Abstract
The microphytoplankton assemblages were studied from water samples collected at eight discrete depths in the top 120 m at five central (open ocean) and four western (shelf/slope region) locations in the Bay of Bengal. The Bay is a low-productive warm pool regime with poor inorganic nutrient inputs to its intensely stratified surface layer despite the very large riverine influx. In addition, the prolonged cloud cover has an adverse effect on the top 25–40 m, on primary production, chlorophyll concentration and phytoplankton assemblages. Microphytoplankton were the most abundant in the northern area of the Bay. A total of 153 phytoplankton species were identified during this study. The most abundant species (at least 1,800 individual cells belonging to a given species or ≥2% of the total counts of identified specimens) during this study were: Thalassiothrix longissima, Thalassiothrix fauenfeldii, Rhizosolenia styliformis, Nitzschia angularis, Thalassionema nitzschioides, Coscinodiscus radiatus, Chaetoceros eibenii, Skeletonema costatum, Coscinodiscus concinnus and Chaetoceros coarctatus. Similarly, there were 20 moderately abundant (≥0.5 but <2%) species. Thirty-three of the least abundant species (<0.5%) occurred at one station. Diatom species, T. fauenfeldii and T. longissima were ubiquitous in the study area. The exclusive occurrence of S. costatum in the northernmost stations suggests that it proliferates only in the low-salinity regions with adequate silica from the land inputs. Abundance of pennate diatoms was higher in the open Bay compared to that of centric diatoms in the more productive northern locations and the western Bay. There appears to be a basic difference between near shore flora and offshore flora. This first analysis of phytoplankton assemblages from the offshore Bay suggests that while there is predominance of only a few species, the Bay harbors very diverse diatom communities that seem to be syntrophic, non-competitive and co-habiting in the generally low nutrient, stratified surface waters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
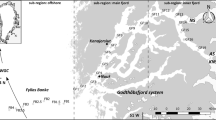
The Bay of Bengal forming the eastern arm of the northern Indian Ocean is a relatively small basin, of 2.2 × 106 km2 area (Shetye et al. 1991; Fig. 1). It has several distinguishing features that make it different from the Arabian Sea. The surface salinity is quite low (22–33 psu) compared to that in the Arabian Sea (31–35 psu). Unlike the Arabian Sea, the southwest monsoon during June–September and northeast monsoon during November–February bring about large-scale precipitation far surpassing evaporation (Shetye 2000). Surface winds are generally weak (0–10 m s−1) and highly variable, with warmer (30°C) and low saline (<34.0 psu) surface waters leading to strong stratification in the upper 50 m (Prasanna Kumar et al. 2002). Thus, the Bay experiences contrasting physical conditions with changing seasons and is a particularly interesting oceanic area to study.
Map showing the locations sampled during the 2001 summer monsoon for analyzing phytoplankton cell counts, chl a and primary productivity in the Central (along 88°E) and Western Bay of Bengal. The arrows indicate the observed wind vectors at the sampling locations. Figure modified from Madhupratap et al. (2003)
Although a large area of the Western Indian Ocean was studied during International Indian Ocean Expedition (IIOE) in 1964, when 237 phytoplankton taxa were recorded (Thorrington-Smith 1971), the attention on the phytoplankton composition in the Bay of Bengal was the least. Earlier studies from the West Coast and off Madras in the East Coast have recorded 134 species of phytoplankton with some new records of diatom species (Venkataraman 1939; Menon 1945; Subramanyan 1946; Subba Rao 1976; Devassy and Goes 1988; Tarran et al. 1999). Subba Rao (1973) reported the composition of phytoplankton for the years 1957, 1958, 1960 and 1962 from Lawson’s Bay off Vizag in the East Coast. The phytoplankton composition analyses in Andaman waters (Devassy and Bhattathiri 1981; Sarojini and Sarma 2001) and coastal waters off Orissa (Gouda and Panigrahy 1996) and off Krishna and Godavari rivers (Umamaheshwararao and Sarojini 1992) revealed a variety of phytoplankton species. It is discernible from the foregoing that most studies in the Bay have been carried out in the coastal areas. There thus exists a gap in information on open ocean phytoplankton composition.
Analyses of phytoplankton composition are helpful in determining the fertility of the seas (Qasim and Kureishy 1986). Because phytoplankton are at the base of the food chain, compositional analyses are important to relate with types and biomass of micro and mesozooplankton. Along with the recent information on physico-chemical processes and biological productivity characteristics from the Bay of Bengal (Ittekkot et al. 2003; Prasanna Kumar et al. 2002, 2004; Madhupratap et al. 2003), analyses of phytoplankton composition and their regional differences will help in realizing the influences that the above processes bear on autotrophic community structure. The objectives of this study were: (1) to characterize the microphytoplankton distribution and composition and to contrast differences between Central Bay (CB) and Western Bay (WB), and (2), to delineate influences, if any, of the physical and chemical parameters on distribution of microphytoplankton.
