence of a conspecific both increased the physiological stress responses over the longer term of 24 h. In the third, de-clawing was shown to be more stressful than ‘induced autotomy’ of claws. Further, the former practice caused larger wounds to the body and significantly higher mortality than the latter. Since the fishery practice is to remove both claws, the stress response observed and mortality data reported are conservative.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Animal welfare concerns have led to legal protection against the imposition of stress being afforded to vertebrates. For example, the UK Animals (Scientific Procedures) Act 1986 limits their use in scientific investigations. Whether invertebrates are stressed in an analogous way is less clear (Sherwin 2001) and legal protective measures are subsequently limited. The UK Animals (Scientific Procedures) Act (Amendment) Order 1993 gave protection to the common octopus, Octopus vulgaris, after much deliberation by the Home Office committee. However, the Norwegian Animal Welfare Act (Code 750.000) now applies to crustaceans as well as many other animals, a decision that has also been implemented in Queensland with the Animal Care and Protection Act 2001.
Crustaceans subject to stressors release crustacean hyperglycaemic hormone (CHH), which elevates haemolymph glucose concentrations (Webster 1996; Bergmann et al. 2001; Toullec et al. 2002). This occurs by mobilisation of intracellular glycogen, liberated glucose either moving to extracellular fractions or being converted intracellularly to lactate via glycolysis (Stentiford et al. 2001; Verri et al. 2001), analogous to the responses of vertebrates. The activity and effects of CHH have been reported in a variety of crustaceans during hypoxia (Albert and Ellington 1985), temperature and salinity changes (Keller et al. 1994), capture by trawling (Paterson and Spanoghe 1997) and changes in light intensity (Fanjul-Moles et al. 1998).
Here, we examine the potential for stress caused by ‘de-clawing’, a common crustacean fishery practice. De-clawing had been controlled by the UK Crab Claws (Prohibition of Landing) Order 1986, which prohibited landing claws that had been detached from live edible crabs, Cancer pagurus. However, in 2000, this was revoked in Scotland, England and N. Ireland, followed by Wales in 2001 (The Crab Claws (Prohibition of Landing) (Revocation) Order 2000). The practice of live de-clawing is also found in the fishery of the southern Florida stone crab, Menippe mercenaria. In C. pagurus, both claws may be removed and the live animal returned to the sea (Fahy et al. 2004), whereas in M. mercenaria only one claw is removed (Muller and Bert 2001). In Portugal, the major claws of the fiddler crab, Uca tangeri, are harvested and the animals released on mudflats (Oliveira et al. 2000). In cultivation of the freshwater prawn, Macrobrachium rosenbergii, claw ablation is induced to ensure uniformity in growth of males and increased survival in holding tanks (Manush et al. 2005).
The practice of de-clawing has been defended as crustaceans may autotomise claws naturally, however, the manner of appendage loss may determine survival (Bergmann and Moore 2001). In addition, because regeneration of limbs occurs in crustaceans, fishermen believe they are contributing to the sustainability of this resource (Carroll and Winn 1989), representing a lower source of mortality than a fishery based on the whole crab. However, the immediate and longer-term welfare and mortality implications of de-clawing, until now, have received little objective study. Mortality as a direct result of de-clawing is an important issue in the pot fishery, which is considered one of the few sustainable fishery practices when compared with mobile fishing gear (Watling and Norse 1998), causing limited damage to the environment, benthic communities and by-catch species (Eno et al. 2001).
The aims of the present study were thus to assess, in the edible crab, C. pagurus: (1) short term (1–10 min) physiological stress responses of crabs to removal of one claw; (2) longer term (24 h) stress responses and effects of the presence of a conspecific; (3) stress responses of manual ‘de-clawing’ as compared to ‘induced’ autotomy. We also compare mortality over 24 h and wound size caused by these two methods. In all experiments we assay glucose, glycogen and lactate and assess glucose relative to glycogen concentrations as this reflects the action of CHH (Stentiford et al. 2001; Briffa and Elwood 2004).
Methods
Ethical note
Investigations into stress can sometimes utilise animals that have undergone the ‘normal’ practice being investigated, such as fishery by-catch (Fahy et al. 2004). However, this is not always possible and thus animals are subject to controlled experiments (Telford 1974; Webster 1996; Sneddon 2003; Manush et al. 2005). Here, we use the latter approach, keeping the number of animals used to a minimum and argue that the gain in knowledge and long term benefit to the subject species is high (see ASAB guidelines 2006).
