Abstract
Molluscan assemblages were studied on fringing reefs (reef flats, Millepora-fringing reefs, fringing reefs with massive corals) and fore-reef hard substrata (coral patches, coral carpets and small patch reefs) in the Gulf of Aqaba at water depths ranging from the intertidal to 26 m. A total of 1,665 molluscan individuals from 51 taxa was counted on 44 transects, which covered 220 m² at eight diving sites. The most important molluscs in the assemblage were the parasitic gastropod Coralliophila neritoidea, the encrusting gastropod Dendropoma maxima and the coral-associated bivalve Pedum spondyloideum. The dead assemblage, in contrast, was dominated by encrusting bivalves (Ostreoidea, Chamoidea, Spondylidae) and the coral-predating gastropod Drupella cornus. Distinct molluscan assemblages inhabit each of the three fringing reef-habitats and most of the important depth-related community changes occurred within the uppermost 5 m. In contrast, the three deeper fore-reef habitats are characterized by a more uniform molluscan composition. Molluscan assemblages were more dependent on substrata and their coral associations than on water depth. Comparisons with other published studies indicate that reefoidal hard substrata in the northern Red Sea are largely characterized by similar species-abundance patterns. The minor differences to other Red Sea studies probably reflect the northern, isolated position of the Gulf of Aqaba, the lack of certain molluscan habitats, and the differential impact of anthropogenic influences. Strong differences between living and dead assemblages in Aqaba are similar to those observed in other regions and are due to distinct biases in the dead assemblage. Molluscs closely associated with living corals (mostly bivalves and Dendropoma) can easily be overgrown after death and are thus undetectable in visual censuses. Some gastropod taxa are preferentially transported into surrounding soft-substrata postmortem or redistributed by hermit crabs. Such complex relationships between ecology and taphonomy are crucial in evaluating the quality of the molluscan fossil record in coral reef environments. The comparison of our results with literature data documents an increase in coral predators during the last two decades in the northern Red Sea. Due to the greater mollusc biodiversity in the shallower Aqaba reef habitats, damage to this coral reef zone would have the greatest impact on the overall mollusc community.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Molluscs are the most diverse marine phylum and their diversity is particularly high in the tropical waters of the Indo-Pacific (Gosliner et al. 1996). They are also considered to be an indicator group for the rapid assessment of coral reef diversity (Wells 1998). In the Red Sea, they occupy almost every ecological niche provided by the complex structures of different reef zones and substrata (Mastaller 1987). General information is available about molluscan assemblages on various supratidal to shallow-water non-reefal hard- and soft substrata of the Red Sea (Fishelson 1971), and the molluscan composition of the rocky intertidal, shallow lagoons and reef flats are comparatively well studied (Hughes 1977; Mergner 1979; Ayal and Safriel 1981; Taylor and Reid 1984; Hulings 1986; Zuschin and Piller 1997a). Less information is available on deeper, subtidal molluscan composition (northern and central Red Sea: Mastaller 1978, 1979; Zuschin and Hohenegger 1998; Zuschin and Oliver 2003a), and only a few studies have systematically treated the molluscan distribution pattern on subtidal coral reef-associated hard substrata (Mergner and Schuhmacher 1974; Mastaller 1979; Zuschin et al. 2001). Although coral colonies provide important habitats for molluscs, most studies on coral reef-associated molluscs have focused on specific coral–mollusc interactions (e.g., Antonius and Riegl 1997; Schuhmacher 1992; Kleemann 1990, 1992; Al-Moghrabi 1996) or the autecology of specific molluscan taxa (e.g., Hughes and Lewis 1974; Kappner et al. 2000). Similarly, changes in coral composition with water depth are well known (e.g., Loya 1972; Riegl and Velimirov 1994), but only a few studies relate molluscan composition on reefoidal hard substrata to water depth categories (e.g., Zuschin and Piller 1997b).
The aim of this paper was to contribute to our knowledge of coral-associated molluscan communities in the Red Sea. Specifically, we wanted to study the dependence of molluscan assemblages on bottom types (i.e., different coral associations) and on water depth. We also investigate the pathways of empty shells—a prerequisite in estimating the fossilisation potential of molluscs (Kidwell and Flessa 1995; Martin 1999). Our approach underlines the importance of examining reefs in a more comprehensive faunistic manner and, from a management and conservation perspective, demonstrates that determining the status of a reef must go beyond the corals themselves to encompass other key invertebrate groups whose presence, abundance and distribution are intimately linked to reef health.
Materials and methods
Study area
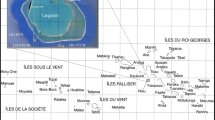
The Gulf of Aqaba (Fig. 1) at the northern end of the Red Sea is a >1,000 m deep, oligotrophic marine basin (Braithwaite 1987; Medio et al. 2000) characterized by fringing reefs (Head 1987). Annual sea surface temperature in the gulf ranges from ∼21 to 27°C; salinity is about 40‰ (Edwards 1987). Due to its morphology, oceanography and hydrodynamics, the northern end of the Red Sea and particularly the Gulf of Aqaba are sometimes considered as a small sub-region of the Red Sea (Medio et al. 2000). For the Gulf of Aqaba, isolation, high salinities and strong temperature fluctuations have zoogeographic consequences: the coral communities differ somewhat from those further south (Sheppard and Sheppard 1991) and molluscan species numbers are somewhat lower than in the rest of the Red Sea (Mastaller 1987).
