Abstract
Low temperature limits the rate of biochemical reactions and aerobic scopes of cold water ectotherms. To compensate for this limiting effect, animals living in cold environments often possess physiological or morphological adaptations to maintain vital functions. Cross-latitudinal comparison of aerobic capacities is one method to test which factors constrain activity in thermally distinct environments particularly when congeneric studies are carried out on related species with conservative ecology and habitat. Burrowing is a major aerobic activity of bivalve molluscs that is described here for the first time for the tropical mangrove species Laternula truncata and Laternula boschasina and then compared with their Antarctic congener Laternula elliptica. About 80% of L. truncata (16.3–46.1 mm shell length) and 63% of L. boschasina (11.3–27.7 mm shell length) buried within 24 h at 28°C. The burrowing rate index (BRI = [3√wet weight/time to bury]×104) ranged between 1.1 and 20.2 for L. boschasina and 1.1–32.9 for L. truncata. These values are 2–3 orders of magnitude less than other tropical bivalve molluscs and are amongst the lowest recorded for any bivalve. Comparisons with the Antarctic L. elliptica showed little or no differences in BRI (Q10 of 1.0–1.2 for specimens of the same size). This is contrary to the general pattern over a wide range of bivalves, where BRI increases with a Q10 of between 2.9 and 6.4 between high latitudes and the equator. L. elliptica has 25–30% longer relative foot length than tropical congeners of the same size, which could be a morphological adaptation compensating for reduced burrowing speeds in a colder environment. Burrowing rates within the genus Laternula could, however, also be maintained by differing habitat, ecological and physiological constraints on burrowing capability.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The cold temperatures experienced by polar ectotherms limit the rates of physiological reactions, which lead to a cold-induced reduction in aerobic scope, i.e. a reduction in the body’s capacity to conduct aerobic work (Pörtner 2002). This cold limitation can be compensated for by morphological adaptation or at the cellular level by up-regulation of mitochondrial densities or adjustments at the molecular level (e.g. Antarctic perciform fish, Johnston et al. 1998). However, compensation is often incomplete and polar ectotherms are characterised by temperature-limited physiological rates and reduced aerobic capacity (Pörtner 2001, 2002; Peck et al. 2004; Pörtner et al. 2004; Peck 2005). Investigating mechanisms of temperature compensation is complicated by ecological, morphological, phylogenetic and habitat differences between species. However, latitudinal comparisons of closely related species can minimise many of these confounding factors and are amongst the best ways of providing insights into adaptive mechanisms that may otherwise be difficult to resolve (e.g. growth compensation in fish, Yamahira and Conover 2002).
There are very few genera with representatives ranging from Antarctica to the tropics. Laternula is a genus of infaunal bivalve molluscs with an exceptionally wide latitudinal distribution from tropical and temperate Australasia to the Antarctic (Morton 1976). At the same time, Laternula species are conservative in shell form and habitat, suggesting that these bivalves share similar morphological constraints at different latitudes (McLachlan et al. 1995; Peck et al. 2004). Burrowing is a quantifiable activity of bivalves that has frequently been used for comparative studies (e.g. Peck et al. 2004). Burrowing is arguably the major locomotor activity of infaunal bivalves as they rebury after removal from the substratum or move within the sediment. The central importance of burrowing for predator avoidance and feeding, as well as the stereotypic nature of the burial process, makes it an ideal activity for comparing temperature compensation of aerobic activity between tropical and polar latitudes. Bivalve burrowing has been studied in a wide range of temperate species (Quayle 1949; Ansell 1962; Trueman et al. 1966) and to a limited extent in sub-tropical (Ansell 1983) and polar species (Peck et al. 2004). However, these comparisons were made across phylogenetically distant bivalve genera with disparate morphologies living in a variety of habitats.
If physical activity is limited by aerobic capacity we might expect tropical Laternula species typically inhabiting environments with temperatures exceeding 25°C to have an aerobic scope eight times higher than the Antarctic Laternula elliptica living at 0°C (assuming a Q10 of 2, Peck 2002). However, for each species, ecological and functional limitations are likely to impose (a) a physiological upper limit to sustainable aerobic scope (Hammond and Diamond 1997) and (b) morphological constraints to burrowing capability (McLachlan et al. 1995). Tropical Laternula species therefore provide a good test of which factors limit burrowing in the Antarctic species L. elliptica.
The aims of this study were to (1) characterise burrowing in tropical Laternula species to facilitate latitudinal comparisons, and (2) use latitudinal comparisons of burrowing within the genus Laternula to evaluate factors constraining burrowing rates.
Materials and methods
Methods were designed to mirror those of Peck et al. (2004) as closely as possible to enable ecological comparisons to be made between tropical and Antarctic Laternula populations.