Animal collection/husbandry
Edible crabs, Cancer pagurus, were collected with baited pots from Strangford Lough, Co. Down, N. Ireland, during January 2005–January 2006 and kept communally (15–20) in circular (191 cm diameter × 71 cm height) outdoor mesh-covered tanks with 20 cm (diameter) × 20 cm (length) plastic pipes for shelter. Seawater was piped continuously from the lough to maintain ambient conditions. Animals were acclimated for 5 days, fed ad libitum with fish carcasses, but not fed for 96 h prior to experiments. Only inter-moult animals with complete sets of undamaged limbs and free from obvious infections/abnormalities were used, in the size range (carapace width) 110–150 mm. Animals were returned to the lough following experiments.
Experiment 1: Short term effects of de-clawing
In January–April 2005 (mean lough water temp. 8°C ± SE 0.3°C), crabs were either ‘handled only’ or ‘de-clawed’ (matched times). De-clawing (right claw only) was performed as in the current fishery practice; the crab was held ventral side down and a claw twisted sharply until separation from the body. Haemolymph and tissue samples were taken, for separate groups of crabs, at 1, 5 and 10 min after treatment (n = 10 per experimental group). Crabs were returned to seawater between treatment and physiological sampling.
Experiment 2: Long term effects of de-clawing and presence of a conspecific
In September–November 2005 (mean lough water temp. 13°C ± SE 0.2°C), crabs were either ‘handled only’ or ‘de-clawed’ and then placed either alone or with a similar sized (fully clawed) conspecific (n = 14 per experimental group). Animals were placed in outdoor tanks (152 cm length × 51 cm width × 46 cm height) with piped seawater, a constant air supply and tubing (20 cm dia × 20 cm length) as shelter and physiological samples taken after 24 h.
Experiment 3: Stress responses of crabs to de-clawing versus induced autotomy
In November 2005–January 2006 (mean lough water temp. 10°C ± SE 0.2°C), animals had either a claw removed manually as above (n = 28) or autotomy was induced (n = 23). The latter is achieved by cutting the joint at the top of the merus, i.e. distal to the autotomy joint; the claw was then ‘cast off’ by the crab within a few seconds. Whilst the claw is shed at the same joint as when de-clawed manually, only in autotomy induced crabs is the limb released at a pre-formed fracture plane limiting blood loss and damage to the tissues. Wound area on the body was estimated by taking the radius as the long plus short axes divided by four. Animals were then maintained in outdoor tanks (50 cm length × 38 cm width × 30 cm height) with piped seawater (aerated) for 24 h prior to physiological sampling. Mortality was recorded for all animals, but physiological samples were only taken from survivors in the manually de-clawed group and from eleven crabs in the autotomised group because the others were used in a further experiment not reported here.
Physiological sampling and assays
Haemolymph was withdrawn from crabs from the sinus at the base of the fifth pereiopod using a 2 ml syringe and a 25-gauge needle. Samples were stored in 0.5 ml microcentrifuge tubes and placed in liquid nitrogen, then stored at −20°C. Glucose concentration (μmol l−1) was determined with the liquid glucose hexokinase method (Kit from Randox Laboratories Ltd., Crumlin, Co. Antrim; Briffa and Elwood 2004). Lactate concentration (μmol l−1) was determined by the enzymatic technique of Gutmann and Wahlefeld (1974), as modified by Engel and Jones (1978) and Briffa and Elwood (2002). The fifth pereiopod from the opposite side to the haemolymph extraction site was removed for glycogen determinations (μg/g) using the method of Briffa and Elwood (2004), adapted from Keppler and Decker (1974). In addition, the concentration of glucose was assessed relative to the concentration of glycogen as this provides a clear indication of energy mobilisation (Briffa and Elwood 2004).
Data analyses
Data were log10 transformed for analyses, but untransformed means are shown in the Figures for clarity. Gender was balanced in the experiments, but since no gender effects emerged in the analyses, this factor is not reported. For each experiment, overall treatment effects on the four physiological variables were examined by MANOVA, followed by individual ANOVAs in which all factors are reported irrespective of the overall significance of the MANOVA. This approach should detect effects on each aspect of the physiological response that might not be noted from the MANOVA alone. We used least squares means tests for pair-wise comparisons of means.