Study area with sampling stations. Station GPS coordinates from Google Earth: Saudi border N 29° 21′ 53′′, E 34° 57′ 42′′; Royal Diving Club 29° 24′ 02′′, 34° 57′ 58′′, Eel Canyon 29° 25′ 01′′, 34° 58′ 24′′, New Canyon 29° 25′ 24′′, 34° 58′ 15′′, Gorgonia II 29° 25′ 32′′, 34° 58′ 15′′, Gorgonia I 29° 25′ 36′′, 34° 58′ 17′′, Rainbow reef 29° 26′ 00′′, 34° 58′ 22′′, Black rock 29° 26′ 05′′, 34° 58′ 18′′
Field work
Different subtidal hard substrata were sampled for shelled molluscs at eight diving sites in the Gulf of Aqaba (Fig. 1, Table 1) with a 0.25 m2 aluminium quadrat. Forty-four transects were laid to cover all major molluscan habitats on fringing reefs (reef flats, Millepora fringing reefs, fringing reefs with massive corals) and fore-reef hard substrata (coral patches, coral carpets and small patch reefs) in water depths ranging from intertidal to 26 m (Table 2). For each transect, a diver haphazardly dropped the frame from a few meters above the substratum. After this, the subsequent frames were positioned contiguously along a line extending from that point. Transects were laid parallel to the coastline. Five square meters of seafloor were investigated per transect (Table 1). Samples were taken during daylight (usually between 10 a.m. and 5 p.m.). The molluscs were identified either in situ (most bivalves) or were collected (many gastropods) for more reliable identification. The fauna was separated into living and empty individuals and hermit crab-occupied shells. The information on live and dead individuals was used for statistical analysis; shells occupied by hermit crabs were treated as an important descriptive feature affecting the distribution of dead gastropod shells. Fieldwork was performed in October and November 2001 and 2002.
Habitat and water depth categories
Gulf of Aqaba coral habitats can be divided into fringing reefs and fore-reef hard substrata. The former consist of reef flats with depauperate coral assemblages and thick crusts of coralline red algae, reef edges and slopes dominated by massive corals (mostly Porites) and Millepora-dominated slopes (Table 2). Fore-reef hard substrata consist of coral carpets, coral patches and small patch reefs. Coral carpets (sensu Reiss and Hottinger 1984) build a framework of (mostly faviid) corals but lack distinct zonation; laterally they grade into coral patches, which are scattered aggregates of faviid coral colonies. Two patch reefs were studied, which elevate from sandy substrata, with diameters and heights of a few meters; they consisted of platy scleractinian associations (Table 2).
Water depth categories were divided into 5 m intervals with a notable exception being the shallowest category (<1 m), which was chosen to reflect the tidal influence in the study area. Tidal amplitudes in the Red Sea are generally small, with highest spring ranges occurring in the Gulfs of Suez (1.5 m) and Aqaba (1.2 m) (Medio et al. 2000). The deepest category (>20 m) included one diving site at 26 m.
Taxonomy
The molluscs were identified to species level wherever possible. Due to questionable taxonomies, Chamoidea, Spondylidae and Ostreoidea were identified to the family and superfamily levels only. Certain other taxa were also identified above the species level due to problems with identification in the field or poor preservation (e.g., Conus spp.).
It is difficult to recognize small molluscs when SCUBA diving. Therefore, to provide a consistent database, molluscs <1 cm were excluded from the quantitative treatment. In addition, most boring bivalves (e.g., gastrochaenids, lithophagines) have been excluded from the quantitative collection because they are difficult to identify using hole characteristics. Species identification followed Sharabati (1984), Oliver (1992), Bosch et al. (1995), and Zuschin and Oliver (2003a).
Analysis of faunal composition
The data set was explored to study differences between molluscan habitats (i.e., bottom types), water depth categories and living and dead fauna using analysis of similarity (ANOSIM, Clarke and Warwick 1994) based on the Bray–Curtis similarity coefficient (Bray and Curtis 1957). The important message of the pairwise tests of the ANOSIM analysis is less the significance level (which can often be low because of few replicates in each group), but the pairwise R-values; the latter give an absolute measure of how separated the groups were, on a scale of 0 (indistinguishable) to 1 (all similarities within groups are less than any similarity between groups). With R values >0.75, groups are well separated; with R values >0.5, groups overlap but are clearly different; with R values >0.25, groups strongly overlap; and with R values <0.25, groups are barely separable (Clarke and Gorley 2001). Non-metric multidimensional scaling (MDS, Kruskal 1964), used as an ordination method to provide a visual comparison of the pattern of Bray–Curtis values among the 44 transects, was run with 30 random starting configurations. ANOSIM and MDS were performed using the software package PRIMER (Clarke and Warwick 1994). Similarities between the living assemblages in Aqaba and Safaga (northern Red Sea), where a similar study has been conducted (Zuschin et al. 2001), were also explored by rank order correlation of the datasets using the software package SPSS 10.0 (SPSS 1999). Species contributing more than 1% to the living assemblages in Aqaba or Safaga were used to explain similarities and differences between these locations. These quantitatively important species are finally also compared with records from other Red Sea studies.