Materials and methods
Samples were collected for this study onboard ORV Sagar Kanya during Southwest monsoon (SM, from July 6 to August 2), 2001. The sampling was along two transects, one in the Central Bay (CB, along 88°E), and another along the Western Bay (WB, 81°–85°E, Fig. 1). In general, the stations south of 17°N in the CB represent a low productive region. The WB stations on the shelf represent, in general, moderately productive waters throughout the year. At all nine stations (Fig. 1), data on salinity, temperature and nutrients were collected. Further, water samples were collected from eight discrete depths (near surface, 10, 20 m and thereafter at 20 m intervals till 120 m) using a rosette sampler (Sea Bird Electronics) with CTD attached. These samples were used for estimating chlorophyll a (chl a), primary production (PP) from dawn to dusk in situ incubation, and phytoplankton cell counts (PPCC). Data on distribution of chl a and PP from this sampling are reported earlier by Madhupratap et al. (2003) during the same study.
For quantitative and qualitative analyses of phytoplankton cell counts and composition, water samples from each of the above depths were fixed with both Lugol’s solution (1% w/v) and 3% formaldehyde and stored in the dark until analyzed. A settling and siphoning procedure was followed to concentrate samples from 250 to 10 ml. For counting nanophytoplankton (>5 to ∼20 μm) and microphytoplankton cells (>20 μm) and identification of genera and species, two 1-ml replicates of concentrated samples were transferred to a Sedgwick-rafter plankton counting chamber and examined microscopically at 200–400× magnification. All 1,000 squares on the chamber were screened. Empty frustules were not included in the total counts. Oil immersion 100× objective on a Zeiss (Axioskop, 2plus, Germany) microscope was used to identify the genera or species. Since both abundance and types (see Results) of nanophytoplankton were sparse, the main focus in this paper is on microphytoplankton whose generic and species identification were done according to various keys (Subramanyan 1946, 1968; Subramanyan and Sarma 1961; Lebour 1978; Constance et al. 1985a, b; Desikachary and Ranjithadevi 1986; Desikachary and Prema 1987; Desikachary et al. 1987; Tomas 1997).
For comparing the diversity of the phytoplankton in the Bay, the species diversity was calculated by the Shannon Weaver formula:
where, S is total number of species and Pi is the proportion of the numbers of individuals of species i to the total number of individuals Pi = ni/N (Omori and Ikeda 1984).
Species richness (SR), the number of species recorded from a region, was calculated by Margalef’s formula (Margalef 1951):
where, S = total number of species and, N = total individuals present in the sample.
For the convenience of grouping the assemblages, the species were categorized as the most (when ≥2% of total), moderate (>0.5–1.99%) and least (≤0.49%) abundant. For this, individual species with ≥1,800 cells in the total phytoplankton counted, were categorized as the most abundant; any species in the range of ≥450 to ≤1,800 cells as moderately abundant and those <450 cells as least abundant.
Results
Hydrography
Hydrographic parameters such as salinity, temperature and density; chemical: nutrients, and DO, were also analyzed during this study. These results are published elsewhere (Prasanna Kumar et al. 2002; Madhupratap et al. 2003). In brief, surface salinity along the CB (88°E transect) showed a steady decrease from ∼34.0 psu at 7°N to 32.0 psu at 16°N. From 16°N to 17°N, it decreased by 3 psu and further north, at 20°N it was around 28. Along the WB, the average salinity was 33.7 psu up to 16°N in the surface layer but decreased northwards reaching as low as 29.6 at 19°N. The salinity gradient in the upper 50 m was ca. 1 at 7°N and 7 psu at 20°N.
The temperature along the WB ranged from 28–29°C in the south and from 27–28°C in the north. A similar range was also found in the CB except at 9°N where an upward shoaling of a cold core eddy was observed (Prasanna Kumar et al. 2004). The euphotic depths ranged from 20 m in the north to about 45 m at the southernmost location.