Results
Experiment 1: Short term effects of de-clawing
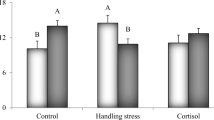
De-clawing had a significant overall effect on the physiological responses (PF4,51 = 4.9, P < 0.002; Fig. 1a–d). There was no significant overall time effect (PF8,104 = 1.69, NS; Fig. 1a–d) or treatment × time interaction effect (PF8,104 = 1.48; NS; Fig. 1a–d).
Glucose was significantly higher in de-clawed crabs (F1,54 = 6.62, P < 0.01; Fig. 1a), with no time effect (F2,54 = 2.21, NS; Fig. 1a) or interaction effect (F2,54 = 0.72, NS; Fig. 1a). De-clawed crabs significantly exceeded handled only crabs in glucose concentration by 10 min post treatment (Fig. 1a). Lactate was also significantly higher in de-clawed crabs (F1,54 = 10.07, P < 0.003; Fig. 1b), with a trend for lactate to increase over time (F2,54 = 2.97, P = 0.06; Fig. 1b), however, there was no interaction effect (F2,54 = 1.62, NS; Fig. 1b). De-clawed crabs significantly exceeded handled only crabs in lactate concentration at 1 min post treatment (Fig. 1b). We did not detect any effect on glycogen (F1,54 = 0.34, NS; Fig. 1c), nor was there a time effect (F2,54 = 0.16, NS; Fig. 1c) or interaction effect (F2,54 = 1.3, NS; Fig. 1c). There was a strong overall trend for de-clawed crabs to mobilise a higher proportion of glycogen (F1,54 = 3.41, P = 0.07; Fig. 1d), with no time effect (F2,54 = 2.36, NS; Fig. 1). There was, however, a significant interaction effect (F2,54 = 4.44, P < 0.02; Fig. 1d), crabs clearly mobilising significant amounts of glycogen by 10 min after de-clawing (Fig. 1d).
Experiment 2: Long term effects of de-clawing and presence of a conspecific
De-clawing and the presence of a conspecific both had significant overall effects on the physiological responses (PF4,76 = 4.11, P < 0.005; PF4,76 = 2.51, P < 0.05; Fig. 2a–d). There was no overall significant interaction effect (PF4,76 = 1.65, NS; Fig. 2a–d).
Mean (+ SE) concentrations of: a haemolymph glucose; b haemolymph lactate; c muscular glycogen; d glycogen mobilisation in crabs 24 h after being ‘handled only’ or ‘de-clawed’ in the absence and presence of a conspecific. Pair-wise comparisons of means were carried out using least squares means tests
Glucose was significantly higher in de-clawed crabs (F1,79 = 4.53, P < 0.04; Fig. 2a), with no significant conspecific effect (F1,79 = 2.21, NS; Fig. 2a), or interaction effect (F1,79 = 0.23, NS; Fig. 2a). However, the glucose concentration of de-clawed crabs significantly exceeded handled only crabs when a conspecific was present (Fig. 2a). Lactate was significantly higher in de-clawed crabs (F1,79 = 5.52, P < 0.02; Fig. 2b) and significantly higher when a conspecific was present (F1,79 = 5.88, P < 0.02; Fig. 2b). There was a significant interaction effect (F1,79 = 3.65, P = 0.05; Fig. 2b), the presence of a conspecific having a greater lactate response in de-clawed crabs. Glycogen was significantly lower in de-clawed crabs (F1,79 = 3.85, P < 0.05; Fig. 2c). There was no conspecific effect (F1,79 = 0.55, NS; Fig. 2c), or interaction effect (F1,79 = 1.39, NS; Fig. 2c). However, de-clawed crabs had significantly less glycogen when a conspecific was present (Fig. 2c). De-clawed crabs mobilised significantly more glycogen (F1,79 = 9.4, P < 0.003; Fig. 2d), with no conspecific effect (F1,79 = 0.41, NS; Fig. 2d) or interaction effect (F1,79 = 0.79, NS; Fig. 2d). However, glycogen mobilisation in de-clawed crabs significantly exceeded handled only crabs when a conspecific was present (Fig. 2d).