Results
A total of 1,665 molluscan individuals from 51 taxa was identified from 44 transects, which covered 220 m² at eight diving sites (Table 1). The quantitatively most important living molluscs were the coral-associated parasitic gastropod Coralliophila neritoidea (Lamarck, 1816), the encrusting gastropod Dendropoma maxima (Sowerby, 1825) and the coral-associated bivalve Pedum spondyloideum (Gmelin, 1791); together these species made up 67% of the living assemblage (Fig. 2). All three fringing reef substrata differed significantly from each other and from most fore-reef substrata in terms of the composition of living molluscs, an exception being the overlap between Millepora-fringing reefs, coral carpets and coral patches. Fore-reef hard substrata could not generally be distinguished from each other based on living mollusc composition (Table 3, Fig. 3). Similarly, the two shallower depth categories (<1 m, 1–5 m) differed significantly from each other and from the four deeper depth categories (6–10 m, 11–15 m, 16–20 m, >20 m), an exception being the lack of significant differences between the assemblages in 1–5 m versus 6–10 m. The four deeper depth categories were also indistinguishable based on their composition of living molluscs (Table 3). The global ANOSIM test shows that the compositions of molluscan living assemblages were better explained by hard substratum type than by water depth, as indicated by the higher R-value (Table 3).
Ordination of abundance data of living and dead molluscs from 44 transects on six substrata- and water depth categories using non-metric multidimensional scaling (MDS). Points close to one another represent transects that are more similar in taxonomic composition than points farther away from one another
Fringing reef habitats, occurring in shallow waters (<10 m), were characterized by high molluscan densities (typically >1 individual/0.25 m²) and were dominated by two gastropod species, Coralliophila neritoidea and Dendropoma maxima (Table 2, Fig. 4). Conversely, the three fore-reef hard substrata occurred in greater average water depths (>10 m) and were characterized by lower densities of living molluscs (typically <1 individual/0.25 m²) (Table 2). Coralliophila neritoidea and Pedum spondyloideum were the most abundant species here. Encrusting bivalves (Chamoidea, Ostreoidea and Spondylidae) and crevice-dwelling forms [Ctenoides annulata (Lamarck, 1819), Isognomon legumen (Gmelin, 1791), Barbatia setigera Reeve, 1844] were also important (Fig. 4). Based on their molluscan composition, fore reef hard substrata cannot be distinguished from each other, but fringing reef habitats are characterized by distinct assemblages (Fig. 3, Table 3).
Reef flats are shallow (<1 m), had the second highest density of living molluscs and a high abundance of Dendropoma maxima, which accounted for more than 60% of the total molluscan fauna in this habitat (Table 2, Fig. 5). Only one additional species, the algal-grazing gastropod Turbo radiatus Gmelin, 1791 contributed more than 10% here (Fig. 5). Fringing reefs with massive corals were studied at an average water depth of ∼2 m and were characterized by the highest density of living molluscs (Table 2). By far the most abundant species is Coralliophila neritoidea, which makes up almost 78% of the total of molluscan fauna in this habitat. All other species here contributed <10% (Fig. 5). Millepora-dominated fringing reefs (average depth about 4 m) had a lower density of living molluscs than reef flats and fringing reefs with massive corals, but a higher density than that of any fore-reef bottom type (Table 2). Millepora-fringing reefs were about equally dominated by Pedum spondyloideum, Pteria spp. and Coralliophila neritoidea (Fig. 5).
The most important molluscs in the dead assemblage were the encrusting bivalves Ostreoidea, Chamoidea, and Spondylidae, and the coral-predating gastropod Drupella cornus, which together accounted for 64% of the empty shells (Fig. 2). Most empty gastropod shells (76%) were inhabited by hermit crabs; the two most common species [Drupella cornus (Röding, 1798), Drupa ricnus hadari Emerson and Cernohorsky, 1793] and several others were almost exclusively occupied by crabs (Fig. 6). Fringing reef substrata differed significantly from fore-reef habitats, except for the strong overlap between Millepora-fringing reefs and fore-reef substrata. Among fringing reef substrata, significant differences occurred only between reef flats and Millepora-fringing reefs. No significant differences were recorded among fore-reef substrata (Table 4). Dead molluscan assemblages changed only little with water depth. Only those in <1 m differed significantly from all other depths; among the latter, however, no significant differences were evident (Table 4). The global ANOSIM test showed dead molluscan assemblages to be better explained by hard substrata than by water depth, as indicated by the higher R-value (Table 4).
In terms of abundance, most molluscs (83.1%) were alive; only 16.9% occurred as dead shells. The number of taxa, however, was higher for dead (40) than for living molluscs (33) (Fig. 2). For all substrata and water depth categories, the differences between living and dead assemblages were highly significant (Table 5, Fig. 3).