During the southwest monsoon, the eastward-flowing strong summer monsoon current (Cutler and Swallow 1984) brings high saline Arabian Sea waters (ASHSW) into the southern Bay of Bengal (Prasanna Kumar et al. 2004). Along the western margin, up to north of 10°N, the East India Coastal Current is mostly northward (Shetye et al. 1991). In the northern Bay, however, the low salinity plume moves toward the equator hugging the coast against local winds (Shetye et al. 1991). Thus, most part of the study area experiences 29–33 psu that increased equator-ward. The surface currents are northwesterly from the Pacific through the Malacca Straight in to the Bay during this time of the year.
During the 2001 southwest monsoon, the Bay of Bengal was devoid of nitrate in the upper 30 m, while shoaling of 1 μM NO3–N isopleth to 20 m was observed at 19°N. A nitracline was observed between 50 and 100 m. Along the WB, shoaling of nitracline (1 μM isopleths) occurred between 16°N and 18°N. Silicate distribution was similar to nitrate except for higher surface concentrations (>2 μM) in the north. A nitracline occurred between 40 and 60 m. A 2-μM-isopleth of silicate was observed in the surface waters at the southern stations, but decreased to ca 1 μM toward the north in the CB. There were pockets of 2-μM-isopleths at 17°N and 15°N along the WB. Phosphate was undetectable in the upper 30 m at all stations. Shoaling of isotherms and nitracline north of 12°N in the CB and at 19°N along the WB was due to the presence of cold core eddies at these locations.
Assemblages
A total of 153 phytoplankton taxa were identified from the samples collected during this study (Table 1). Diatoms were the major group contributing to over 97.5%, while dinoflagellates contributed 1.69% followed by silicoflagellates 0.33%. The rest of the phytoplankton, i.e., 0.48% were unidentifiable. The number of species of centric diatoms was greater than that of pennate diatoms. However, the abundance of pennate diatoms was more than that of centric diatoms.
Among diatoms in the CB, Thalassiothrix longissima (20%), Thalassiothrix fauenfeldii (16%), Nitzschia angularis (8%), Chaetoceros coarctatus, Skeletonema costatum and Thalassionema nitzschioides (>4% each) were the most dominant. Nitzschia delicatissima, Coscinodiscus concinnus, Rhizosolenia styliformis were ≥2 but ≤3%. The most abundant species in the WB were, T. fauenfeldii and T. longissima forming 23% each. S. costatum, C. coarctatus and T. nitzschioides, Coscinodiscus radiatus, were >4% in numbers. N. delicatissima and N. angularis contributed 3 and 2%, respectively. Some organisms could be identified as diatoms but could not be identified to species level and are listed as unidentified diatoms (Table 1).
Only a few taxa of dinoflagellates were observed. Among them, Ceratium furca (0.6%) and Peridinium sp. (0.5%) were the main ones. Dictyocha crux and D. speculum were the only two silicoflagellate species observed.
The nanophytoplakton were a minor fraction in the Bay. They were represented by the least abundant Leptocylindrus minimus (0.08% in CB and 0.01% in WB), Chaetoceros danicus (0.32%; found only in CB) and Thalassiosira anguste-lineata (0.02%; only in CB). Further, the contribution of picoplankton [that passes through 10 μm mesh (Hydro-Bios)] to total chlorophyll measured fluorometrically was negligible or at best <0.5% of total chl a. The 10 μm passing autofluorescing cells ranged from 2.40 to 16.70 × 104 l−1 during this study.
A cluster analysis (Ward 1963) was performed to see whether a holistic picture was obtainable on phytoplankton variability between the stations. When all the species from all nine stations were subjected to this analysis, the clustering resolution was very poor (for example, most abundant species clustered together or, the least abundant skewed together) and difficult to interpret. Therefore, based on their abundance, the species were categorized as most, moderate and least abundant as defined in methods and then subjected independently to cluster analysis. This analysis was useful for discerning relatedness among these three categories somewhat more clearly than when all species were taken together for cluster analyses. Results of cluster analysis are not shown here.
Only ten species categorized as most abundant were found in the CB (see Fig. 2). The moderately abundant species, numbering 20, were found throughout the transect except at the southernmost station 9°N 88°E. Among these, five species viz., Thalassiosira condensate, Biddulphia sinensis, B. mobiliensis, N. delicatissima, and Chaetoceros lorenzianus were found only north of 17°N. Coscinodiscus rothii, C. gigas, C. curvatulus and Plagiotropis lepidoptera were present only at the southernmost station. The least abundant species, 85 in all, were distributed throughout the CB from 9°N to 20°N mostly in the top 60 m. Interestingly, S. costatum in particular was found exclusively at the northernmost station (20°N 88°E). Further, C. concinnus, T. fauenfeldii and C. coarctatus were found north of 17°N 88°E only and were totally absent in the southern stations along the CB. Thus, it is likely that they also prefer the low saline waters.