Experiment 3: Stress responses of crabs to de-clawing versus induced autotomy
Compared to induced autotomy, de-clawing had a significant overall effect on the physiological responses (PF4,29 = 4.5, P < 0.006; Fig. 3a–d).
Glucose and lactate were higher in de-clawed crabs (F1,32 = 5.99, P < 0.02; F1,32 = 7.49, P < 0.01; Fig. 3a,b), while glycogen was lower (F1,32 = 4.82, P < 0.04; Fig. 3c), due to higher mobilisation (F1,32 = 5.32, P < 0.03: Fig. 3d).
Mortality was significantly higher in de-clawed crabs (5/28) compared to those induced to autotomise (0/23) (χ2 = 4.55; P < 0.05). In addition, there were significantly larger wound areas in de-clawed crabs (166.77 mm2 ± 21.9; mean ± SE) compared to those autotomised (58.3 mm2 ± 3.77; t37 = 3.07, P < 0.004). Furthermore, crabs that died after de-clawing had significantly larger wounds than those that survived (311.7 mm2 ± 71.45 and 135.26 mm2 ± 16.2; t26 = 3.76, P < 0.001).
Discussion
In the first experiment, removal of a single claw from the edible crab, C. pagurus, caused marked short-term physiological changes that are consistent with a stress response. The increase in glucose was clear after 5–10 min, while the lactate response was particularly rapid and significant within 1 min. Webster (1996) demonstrated a similar immediate hyper-lactaemia preceding hyper-glycaemia 30 min post air exposure in C. pagurus. Also, claw ablation studies on the second chelae of the freshwater prawn, Macrobrachium rosenbergii, reported increases in glucose concentration (Manush et al. 2005). Indeed, this short-term effect is typical of the hyper-glycaemic response used to monitor stress in crustaceans, with an increase in glucose mediated by CHH detectable within 5–10 min (Keller and Andrew 1973; Carefoot 1994). Lactate is a good indicator of the stress response in crustaceans as it is the major end product of anaerobic metabolism, with higher concentrations indicating an attempt by the animal to mediate the effect of a stressor (Albert and Ellington 1985). In normal un-stressed conditions, lactate concentrations should cycle with those of glucose during daily patterns of internally driven circadian rhythms (Tilden et al. 2001). Whilst glycogen in the sampled fifth pereiopod did not appear to decrease significantly by 10 min post claw removal, the ratio of glucose to glycogen increased significantly by this time, indicating mobilisation of glycogen energy reserves within 10 min. Several authors have questioned whether the energy demands of a stress response are attained by this mobilisation process (Briffa and Elwood 2004; Manush et al. 2005), but it appears that this is the case for C. pagurus, with liberated glucose leading to hyper-glycaemia. Elevated haemolymph glucose due to glycogen mobilisation after exposure to a stressor in crustaceans is remarkable when compared to the system in vertebrates, where significant amounts of glucose are not recorded in the blood due to low glucose-6-phosphatase activity (Keller and Andrew 1973).
Similar short-term responses to a range of other stressors have been reported for other crustaceans. For example, glucose concentrations are high immediately after trawling in Liocarcinus depurator and Munida rugosa, with a simultaneous rise in lactate (Bergmann et al. 2001). Significantly elevated glucose concentrations were measured two minutes post capture in the crayfish Orconectes propinquus, O. immunis and Cambarus robustus (Telford 1974) and in the lobster, Homarus americanus (Telford 1968). Handling post capture also has a marked short-term effect on methyl farnesoate concentrations in the green crab, Carcinus maenas, (Lovett et al. 2001) and lactate concentrations in the lobster, Panulirus cygnus (Paterson and Spanoghe 1997). Elevated glucose derived from the mobilisation of glycogen reserves has been reported in the river crab, Potamonautes warreni, during periods of anoxia (van Aardt 1988), and in the crayfish, Orconectes limosus, and the Atlantic sand fiddler crab, Uca pugilator, under hormonally induced hyper-glycaemia (Keller and Andrew 1973).