Rank order correlations between the abundances of living assemblages of Aqaba and Safaga were highly significant (Spearman’s rho = 0.615, p < 0.01). The three most abundant species were the same in Aqaba and Safaga, although in different rank orders (Table 6). The proportional abundance of Coralliophila neritoidea was much higher in Aqaba than in Safaga, and the opposite was true for Pedum. Among the less important species, a striking difference was the much higher proportional abundance of Tridacna maxima (Röding, 1798) in Safaga. Lopha cristagalli (Linnaeus, 1758) was absent in our survey of Aqaba and certain other taxa [Barbatia setigera, Barbatia foliata (Forsskål, 1775), Cerithium spp.] were also distinctly more abundant in Safaga. Conversely, oysters, pteriid bivalves, and the gastropods Turbo radiatus and Tectus virgatus (Gmelin, 1791) were more abundant in Aqaba than in Safaga (Table 6).
Discussion
Hard substrata versus water depth
At our Red Sea site, the composition of both molluscan living and dead assemblages was better explained by hard-substratum type than by water depth. This is not surprising because coral reef molluscs depend on their coral habitat (e.g., Hadfield 1976; Morton 1983). While these do change with depth-related factors like light penetration and water flow, they are also influenced by other environment parameters (Perrin et al. 1995). Nevertheless, the two basic habitats investigated—fringing reef and fore-reef substrata—mark different depth categories and thus also differ strongly in terms of molluscan composition and density. Within fringing reefs themselves, the bottom types and their coral assemblages change with water depth. Accordingly, the three fringing reef categories feature distinct molluscan compositions. In contrast, depth is not a structuring variable for fore-reef hard substrata: water depths overlap in the three fore-reef categories, yielding similar coral compositions. Here, the rather uniform molluscan composition did not change within the depth range studied.
Life habits of the important taxa in Aqaba and the Red Sea
From the habitat perspective, coral assemblages and water flow are the key environment parameters influencing distribution patterns of the most important gastropod and bivalve species in this study. The comparison between the Aqaba and Safaga data sets (Table 6) reveals that molluscan communities on reefoidal hard substrata in the northern Red Sea are largely characterized by similar species-abundance patterns. Certain differences–—like those in rank order of the three most important species and higher proportional abundances in Aqaba for pteriid bivalves and oysters–—may reflect different sampling intensities at particular habitats.
The parasitic gastropod Coralliophila neritoidea, feeding on Porites (Robertson 1970; Schuhmacher 1992), was therefore mostly found on Porites-rich fringing reefs with massive corals in Aqaba. However, the high abundances we observed could be a relatively new phenomenon here: this gastropod was not listed by Mergner and Schuhmacher (1974), is mentioned as occurring only regularly by Mastaller (1979) and in only low abundances by Schuhmacher (1992) and Al-Moghrabi (1996).
The encrusting gastropod Dendropoma, inhabiting dead coral rock but also embedded in massive coral colonies, requires agitated water conditions to spread its mucous net for suspension feeding (Hughes and Lewis 1974; Kappner et al. 2000). Dendropoma was therefore only abundant on reef flats in Aqaba, with rare occurrences in the shallowest parts of fringing reefs. Its high densities and abundances are typical features in Aqaba (Mergner and Schuhmacher 1974; Mastaller 1979; Kappner et al. 2000) and are known elsewhere from the Red Sea (Hughes and Lewis 1974; Hughes 1977; Mastaller 1978; Taylor and Reid 1984; Zuschin et al. 2001), but have otherwise only been reported from the SE Pacific (e.g., Salvat 1971).
The host-specific bivalve Pedum bores into living corals, where it attaches with the byssus; it is associated with a variety of scleractinians in the Red Sea, but prefers Montipora (Kleemann 1990). Correspondingly, Pedum occupied all studied hard substrata, yet with distinctly lower abundances on reef flats. It was recorded as occurring in only scattered (Mergner and Schuhmacher 1974) and regular patterns (Mastaller 1979) for Aqaba.
Species of Chamoidea, Ostreoidea and Spondylidae encrust dead hard substrata, mostly dead coral colonies, but also bare rocky surfaces (Zuschin and Oliver 2003a). In Aqaba, they were found on all bottom types but preferred fore-reef hard substrata; oysters were also abundant on fringing reefs with massive corals.
Pteria spp. are bysally attached epizoic bivalves on Millepora fringing reefs, on fore-reef habitats and on octocorals at about 20 m depth (see also Zuschin and Oliver 2003a). The bivalve Ctenoides annulata is a crevice dweller in dead coral colonies (Zuschin and Oliver 2003a): in the study area it was restricted to fore reefs. The low abundance of Ctenoides and other bivalve crevice dwellers (B. setigera, I. legumen, Lima paucicostata Sowerby, 1843) in Aqaba is most likely a long-term feature because, except for B. setigera, all the above species were either listed as being rare by Mastaller (1979) or were apparently absent. The greater importance of crevice dwellers in Safaga (9%) than in Aqaba (3%) could reflect distinct habitat differences. Coral carpets are particularly well developed in Safaga, and bivalve crevice dwellers preferred such habitats, especially in areas tentatively associated with high suspension loads. No such areas were observed in Aqaba, perhaps explaining why Lima paucicostata was missing and Barbatia setigera occurred rarely in our Aqaba survey; Ctenoides and Isognomon were also present in distinctly lower proportions than in Safaga. This interpretation is supported by other observations. The same habitat was also preferred by Lopha cristagalli and Cerithium spp. in Safaga, and both taxa were either absent or quantitatively unimportant, respectively, in Aqaba. Finally, up to 30-cm-long specimens of Hyotissa hyotis (Linnaeus, 1758) were found in Safaga on this habitat type and tentatively related to the high suspension load: no such shells were found in Aqaba.