As for the WB, four of the eight most abundant species viz., C. radiatus, T. fauenfeldii, T. longissima, and Thalassionema nitzschioides were present at all the stations (Fig. 3). The other four species, C. coarctatus, N. delicatissima, N. angularis and S. costatum were mostly in the two northern locations indicating their preferences to regions with <32 psu. Interestingly, S. costatum was the only species that was exclusively found in the northernmost stations of both CB and WB apparently preferring low saline waters. From the cluster analysis it was discernible that all 15 of the moderately abundant species (see serial numbers 21, 23, 26, 36, 51, 52, 70, 71, 88, 99, 110, 125, 127, 135 and 150 in Table 1) showed a restricted pattern of distribution.
The least abundant species constituted the bulk of the phytoplankton composition along both the CB and WB suggesting that they are responsible for high diversity in the Bay.
The lowest numbers of species were observed at 15°N 81°E, nearly half of the153 species observed during this season in the Bay occurred at 17°N 88°E (Table 2). As expected, the maximum and minimum H′ and SR corroborated with high and low numbers of species along both the transects. Overall, the diversity was higher in the CB (H′: 4.93; SR: 10.43) compared to the WB (H′: 4.18; SR: 7.29). It was also inferable that the northernmost stations, receiving large influx of river water, had greater H′ and SR than the southern stations along both transects (Table 2).
Phytoplankton abundance
In the CB, the highest cell counts (13.8 × 103cells l−1 or 13.8 × 106 cells m−3) were observed at 20°N 88°E. T. fauenfeldii (with 10.5 × 103 cells l−1 or 35.7% of the total counts) was the dominant species at this station. This was followed by T. longissima and S. costatum with 5.5 × 103 and 4.1 × 103 cells l−1 (with their maxima between 20 and 40 m). In general, phytoplankton counts decreased with depth (Fig. 4). The subsurface chlorophyll maximum at 9°N, the southern most station, was around 40 m and shoaled northerly to 10 m at 20°N. The subsurface chlorophyll maximum did not generally coincide (Fig. 4) with the phytoplankton abundance except at 20°N, the northernmost station.
In the WB (Fig. 4), a subsurface maximum (23.2 × 103 cells l−1) was observed at 20 m at 19°N 85°E. T. fauenfeldii (17.8 × 103 cells l−1 or 42% of total counts) and S. costatum (4.1 × 103 cells l−1 or 12% of total) were the major species at this depth. Their maximal abundances were between 20 and 40 m, usually coinciding with subsurface chlorophyll maxima.
Thalassiothrix longissima and N. angularis were dominant at the southernmost station (9°N 88°E) with 4.4 × 103 and 3.5 × 103 cells l−1 respectively, with a maximum density observed at the surface for both the species in the CB. T. longissima (13.4 × 103 l−1), C. radiatus (3.0 × 103 l−1) and T. fauenfeldii (1.6 × 103 cells l−1) were the dominant species in the southern WB. Their maximum numbers were between 40 and 60 m coinciding with the subsurface chlorophyll maxima.
Integrated abundance of phytoplankton cell counts for the upper 120 m for different stations decreased from 20°N (37 × 107 cells m−2) to 15°N (8.1 × 107 cells m−2) and thereafter, increased toward 9°N (12.6 × 107 cells m−2) in the CB (Fig. 5). Along the WB, abundance decreased southwards till 15°N 81°E and, thereafter, increased at 12°N 81°E. The integrated counts ranged from 5.3 × 107 to 82.1 × 107 cells m−2 in the WB.
Discussion
The highest phytoplankton abundance was observed at the northernmost stations viz. 20°N 88°E in the CB and 19°N 85°E along the WB. This could be attributed to the high concentrations of silica (ca. 2 and 4 μM, respectively) input from the rivers (Madhupratap et al. 2003). Apart from higher silica concentrations, the 1-μM-isopleth of NO3 was also observed to shoal to less than 30 m along the WB between 16°N and 18°N (Madhupratap et al. 2003), resulting in higher phytoplankton abundance in the WB compared to the CB. Since the early days of phytoplankton ecology, nutrients have been known for controlling the phytoplankton community structure and biomass (Raymont 1980; Tilman 1982; Gouda and Panigrahy 1996), which is also evident in the Bay during this study. That the nutrients have the greatest influence on phytoplankton abundance is clear from the strong negative correlation between phytoplankton abundance and nitrate (r = −0.73; P < 0.005), silicate (r = −0.62; P < 0.005) and phosphate (r = −0.57; P < 0.005). When the phytoplankton become abundant, nutrients in the water column are low or undetectable. In the present study, as also observed from the coastal Bay by Gouda and Panigrahy (1996) and Panigrahi et al. (2004), the diatoms (Bacillariophyceae) predominated owing mostly to the availability of adequate concentrations of silicate (Madhupratap et al. 2003).