The second and third experiments revealed that these physiological effects were still present 24 h after claw removal. Both glucose and lactate were higher, glycogen lower and glycogen mobilisation higher in de-clawed as compared to intact handled only crabs and crabs induced to autotomise. In M. rosenbergii, a decrease in glycogen concentrations was demonstrated at 6 h post claw ablation (Manush et al. 2005), whilst lactate concentrations peaked at 18 h in the swimming crab, Necora puber, exposed to air, and remained high over 24 h (Durand and Regnault 1998). The increase in lactate concentrations recorded is indicative of a switch to energy production by anaerobiosis required, in the case of N. puber, to meet the energetic needs of the animals when emersed (Durand and Regnault 1998). The extent of lactate accumulation depends on the function, mode, duration and intensity of exposure to the stressor, whilst the rate of recovery is determined by its clearance from the haemolymph (Albert and Ellington 1985). The slow return to normal haemolymph lactate concentrations in crustaceans indicates that these species lack efficient systems for metabolising lactate (Albert and Ellington 1985).
The third experiment showed that induced autotomy of a single claw results in much lower physiological stress responses than manual claw removal. Indeed, although not statistically tested, the physiological measures after autotomy were not markedly different to handled only crabs in experiment 2 (c.f. Figs. 2, 3). Thus, the stress response is not due to the loss of a claw per se, rather it seems to be due to the nature of claw removal. This may be at least partially explained by autotomy resulting in a very small wound compared with the normal fishery method of manual claw removal. Thus the defence of claw removal on the grounds that the effects are similar to natural autotomy is refuted.
In Experiment 3, 17.8% of crabs that had a single claw forcibly removed died, however, we found no deaths of crabs induced to autotomise a claw. In the stone crab, M. mercenaria, mortality increased with severity of claw break and consequential wound exposure (Juanes and Smith 1995). Munida rugosa and Liocarcinus depurator were more likely to survive breaks at the fracture plane compared to those where the wounds extended into the body (Davis 1980). The wounds of animals that died were significantly larger than those that survived suggesting that it is the degree of wounding that is the main factor in mortality. This is important because in the edible crab fishery, where two claws are commonly removed, the degree of wounding and consequent mortality is likely to be substantially higher than in the present study. This has implications for the sustainability of the fishery because the animals do not survive to regenerate their claws (Carroll and Winn, 1989). Again, the marked difference between the effects of de-clawing and autotomy are apparent.
Similar physiological responses to those described here are recorded after exercise (Bridges and Brand 1980; Onnen and Zebe 1983; Head and Baldwin 1986; Briffa and Elwood 2004), however, the effects of claw removal cannot be explained by high levels of activity in the de-clawed groups. In experiment 1, the animals were monitored and it was noted that de-clawed crabs remained motionless. In the longer term experiments, crabs were not monitored for the entire period, however, in most cases de-clawed crabs had not moved far from their original position over the 24 h period, in marked contrast to the intact, handled only crabs. Indeed, when two intact crabs were paired the animals were observed to grasp at each other when an encounter occurred, an activity, which was not recorded if one of the crabs had been de-clawed. However, no injuries were recorded in any of the groups. De-clawed crabs were occasionally found underneath the shelter after the 24 h period but were never seen inside the tubing. Thus, we conclude that the physiological effects noted were due to claw removal and not general activity.
Conspecific effects were considered here because crabs are often de-clawed and immediately returned to the sea, where they will encounter conspecifics and other species during foraging, aggressive encounters and mate attraction. Crabs may also be stored in communal holding tanks following capture, where their claws may be nicked or removed to prevent them damaging each other during storage in abnormally crowded conditions. Assessing the effect of a conspecific showed that the presence of a similar sized intact edible crab (see Experiment 2) had a physiological stress effect, especially in de-clawed animals. Thus, when a de-clawed crab was grouped with an intact conspecific a significant physiological response was recorded that was not present when the crabs were held individually. Clearly, if the animals are returned to the sea or held in storage following claw removal there is considerable risk of encountering conspecifics which will exacerbate the stress response reported in individually held crabs.
We have demonstrated both short-term and longer-term physiological stress responses to claw removal in the edible crab, C. pagurus, which may be likened to the stress response of vertebrates in extreme situations (Bateson and Bradshaw 1997). However, this stress response is specific to manual de-clawing and not to induced claw autotomy. The stress response was greater in crabs exposed to an intact crab suggesting that perceived threat is stressful. Of particular concern was that claw removal resulted in a substantial mortality that appeared to occur when the wound size was large. Mortality was not observed with induced autotomy, presumably because this results in small wounds at the fracture plane. Thus, the typical fishery practice of removing two claws is likely to result in a much higher mortality than that observed here and thus will have marked implications for the sustainability of crab-claw fisheries.