Turbo radiatus, Tectus dentatus (Forsskål, 1775) and Tectus virgatus are herbivores and algivores on rocky substrata (Mastaller 1979; Taylor and Reid 1984), each with distinct habitat preferences. Turbo radiatus was virtually restricted to reef flats, with a single occurrence on fringing reefs with massive corals, whereas Tectus virgatus preferred fringing reefs, being rare on the reef flat and occurring once on the fore reef. Tectus dentatus, finally, was about equally distributed among reef flat, fringing reef and fore-reef substrata.
The relatively high abundances of Turbo radiatus and Tectus virgatus in Aqaba are probably a long-term feature (see Mastaller 1979), and the distinctly lower abundances in Safaga may be due to shell collecting by diving tourists there.
The giant clam Tridacna maxima is usually bysally attached within or between living coral colonies (Zuschin and Oliver 2003a). In the study area it preferred fore-reef hard substrata, with few occurrences on reef flats and fringing reefs. Its strikingly low abundance in Aqaba versus Safaga seems to be a long-term feature at the former site (Mergner and Schuhmacher 1974; Mastaller 1979). More recent studies, however, consider Tridacna species as endangered due to souvenir collecting (Kilada et al. 1998; Al-Horani et al. 2006). The low abundance of Barbatia foliata in Aqaba is also a long-term feature (see Mastaller 1979) but more difficult to explain because Porites colonies in very shallow water are abundant. In Safaga (Zuschin et al. 2001) and the central Red Sea (Taylor and Reid 1984), the abundant B. foliata is bysally attached to these corals. The low abundance of both bivalves could reflect the northern, somewhat isolated, position of the Gulf of Aqaba, with its characteristic high salinities and fluctuating shallow-water temperature (Mastaller 1987).
The influence of over-fishing by local fishermen and tourists is probably responsible for the low abundance of three other attractive species in the northern Red Sea: the pearl oyster Pinctada margaritifera (Linnaeus, 1758) and the gastropods Strombus tricornis Humphrey, 1786 and Lambis truncata (Kiener, 1843) were absent in our quantitative survey of Aqaba. Scattered and rare occurrences are recorded for Aqaba (Mergner and Schuhmacher 1974; Mastaller 1979) and rare finds in Safaga (Zuschin et al. 2001).
In Aqaba, the coral-predator Drupella was widely distributed on reef flats, fringing reefs and fore reefs, but larger individuals only on acroporans. Drupella cornus was not listed by Mergner and Schuhmacher (1974) or by Mastaller (1979). Abundances were low in the 1970s and 1980s—partly estimated based on distinct feeding marks—and increased distinctly, mostly in the shallowest reef parts, in 1992 and 1993 (Schuhmacher 1992; Schuhmacher et al. 1995). Al-Moghrabi (1996), using a more destructive sampling method, which enabled him to collect many cryptic specimens in 1994 and 1995, recorded a Drupella outbreak in the northern Gulf of Aqaba. Based on semi-quantitative sampling strategies, a Drupella outbreak was recorded for 1996 further south on the Sinai side of the Gulf, around Ras Mohammed (Antonius and Riegl 1997). Our study, and a similar survey in the northern Red Sea at Safaga (Zuschin et al. 2001), which are methodologically more comparable to that of Schuhmacher (1992), now suggest that Drupella regularly occurs on acroporans in this region.
Conus spp., invertebrate predators that favour reefs with <20% living coral cover (Kohn 1983), were found mostly on reef flats, with two occurrences on fore-reef substrata. This reflects their reported occurrence on reef flats with rocky substrata, sand pockets and sparse coral coverage here and elsewhere in the Red Sea (Mergner and Schuhmacher 1974; Hughes 1977; Taylor and Reid 1984). In Safaga they were also abundant on subtidal rock bottoms of the fore reef (Zuschin et al. 2001).
Streptopinnasaccata (Linnaeus, 1758) was typically embedded in living massive corals, but sometimes also occurred in dead coral head crevices (see also Zuschin and Oliver 2003a); it was about equally distributed among the studied hard substrata. Around Port Sudan, the muricid Drupa ricinus hadari feeds on a broad range of invertebrates (mostly crustaceans) (Taylor and Reid 1984), and in Aqaba it preferred reef flats, with two occurrences on Millepora-fringing reefs and on the fore reef, respectively.
Differences between living and dead assemblages
Comparable studies—using the same field methods—on the degree of coincidence between molluscan living and dead assemblages on coral reef-associated hard substrata are available from Safaga, northern Red Sea (Zuschin et al. 2000; Zuschin and Oliver 2003a) and the Seychelles (Zuschin and Oliver 2003b). Water depths and molluscan habitats were similar to the Safaga study and somewhat different from the Seychelles one (see Zuschin and Oliver 2003b). All three studies, including the present one, however, revealed strong differences in the abundances of living and dead molluscs, largely due to distinct biases in the dead assemblage.
(1) Dead bivalves that lived in close association with living corals (mainly byssate pteriomorph bivalves) are easily overgrown after death. They were therefore overlooked by our sampling regime. (2) Some gastropods were under-represented in the studied dead assemblages, presumably because of rapid post-mortem transport into surrounding sediments or into crevices within corals (mainly parasitic coralliophilids).