Kricher (1972), De Jong (1975) and Ilangovan (1987) reported that an increase in diversity is a function of increasing species numbers, environmental heterogeneity and, incomplete mixing of waters. Also, low levels of nutrients (Tilman 1994; Huisman et al. 1999) are known to promote species diversity (Raymont 1980). The higher H′ in the northern stations, 20°N and 19°N, might be explained by the intense stratification in the top layers. As Madhupratap et al. (2003) and Prasanna Kumar et al. (2004) report, this stratification leads to incomplete mixing and formation of low saline surface-lenses over the high saline waters. The greatest diversity noticed at 17°N 88°E might be due to higher concentrations of nutrients, in particular below 60 m (NO3: 0–27 μM; PO4: 0–1.88 μM; SiO4: 0–21 μM).
The phytoplankton composition showed a number of temperate—tropical and tropical species (Table 1). T. fauenfeldii, Biddulphia longicuris, Chaetoceros diversus, C. messanenis, C. eibenii, Bacteriastrum comosum, are the known temperate-tropical species observed in the Bay. While some of them, such as Corethron criophilum, Coscinodiscus asteromphalus, Rhizosolenia stolterfothii, R. styliformis, R. hebetata, Bacteriastrum delicatulum, B. furcatum, B. hyalinum, Eucampia zoodiacus, Ditylum brightwellii, Biddulphia mobiliensis, T. longissima, Chaetoceros socialis, C. curvisetus, were some of the known temperate and/or polar/cosmopolitan species found during this study in the Bay of Bengal.
All the species observed during this study have been previously reported in Indian waters by Subramanyan (1946, 1968), Subramanyan and Sarma (1961), Desikachary and Ranjithadevi (1986), Desikachary and Prema (1987), Menon (1945) and Ilangovan (1987). In this respect, the phytoplankton in the Bay of Bengal is composed of a wide mix of species belonging to various biogeographical realms. This could be due to its being open to the Equatorial Indian Ocean, the Arabian Sea, also to the Pacific Ocean via the Malacca Strait. Approximately 50,000 ships traverse this Strait annually. The Bay of Bengal with many ports receiving ships from the world over can add to microphytoplankton communities (through transport on their hulls and in ballast water) in the generally oligohaline regimes of the Bay north of 10°N. It is well recognized that each species is a variable and, on the basis of its relationship with other species, the patterns of structure emerge (Sokal and Rohlf 1981) that implicitly describes the assembling or grouping at related species.
Higher silicate concentrations in the north of 18°N appear to be important in sustaining higher abundance of phytoplankton in the Bay. Further, the ubiquity and sheer abundance of T. fauenfeldii and T. longissima in the Bay seem to be important in contributing to dimethyl sulphide (DMS) concentrations in the Bay. Although diatoms are considered to be insignificant in DMS production (Liss et al. 1993), there was a strong relationship between T. fauenfeldii and the DMS concentrations in the Bay during the sampling period (Shenoy et al. 2006). Contribution of diatoms to DMS production and fluxes may be lower compared to coccolithophores, prymnesiophyceans (Phaeocystis spp.) and dinoflagellates.
Presence of high numbers of T. longissima and N. angularis appears to contribute in a large way to the biomass along both transects as well as to the material fluxes. Ramaswamy and Nair (1994) reported the highest particle flux and organic carbon to carbonate carbon ratios in the deep traps during the summer monsoon. Although there can be inter-annual differences in the dominant flora, it is possible to suggest that the large sizes, chain-forming characteristics and predominance of microphytoplankton such as T. longissima, C. radiatus and T. fauenfeldii, among others, are important for material fluxes to the deep. The percentage contribution of Dinophyta to the total microphytoplankton counts was not very high. We think that their abundance is low in the Bay of Bengal. The 25-fold concentration of the water samples and careful screening of the entire 1,000 cells in the Sedgewick-rafter are adequate for measuring the abundance of microphytoplankton in general. However, further confirmation of low abundance of Dinophyta will be useful.