References
Albert JL, Ellington WR (1985) Patterns of energy-metabolism in the stone crab, Menippe mercenaria, during severe hypoxia and subsequent recovery. J Exp Zool 234:175–183
ASAB guidelines (2006) Guidelines for the treatment of animals in behavioural research and teaching (ASAB). Anim Behav 71:245–253
Bateson P, Bradshaw EL (1997) Physiological effects of hunting red deer (Cervus elaphus). Proc R Soc Lond 264:1707–1717
Bergmann M, Moore PG (2001) Survival of decapod crustaceans discarded in the Nephrops fishery of the Clyde Sea area, Scotland. ICES J Mar Sci 58:163–171
Bergmann M, Taylor AC, Moore PG (2001) Physiological stress in decapod crustaceans (Munida rugosa and Liocarcinus depurator) discarded in the Clyde Nephrops fishery. J Exp Mar Biol Ecol 259:215–229
Bridges CR, Brand AR (1980) The effect of hypoxia on oxygen consumption and blood lactate levels of some marine crustacea. Comp Biochem Physiol 65:399–409
Briffa M, Elwood RW (2002) Power of shell-rapping signals influences physiological costs and subsequent decisions during hermit crab fights. Proc R Soc Lond 269:2331–2336
Briffa M, Elwood RW (2004) Use of energy reserves in fighting hermit crabs. Proc R Soc Lond 271:373–379
Carefoot TH (1994) Effects of environmental stressors on blood-glucose levels in sea hares, Aplysia-dactylomela. Mar Biol 118:579–583
Carroll JC, Winn RN (1989) Species Profiles: Life histories and environmental requirements of coastal fishes and invertebrates (Pacific Southwest): brown rock crab, red rock crab, and yellow crab. U.S. Fish and Wildlife Service. Biology Report 82(11.117), 16 p
Davis GE (1980) Effects of injuries on spiny lobster, Panulirus argus, and implications for fishery management. Fish B-NOAA 78:979–984
Durand F, Regnault M (1998) Nitrogen metabolism of two portunid crabs, Carcinus maenas and Necora puber, during prolonged air exposure and subsequent recovery: a comparative story. J Exp Biol 201:2515–2526
Engel PC, Jones JB (1978) Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites. Anal Biochem 88:475–484
Eno CN, MacDonald DS, Kinnear JAM, Amos SC, Chapman CJ, Clark RA, Bunker Fspd Munro C (2001) Effects of crustacean traps on benthic fauna. ICES J Mar Sci 58:11–20
Fahy E, Hickey J, Perella N, Hervas A, Carroll J, Andray C (2004) Bionomics of brown crab Cancer pagurus in the south east Ireland inshore fishery. Irish Fisheries Investigations No 12, 36pp
Fanjul-Moles ML, Bosques-Tistler T, Prieto-Sagredo J, Castanon-Cervantes O, Fernandez Rivera-Rio L (1998) Effect of variation in photoperiod and light intensity on oxygen consumption, lactate concentration and behavior in crayfish Procambarus clarkii and Procambarus digueti. Comp Biochem Physiol A 119:263–269
Head G, Baldwin J (1986) Energy metabolism and the fate of lactate during recovery from exercise in the Australian fresh water crayfish Cherax destructor. Aust J Mar Fresh Res 37:641–646
Gutmann I, Wahlefeld AW (1974) L-(+)-lactate determination with lactate Dehydrogenase and NAD+. In: Bergemeyer HU (ed) Methods of enzymatic analysis. 2nd edn. Academic, New York, pp 1464–1468
Juanes F, Smith LD (1995) The ecological consequences of limb damage and loss in decapod crustaceans: a review and prospectus. J Exp Mar Biol Ecol 193:197–223
Keller R, Andrew EM (1973) The site of action of the crustacean hyperglycemic hormone. Gen Comp Endocrinol 20:572–578
Keller R, Haylett B, Cooke I (1994) Neurosecretion of crustacean hyperglycemic hormone evoked by axonal stimulation or elevation of saline K+ concentration quantified by a sensitive immunoassay method. J Exp Biol 188:293–316
Keppler D, Decker K (1974) Glycogen determination with amyloglucosidase. In: Bergmeyer HU (ed) Methods in enzymatic analysis, 2nd edn. Academic, New York, pp 1129–1311