(3) Most gastropods, on the other hand, were strongly overrepresented in the dead assemblage (mostly cerithiids and thaidids). Most were inhabited by hermit crabs, and thus important—as secondary inhabitants—in forming the dead assemblage.
The comparison of living and dead molluscs yielded important insights into the temporal and spatial dynamics of the taphonomic fate of reef biota. Taxa preferentially overgrown by a living substratum should provide considerable temporal and ecological information: they will be preserved within a rapidly growing reef framework (e.g., Crame 1980, 1981). Fauna preferentially transported into surrounding soft substrata will be affected by the processes of time-averaging and taphonomic disintegration that typically occur in such sediments: much temporal information will be lost (e.g., Perry 1996; Kidwell et al. 2005). Gastropod shells inhabited by hermit crabs may strongly alter the fossil gastropod community structure (for review see Walker 1989). Almost all dead gastropod shells in Aqaba were occupied by hermit crabs, except for those that are structurally unsuitable (e.g., Cypraea) to house the crabs’ abdomen.
Such complex relationships between ecology and taphonomy have to be considered when evaluating the quality of the fossil record of molluscs in coral reef environments.
Conservation implications
Molluscs are increasingly being recognized as a major component in the overall biodiversity of coral reefs (Bouchet et al. 2002; Zuschin and Oliver 2005). Accordingly, molluscs should be better used in evaluating the status of reefs (Wells 1998) and in making prognoses about the effects of reef degradation (Zuschin et al. 2001). Our results have clear management and conservation implications. First, the comparison of this and other recent studies (Schuhmacher 1992; Al-Moghrabi 1996; Zuschin et al. 2001) with data from the literature (Mergner and Schuhmacher 1974; Mastaller 1979) suggest that coral predators (Coralliophila and Drupella) have increased considerably during the last two decades in the northern Red Sea. This points to higher stress levels for corals and the prevalence of coral diseases (Antonius and Riegl 1998). Second, the major substrate-related changes in molluscan composition in reef flats, Millepora-fringing reefs, fringing reefs with massive corals and fore-reef substrata mean that any loss of these habitat types will impact distinct molluscan assemblages. Third, the strong depth-related changes in the shallow fringing reef substrata means that damage in shallow water will have the greatest impact on the overall mollusc community. These depths are precisely the most vulnerable to an array of anthropogenic impacts. Tourism-related impacts in the shallowest zones range from trampling (wading beachgoers or scuba divers entering the water), fin damage by inexperienced snorkelers, to boat- and footbridge-related damage. Currently, the Gulf of Aqaba is unevenly affected by anthropogenic factors, with the Israeli coast from Eilat southward having deteriorated extensively during the last decade and being heavily impacted (Fishelson 1995; Wielgus 2003; Loya 2004; Loya et al. 2004). At present the Jordanian coast is in good condition (Al-Horani et al. 2006). Those authors, however, identify rapid development of tourism, industry and construction sectors along the coast as key problems. This coast may be one of the fastest growing resort areas in the world (Shackley 1999). Specifically, the major current and planned tourism development along the Jordanian coast, including the creation of major hotel complexes directly on the shoreline, artificial harbors and new lagoons, threaten the reefs here. Our study demonstrates that the potential degradation of such shallower coral habitats will pose the greatest threat to molluscan biodiversity. Degradation of coral cover would result in a loss of coral-associated molluscs in favour of bivalve crevice dwellers in dead coral heads and of encrusters on dead hard substrata (Zuschin et al. 2001). The results once again underline a general conclusion that coral reef decline is directly correlated to a concurrent decline in the full range of associated fauna. This study, conducted in a pre-exploitation phase, also provides a baseline for future evaluations of the status of coral reef-associations–—a particular concern in an area at the northernmost limits of coral reef distribution in the Red Sea. This calls for a more inclusive approach to evaluating reef status, one that considers molluscs and other invertebrate groups in monitoring studies, reef surveys and general research efforts.