The abundant pennate diatoms viz., T. longissima, T. fauenfeldii and Nitzschia angularis usually with high surface-to-volume ratio might be absorbing nutrients rapidly. But, the highly diverse centric diatoms viz., Skeletonema, Coscinodiscus, Chaetoceros, with low surface to volume ratio might begin multiplying after the optimal nutrient concentrations are attained. Mixture of these communities, therefore, may be well suited to inhabit the low nutrient, generally stratified upper water column in the Bay. Although this is the first detailed phytoplankton compositional analysis from the offshore Bay, it is possible to suggest that the hydrographic conditions during the monsoon months of June–September permit non-competitive-coexistence of diverse microphytoplankton in this nutrient-limited regime. It is probable that the abilities of different species of phytoplankton to utilize nutrients and other essential inorganic compounds effectively at low concentrations account for the high diversity in the Bay. It is also well known that oligotrophic waters are more diverse compared to mesotrophic and/or eutrophic waters (Raymont 1980). From the abundance of many species at certain depths, they appeared to be stratified by depth. However, it is difficult to recognize any one depth as their stratum in different locations or regions.
Contrastingly, all four of the most dominant species (i.e., S. costatum, C. radiatus, T. longissima, T. fauenfeldii) during the monsoon 2001 were very low in abundance during the post-monsoon months of September–October 2002 (unpublished data). For example, the total number of species along the cruise tracks of this study area during the post monsoon months of September–October 2002 were 129 and those during the pre monsoon months of April–May 2003 were 69. During the post monsoon, species such as T. nitzschioides, Navicula sp., R. styliformis, Synedra hennedyna, Rhizosolenia shrubsolei, Rhizosolenia sp., Chaetoceros didymus, Pseudonitzschia sp., T. fauenfeldii, Thalassiosira sp. were the most abundant ((≥2%)in the CB. Chaetoceros lorenzianus, C. curvisetus, Navicula sp., Thalassiosira sp., T. gravida, Chaetoceros eibenii, Ditylum brightwelli, Chaetoceros didymus, Pseudonitzschia sp., Nitzschia longissima, C. coarctatus, C. paradoxus were the most abundant in the WB. During the pre monsoon, Navicula sp., Coscinodiscus sp., Pseudonitzschia sp., Leptocylindrus meditterraneans, T. nitzschioides, Navicula distans, Rhizosolenia cylindrus, Fragilariopsis doliolus and Trichodesmium sp., were the most abundant ones in the CB. Chaetoceros didymus, Navicula sp., Bacteriastrum comosum, B. furcatum, B. hyalinum, Chaetoceros curvisetus, Rhizosolenia striata, R. cylindrus, Navicula messanensis, Rhizosolenia flaccida, Stephanopyxis palmeriana, Chaetoceros sp., Nitzschia sp. were the most abundant in the WB.
Few detailed analyses of microphytoplankton communities from the offshore regions in the tropics are available. For this reason it is not possible to compare the results of this study with existing literature. From the Arabian Sea, Sawant and Madhupratap (1996) reported that the most abundant diatoms during the summer monsoon were, T. longissima (22%), Chaetoceros sp. (5%), Rhizosolenia sp. (5%), Rhizosolenia stolterfothii (4%), Nitzschia serriata (4%) and Thalassiosira sp. (2%). Interestingly, the same species, T. longissima contributed up to 20 and 23% in the CB and WB respectively in the Bay of Bengal. Apparently, oligohaline waters (<30 psu) and warm temperatures around 28–30°C at this location are ideal for S. costatum. Low salinity near-coastal tropical regions are reported to aid the proliferation of this species (Mitbavkar and Anil 2000; Babu et al. 2001). Apart from a few commonalities, the phytoplankton composition in these hydrographically differing areas is quite different. From these observations it is suggested that the hydrological regimes of the Bay strongly influence the microphytoplankton communities. In general, the abundance of centric diatoms was more abundant in the CB compared to the abundance of pennate diatoms in the more productive northern locations and the WB. Thus, there appears to be a basic difference between near shore flora and offshore flora.