Lovett DL, Verzi MP, Clifford PD, Borst DW (2001) Haemolymph levels of methyl farnesoate increase in response to osmotic stress in the green crab, Carcinus maenas. Comp Biochem Physiol 128:299–306
Manush SM, Pal AK, Das T, Mukherjee SC (2005) Dietary high protein and vitamin C mitigate stress due to chelate claw ablation in Macrobrachium rosenbergii males. Comp Biochem Physiol 142:10–18
Muller, RG, Bert TM (2001) Update on Florida’s stone crab fishery. Report to Florida Fish and Wildlife Conservation Commission. Florida Marine Research Institute. St. Petersburg, Florida
Oliveira RF, Machado JL, Jonhão JM, Burford FL, Latruffe C, McGregor PK (2000) Human exploitation of male fiddler crab claws: behavioural consequences and implications for conservation. Anim Conserv 3:1–5
Onnen T, Zebe E (1983) Energy metabolism in the tail muscles of the shrimp Crangon crangon during work and subsequent recovery. Comp Biochem Physiol 74:833–838
Paterson BD, Spanoghe PT (1997) Stress indicators in marine decapod crustaceans, with particular reference to the grading of western rock lobsters (Panulirus cygnus) during commercial handling. Mar Freshwater Res 48:829–834
Sherwin CM (2001) Can invertebrates suffer? Or, how robust is argument-by-analogy? Anim Welfare 10:S103–S118
Sneddon LU (2003) The evidence for pain in fish: the use of morphine as an analgesic. Appl Anim Behav Sci 83:153–162
Stentiford GD, Chang ES, Chang SA, Neil DM (2001) Carbohydrate dynamics and the crustacean hyperglycemic hormone (CHH): effects of parasitic infection in Norway lobsters (Nephrops norvegicus). Gen Comp Endocrinol 121:13–22
Telford M (1968) The effects of stress on blood sugar composition of the lobster, Homarus americanus. Can J Zool 46:819–826
Telford M (1974) Blood glucose in crayfish-II. Variations induced by artificial stress. Comp Biochem Physiol 48:555–560
Tilden A, McGann L, Schwartz J, Bowe A, Salazar C (2001) Effect of melatonin on haemolymph glucose and lactate levels in the fiddler crab, Uca pugilator. J Exp Zool 290:379–383
Toullec JY, Vinh J, Le Caer JP, Shillito B, Soyez D (2002) Structure and phylogeny of the crustacean hyperglycemic hormone and its precursor from a hydrothermal vent crustacean: the crab Bythograea thermydron. Peptides 23:31–42
van Aardt WJ (1988) Lactate metabolism and glucose patterns in the river crab, Potamonautes warreni Calman, during anoxia and subsequent recovery. Comp Biochem Physiol 91:299–304
Verri T, Mandal A, Zilli L, Bossa D, Mandal PK, Ingrosso L, Zonno V, Vilella S, Ahearn GA, Storelli C (2001) D-Glucose transport in decapod crustacean hepatopancreas. Comp Biochem Physiol 130:585–606
Watling L, Norse EA (1998) Effects of mobile fishing gear on marine benthos introduction. Conserv Biol 12:1178–1179
Webster SG (1996) Measurement of crustacean hyperglycaemic hormone levels in the edible crab Cancer pagurus during emersion stress. J Exp Biol 199:1579–1585
Acknowledgments
This study was financed by a Department of Agriculture and Rural Development (DARD) PhD studentship to LP. The authors would like to extend sincere thanks to Grant Stentiford and Mark Briffa for their advice with the physiological assays and Philip Johnston for his technical assistance whilst field working.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. Atkinson.
Rights and permissions
About this article
Cite this article
Patterson, L., Dick, J.T.A. & Elwood, R.W. Physiological stress responses in the edible crab, Cancer pagurus, to the fishery practice of de-clawing. Mar Biol 152, 265–272 (2007). https://doi.org/10.1007/s00227-007-0681-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0681-5