References
Al-Horani FA, Al-Rousan SA, Al-Zibdeh M, Khalaf MA (2006) The status of coral reefs on the Jordanian coast of the Gulf of Aqaba, Red Sea. Zool Middle East 38:99–110
Al-Moghrabi SM (1996) Bathymetric distribution of Drupella cornus and Coralliophila neritoidea in the Gulf of Aqaba (Jordan). Proc Int Coral Reef Symp 2:1345–1350
Antonius A, Riegl B (1997) Coral diseases and Drupella cornus invasion in the Red Sea. Coral Reefs 17:48
Antonius A, Riegl B (1998) A possible link between coral diseases and a corallivorous snail (Drupella cornus) outbreak in the Red Sea. Atoll Res Bull 447:1–9
Ayal Y, Safriel UN (1981) Species composition, geographical distribution and habitat characteristics of rocky intertidal Cerithiidae (Gastropoda; Prosobranchia) along the Red Sea shores of Sinai. Argamon-Isr J Malacol 7:54–72
Bosch DT, Dance SP, Moolenbeek RG, Oliver PG (1995) Seashells of Eastern Arabia. Motivate Publishing, Dubai, p 296
Bouchet P, Lozouet P, Maestrati P, Heros V. 2002. Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a Caledonia site. Biol J Linn Soc 75:421–436
Braithwaite CJR (1987) Geology and palaeogeography of the Red Sea region. In: Edwards AJ, Head SM (eds) Red Sea, key environments. Pergamon, Oxford, pp 22–44
Bray JR, Curtis JT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Clarke KR, Gorley RN (2001) Primer v5: User manual/tutorial. Primer-E, Plymouth, p 91
Clarke KR, Warwick RM (1994) Changes in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth, p 144
Crame JA (1980) Succession and diversity in the Pleistocene coral reefs of the Kenya coast. Palaeontology 23:1–37
Crame JA (1981) Ecological stratification in the Pleistocene coral reefs of the Kenya coast. Palaeontology 24:609–646
Edwards FJ (1987) Climate and oceanography. In: Edwards AJ, Head SM (eds) Red Sea, key environments. Pergamon, Oxford, pp 45–69
Fishelson L (1971) Ecology and distribution of the benthic fauna in the shallow waters of the Red Sea. Mar Biol 10:113–133
Fishelson L (1995) Elat (Gulf of Aqaba) littoral: life on the red line of biodegradation. Isr J Zool 41:43–55
Gosliner TM, Behrens DW, Williams GC (1996) Coral reef animals of the Indo-Pacific. Sea Challengers, Monterey
Hadfield MG (1976) Molluscs associated with living tropical corals. Micronesica 12:133–148
Head SM (1987) Corals and coral reefs of the Red Sea. In: Edwards AJ, Head SM (eds) Red Sea, key environments. Pergamon, Oxford, pp 128–151
Hughes RN (1977) The biota of reef flats and limestone cliffs near Jeddah, Saudi Arabia. J Nat Hist 11:77–96
Hughes RN, Lewis AH (1974) On the spatial distribution, feeding and reproduction of the vermetid gastropod Dendropoma maximum. J Zool 172:531–547
Hulings NC (1986) Aspects of the ecology of the mollusks of the rocky intertidal zone, Jordan Gulf of Aqaba (Red Sea). The Veliger 28:318–327
Kappner I, Al-Moghrabi SM, Richter C (2000) Mucus-net feeding by the vermetid gastropod Dendropoma maxima in coral reefs. Mar Ecol Prog Ser 204:309–313
Kidwell SM, Flessa KW (1995) The quality of the fossil record: populations, species, and communities. Annu Rev Ecol Syst 26:269–299
Kidwell SM, Best MMR, Kaufman DS (2005) Taphonomic trade-offs in tropical marine death assemblages: differential time averaging, shell loss, and probable bias in siliciclastic vs. carbonate facies. Geology 33:729–732
Kilada R, Zakaria S, Farghalli ME (1998) Distribution and abundance of the giant clam Tridacna maxima (Bivalvia: Tridacnidae) in the northern Red Sea. Bull Natl Inst Oceanogr Fish ARE 24:221–240
Kleemann K (1990) Coral associations, biocorrosion, and space competition in Pedum spondyloideum (GEMLIN) (Pectinacea, Bivalvia). PSZNI Mar Ecol 11:77–94
Kleemann K (1992) Coral communities and coral-bivalve associations in the Northern Red Sea at Safaga, Egypt. Facies 26:1–10
Kohn AJ (1983) Microhabitat factors affecting abundance and diversity of Conus on coral reefs. Oecologia 60:293–301
Kruskal JB (1964) Multidimensional scaling by optimizing goodness of fit to a non-metric hypothesis. Psychometrika 29:1–27
Loya Y (1972) Community structure and species diversity of hermatypic corals on Eilat, Red Sea. Mar Biol 13:100–123
Loya Y (2004) The coral reefs of Eilat-past, present and future: three decades of coral community structure studies. In: Rosenberg E, Loya Y (eds) Coral reef health and disease. Springer, Berlin, p 400
Loya Y, Lubinevsky H, Kramarsky-Winter E (2004) Nutrient enrichment caused by in situ fish-farms is detrimental to coral reproduction. Mar Pollut Bull 49:344–353
Martin RE (1999) Taphonomy: a process approach. Cambridge University Press, Cambridge, p 508
Mastaller M (1978) The marine molluscan assemblages of Port Sudan, Red Sea. Zool Mededelingen 53:117–144
Mastaller M (1979) Beiträge zur Faunistik und Ökologie der Mollusken und Echinodermen in den Korallenriffen bei Aqaba, Rotes Meer, p 344. Unpublished Ph.D. study, University Bochum
Mastaller M (1987) Molluscs of the Red Sea. In: Edwards AJ, Head SM (eds) Red Sea, key environments. Pergamon, Oxford, pp 194–214