References
Babu MM, Kitto MR, Marian PM (2001) Growth performance, salinity tolerance and nutritive values of marine microalga, Skeletonema costatum in the brackish waters of Buckingham canal, Nellore, India. J Aquac Tropic 16:113–120
Constance AS, Carroll SE, Hillman NS, Janal MJ, Vancouvering JA (1985a) Catalogue of diatoms. In: The Ellis and Messina Catalogues of Micropaleontology Book 1. Micropaleontology Press, New York
Constance AS, Carroll SE, Hillman NS, Janal MJ, Vancouvering JA (1985b) Catalogue of diatoms. In: The Ellis and Messina Catalogues of Micropaleontology Book 2. Micropaleontology Press, New York
Cutler AN, Swallow SC (1984) Surface currents of the Indian Ocean (to 25°S, 100°E) compiled from historical data archived by the UK Meteorological Office, Rep. 187, 36 pp., Inst. of Oceanogr. Sci., Godalming, UK
De Jong TM (1975) A comparison of three diversity indices based on their components of richness and eveness. Oikos 26:222–227
Desikachary TV, Prema P (1987) Diatoms from the Bay of Bengal. In: Atlas of diatoms. Madras Science Foundation, Madras
Desikachary TV, Ranjithadevi KA (1986) Marine fossil diatom from India and Indian Ocean Region. In: Atlas of diatoms. Madras Science Foundation, Madras
Desikachary TV, Gowthaman S, Latha Y (1987) Diatom flora of some sediments from the Indian Ocean Region. In: Atlas of diatoms. Madras Science Foundation, Madras
Devassy VP, Bhattathiri PMA (1981) Distribution of phytoplankton and chlorophyll a around little Andaman Islands. Indian J Mar Sci 10:243–247
Devassy VP, Goes JI (1988) Phytoplankton community structure and succession in a tropical estuarine complex (Central West coast of India). Estaur Coast Shelf Sci 27:671–685
Gouda R, Panigrahy RC (1996) Ecology of phytoplankton in coastal waters of Gopalpur, East coast of India. Indian J Mar Sci 25:81–84
Huisman J, Oostveen PV, Weissing FJ (1999) Species dynamics in phytoplankton blooms: incomplete mixing and competition for light. Am Nat 154:46–68
Ilangovan G (1987) A comparative study on species diversity, distribution and ecology of the Dinophyceae from Vellar estuary and nearby Bay of Bengal. J Mar Biol Assoc India 29:280–285
Ittekkot V, Kudrass HR, Quadfasel D, Unger D (2003) The Bay of Bengal—an introduction. Deep Sea Res II 50:853–854
Kircher JC (1972) Bird species diversity: the effect of species richness and equitability on the diverse index. Ecol 53:278–282
Lebour M (1978) The plankton diatoms of northern Seas. Otto Koeltz Science Publishers, D−6240, Koenigstein/W-Germany
Liss PS, Malin G, Turner SM (1993) Production of DMS by marine phytoplankton. In: Restelli G, Angeletti G (eds) Dimethyl sulphide: oceans atmosphere and climate. Kluwer, Dordrecht, pp 1–14
Madhupratap M, Gauns M, Ramaiah N, Prasanna Kumar S, Muraleedharan PM, De Sousa SN, Sardessai S, Muraleedharan U (2003) Biogeochemistry of the Bay of Bengal: physical, chemical and primary productivity characteristics of the central and western Bay of Bengal during summer monsoon 2001. Deep Sea Res II 50:881–896
Margalef R. (1951) Diversidad de especies en las communidades naturals, Publicaciones Insitute Biologia Aplicada 9:5–27
Menon MAS (1945) Observations on the seasonal distribution of the plankton off Trivandrum coast. Proc Indian Acad Sci 22:31–62
Mitbavkar S, Anil AC (2000) Diatom colonization on stainless steel panels in estuarine waters of Goa, West Coast of India. Indian J Mar Sci 29:273–276
Omori M, Ikeda T (1984) Distribution and community structure. In: Methods in marine zooplankton ecology. Wiley-Interscience Publication, pp 253–279
Panigrahi SN, Acharya BC, Das SN (2004) Distribution of diatoms and dinoflagellates in tropical waters of Orissa and West Bengal with emphasis on neretic assemblages. In: Abidi SAH, Ravindran M, Venkatesan R, Vijayakumaran M (eds) Proceedings of the National Seminar on New Frontiers in Marine Bioscience Research, January 22–23, 2004. National Seminar on New Frontiers in Marine Bioscience Research; National Institute of Ocean Technology, Chennai (India); 22–23 Jan 2004). Allied; New Delhi (India), pp 535–543
Prasanna Kumar S, Muraleedharan PM, Prasad TG, Gauns M, Ramaiah N, Desouza SN, Sardessai S, Madhupratap M (2002) Why is the Bay of Bengal less productive during SM as compared to the Arabian Sea? Geophys Res Lett 29:2435–2438
Prasanna Kumar S, Murukesh N, Narvekar J, Kumar A, Sardessai S, De Souza SN, Gauns M, Ramaiah N, Madhupratap M (2004) Are eddies nature’s trigger to enhance biological productivity in the Bay of Bengal? Geophys Res Lett 31:L07309. doi:10.1029/2003 GL019274