Medio D, Sheppard CRC, Gascoigne J (2000) The Red Sea. In: McClanahan TR, Sheppard CRC, Obdura DO (eds) Coral reefs of the Indian Ocean, Oxford University Press, Oxford, pp 231–255
Mergner H (1979) Quantitative ökologische Analyse eines Rifflagunenareals bei Aqaba (Golf von Aqaba, Rotes Meer). Helgoländer wissenschaftliche Meeresuntersuchungen 32:476–507
Mergner H, Schuhmacher H (1974) Morphologie, Ökologie und Zonierung von Korallenriffen bei Aqaba (Golf von Aqaba, Rotes Meer). Helgoländer wissenschaftliche Meeresuntersuchungen 32:238–358
Morton B (1983) Coral-associated bivalves of the Indo-Pacific. In: Russel-Hunter WD (ed) The Mollusca. Ecology, vol 6. Academic, New York, pp 139–224
Oliver PG (1992) Bivalved seashells of the Red Sea. Verlag Christa Hemmen, Wiesbaden, p 330
Perrin C, Bosence D, Rosen, B (1995) Quantitative approaches to palaeozonation and palaeobathymetry of corals and coralline algae in Cenozoic reefs. In: Bosence DW, Allison PA (eds) Marine palaeoenvironmental analysis from fossils. Geol Soc Spec Publ 83: 181–229
Perry CT (1996) The rapid response of reef sediments to changes in community composition: implications for time averaging and sediment accumulation. J Sediment Res 66:459–467
Reiss Z, Hottinger L (1984) The Gulf of Aqaba. Ecological micropaleontology. Springer, Berlin, p 354
Riegl B, Velimirov B (1994) The structure of coral communities at Hurghada in the Northern Red Sea. PSZNI Mar Ecol 15:213–231
Robertson R (1970) Review of the predators and parasites of stony corals, with special reference to symbiotic prosobranch gastropods. Pac Sci 24:43–54
Salvat B (1971) Mollusques lagunaires et récifaux de l’ile de Raevavae (Australes, Polynésie). Malacol Rev 4:1–15
Schuhmacher H (1992) Impact of some corallivorous snails on stony corals in the Red Sea. Proc Int Coral Reef Symp 2:840–846
Schuhmacher H, Kiene W, Dullo WC (1995) Factors controlling Holocene reef growth: an interdisciplinary approach. Facies 32:145–188
Shackley M (1999) Tourism development and environmental protection in southern Sinai. Tour Manage 20:543–548
Sharabati D (1984) Red Sea shells. Routledge, London p 128
Sheppard CRC, Sheppard ALS (1991) Corals and coral communities of Arabia. Fauna Saudi Arabia 12:170
SPSS (1999) SPSS base 10 applications guide. Prentice-Hall, Chicago, p 426
Taylor JD, Reid DG (1984) The abundance and trophic classification of molluscs upon coral reefs in the Sudanese Red Sea. J Nat Hist 18:175–209
Walker SE (1989) Hermit crabs as taphonomic agents. Palaios 4:439–452
Wells FE (1998) Marine molluscs of Milne Bay Province, Papua, New Guinea. In: Werner TB, Allen GR (eds) A rapid biodiversity assessment of the coral reefs of Milne Bay Province, Papua, New Guinea, vol 11. RAP working papers, pp 35–38
Wielgus J (2003) The coral reef of Eilat (northern Red Sea) requires immediate protection. Mar Ecol Prog Ser 263:307
Zuschin M, Hohenegger J (1998) Subtropical coral-reef associated sedimentary facies characterized by molluscs (Northern Bay of Safaga, Red Sea, Egypt). Facies 38:229–254
Zuschin M, Oliver PG (2003a) Bivalves and bivalve habitats in the northern Red Sea. The Northern Bay of Safaga (Red Sea, Egypt): an actuopalaeontological approach. VI. Bivalvia, Naturhistorisches Museum, Wien, p 304
Zuschin M, Oliver PG (2003b) Fidelity of molluscan life and death assemblages on sublittoral hard substrata around granitic islands of the Seychelles. Lethaia 36:133–149
Zuschin M, Oliver PG (2005) Diversity patterns of bivalves in a coral dominated shallow-water bay in the northern Red Sea—high species richness on a local scale. Mar Biol Res 1:396–410
Zuschin M, Piller WE (1997a) Gastropod shells recycled—an example from a rocky tidal flat in the northern Red Sea. Lethaia 30:127–134
Zuschin M, Piller WE (1997b) Bivalve distribution on coral carpets in the Northern Bay of Safaga (Red Sea, Egypt) and its relation to environmental parameters. Facies 37:183–194
Zuschin M, Hohenegger J, Steininger FF (2000) A comparison of living and dead molluscs on coral reef associated hard substrata in the northern Red Sea—implications for the fossil record. Palaeogeogr Palaeoclimatol Palaeoecol 159:167–190
Zuschin M, Hohenegger J, Steininger FF (2001) Molluscan assemblages on coral reefs and associated hard substrata in the Northern Red Sea. Coral Reefs 20:107–116
Acknowledgments
We wish to thank the director and staff of the Royal Diving Club, Aqaba, Jordan, for supporting the fieldwork and W. Waitzbauer, J. Herler, P. Zolda of the University of Vienna for organizational and logistic help. MZ was supported by project H-140/2000 of the Hochschuljubiläumsstiftung der Stadt Wien. The experimental procedure complied with the current national laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne.
Rights and permissions
About this article
Cite this article
Zuschin, M., Stachowitsch, M. The distribution of molluscan assemblages and their postmortem fate on coral reefs in the Gulf of Aqaba (northern Red Sea). Mar Biol 151, 2217–2230 (2007). https://doi.org/10.1007/s00227-007-0656-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0656-6