Qasim SZ, Kureishy TW (1986) Biological productivity in the seas around India: present status and major threats. Proc Indian Acad Sci 64B:1–17
Ramaswamy V, Nair RR (1994) Fluxes of material in the Arabian Sea and Bay of Bengal-Sediment trap studies In: Lal D (ed) Biogeochemistry of the Arabian Sea, pp 91–112. Indian Academy of Sciences Special Publication
Raymont JEG (1980) Plankton and productivity in the oceans, 2nd edn, vol I. Phytoplankton. Pergamon Press Ltd. London, p 489
Sarojini Y, Sarma NS (2001) Phytoplankton distribution in the sea around Andaman and Nicobar Islands at the onset of North East Monsoon. Sea Weed Res Util 23:133–141
Sawant S, Madhupratap M (1996) Seasonality and composition of phytoplankton in the Arabian Sea. Curr Sci 47:869–873
Shenoy DM, Paul JT, Gauns M, Ramaiah N, Dileep Kumar M (2006) The 2001 Spatial variation of DMS, DMSP and Phytoplankton in the Bay of Bengal during the summer monsoon 2001. Mar Environ Res 62:83–97
Shetye SR (2000) The Indian summer monsoon and the waters around India. Kumari L A Meera Memorial Trust, Palghat, p 21
Shetye SR, Shenoi SSC, Gouveia AD, Michael GS, Sundar D, Nampoothiri G (1991) Wind-driven coastal upwelling along the western boundary of the Bay of Bengal during the southwest monsoon. Cont Shelf Res 11:1397–1308
Sokal RR, Rohlf FJ (1981) Biometry: the principles and practice of statistics in biological research, 2nd edn. W.H. Freeman, San Francisco
Subba Rao DV (1973) Effects of Environmental perturbations on short term phytoplankton production off Lawson’s Bay, a tropical embayment. Hydrobiology 43:77–91
Subba Rao DV (1976) Temporal variations in primary production during upwelling season off Waltair, Bay of Bengal. Int J Ecol Environ Sci 2:107–114
Subramanyan R (1946) A systematic account of the Marine plankton diatoms off the Madras Coast. Proc Indian Acad Sci 24B:85–197
Subramanyan R (1968) The Dinophyceae of the Indian Sea. In: Marine Biological Association of India. Mar Biol Assoc India 3:118–133
Subramanyan R, Sarma AH (1961) Studies on the phytoplankton of the West Coast of India. Indian J Fish 7:307–336
Tarran GA, Burkill PH, Edwards ES, Woodward EMS (1999) Phytoplankton community structure in the Arabian Sea during and after the SW monsoon. 1994. Deep Sea Res II 46:655–676
Thorrington-Smith M (1971) West Indian Ocean phytoplankton: a numerical investigation of phytohydrographic regions and their characteristic phytoplankton associations. Mar Biol 9:115–137
Tilman D (1982) Phytoplankton community ecology: the role of limiting nutrients. Ann Rev Ecol Syst 13:349–372
Tilman D (1994) Competition and biodiversity in spatially structured habitats. Ecology 75:2–16
Tomas Carmelo R (1997) Identifying marine phytoplankton. Academic, United Kingdom
Umamaheswararao M, Sarojini Y (1992) Composition, abundance and vertical distribution of phytoplankton and fungi off Krishna and Godavari river mouth, east coast of India. Indian J Mar Sci 21:128–132
Venkataraman G (1939) A systematic account of some South Indian diatoms. Proc Indian Acad Sci 10B:29–67
Ward JH (1963) Hierarchical groupings to optimize an objective function. J Am Stat Assoc 58:234–244
Acknowledgments
We thank the Department of Ocean Development, New Delhi, for financial support for this research under the Bay of Bengal Process Studies programme. We thank Dr. Aditi Pant, Dr. N. Anand, University of Madras and V.T. Shridharan, Reader, University of Tiruchirapally for confirming the identification of many phytoplankton species. JTP and VF acknowledge the Council of Scientific and Industrial Research, New Delhi for an award of Senior Research Fellowship. We are grateful to Late Dr. M. Madhupratap for his leadership and many advices. The crew and all participants of SK 166 are thanked for their help. Comments of three anonymous reviewers were very helpful in improving this manuscript. This is NIO contribution No: 4235.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by A. McLachlan.
Rights and permissions
About this article
Cite this article
Paul, J.T., Ramaiah, N., Gauns, M. et al. Preponderance of a few diatom species among the highly diverse microphytoplankton assemblages in the Bay of Bengal. Mar Biol 152, 63–75 (2007). https://doi.org/10.1007/s00227-007-0657-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0657-5