Collection and handling of Laternula
The infaunal mangrove bivalves Laternula truncata (Lamarck, 1818) and Laternula boschasina (Valenciennes, 1860) were both collected at Sungei Buloh Wetland Reserve fronting the Johor Straits in Singapore (1.45°N, 103.73°E). Temperature in the Johor Straits varies from 27 to 31°C and salinity varies between 20 and 33 depending on river run-off (Chou and Lee 1997). Animals were collected at low water by digging in sandy mud at the mid-intertidal shore between the roots of mangrove trees.
Animals were transported in seawater to aerated aquarium tanks at the Tropical Marine Science Institute on St. John’s Island where they were kept at ambient temperatures, between 27.5 and 29.0°C, and a salinity of 20. Animals were acclimated in these tanks without sediment for at least 24 h before experiments were started. To enable video recording a 24-h light regime was maintained at 900 lux during daylight (12 h light) and 300 lux during the night.
Laternula elliptica were collected by SCUBA diving at the British Antarctic Survey research station at Rothera (67.57°S, 68.13°W) and kept in the station aquarium system at 0.0°C and a salinity of 33.
Video recording and analysis
A glass aquarium tank (30 × 20 × 20 cm) was placed in a large flow-through seawater tank to dampen out daily air temperature fluctuations. The tank was three-fourth filled with washed sand from a man-made beach at St. John’s Island (77–80% retained on a 250 μm sieve), which, although a little finer than the sediment in the mangroves (84–90% retained on a 250 μm sieve), was very similar to the sand used by Peck et al. (2004). Such small differences in sediment grain size are unlikely to affect burrowing rates as preliminary trials found no significant difference in the burrowing rate of Laternula between fine and coarse sediments (31 or 88% retained on a 250 μm sieve).
Animals were placed on the sand and their behaviour was filmed for 24 h using a Panasonic WV-CP412E video camera connected to a Panasonic AG-TL700 24-h time lapse video recorder. After 24 h all animals were removed and fresh animals were used for subsequent trials. Burrowing in bivalves is achieved through a series of cycles of foot extension, pulling down on the anchor formed by the distal region of the foot, and then a period of quiescence until the process is repeated. This is seen as a series of pulses on video recordings. Video recordings were analysed according to Peck et al. (2004) to obtain the total time needed for complete shell burial and also the duration of each burrowing cycle. From the point of initial foot anchor the duration of each burrowing cycle was recorded until the shell was completely buried. The time to complete shell burial (Tb in seconds) was used to calculate the burrowing rate index (BRI) (Alexander et al. 1993; modified after Stanley 1970), which takes animal size (W, whole wet weight, including the shell, in grams) into account and allows comparison between studies:
The number of burrowing cycles and the duration of each burrowing cycle were calculated for standard sized L. truncata from regressions of the number of cycles and the mean cycle duration against shell length. This was to enable size controlled comparisons with data for L. elliptica (Peck et al. 2004). There was no significant relationship between shell size and the number of burrowing cycles, or between shell size and the duration of the burrowing cycle in L. boschasina individuals between 0.2 and 2.6 g, so in this species an average value was calculated.
Morphometrics
Shell length (L) and wet weight were measured for each individual. Power curves were fitted to describe the length–weight relationships, W = aLb. Length–weight relationships were transformed into linear relationships by the \( \root b \of {\,} \) to allow ANCOVA tests between species. Shell length, width and height of L. truncata, L. boschasina and L. elliptica were measured for morphometric comparisons between species. Height to width (H/W) and length to height (L/H) ratios were calculated following Stanley (1970).
Foot length (Lf) of a further set of animals was measured by keeping animals submerged in seawater and carefully removing portions of the shell until the foot was exposed. Animals were left to recover and foot length was measured once the foot had returned to its relaxed state. For the smaller tropical Laternula species this was done under a dissecting stereomicroscope. To allow congeneric comparisons, foot length was divided by shell length. This ratio was arcsine transformed to normalise the data before ANOVA and post hoc Tukey tests were conducted.
Calculation of Q10
Q10 is a useful metric that allows comparisons of the influence of temperature on biological systems both within and across species (e.g. Clarke 2004; Clarke and Fraser 2004; Clarke 2006). It represents the change in the rate of a biological process for a 10°C rise in temperature:
where T1 = upper temperature and T0 = lower temperature, R1 = the rate at T1 and R0 = the rate at T0.
Q10 was calculated for the difference between the BRI of L. elliptica and both L. truncata and L. boschasina over the 28.2°C temperature difference between experiments. The linear regression between the natural logarithm transformed BRI and shell length for L. elliptica (Peck et al. 2004) and L. truncata was used to calculate BRI values for the smallest and largest individuals within the overlapping size range. As the BRI of L. boschasina was not size related, an average BRI was calculated and this compared with an extrapolated value for L. elliptica of the same size as the average L. boschasina.
Results
Morphometrics
Length/weight relationships for L. truncata and L. boschasina were described by W = 0.0004L2.8 (r2 = 0.96, F = 880, P < 0.01, df = 40) and W = 0.0004L2.7 (r2 = 0.92, F = 290, P < 0.01, df = 23), respectively. Data linearised by root transformation showed no significant difference between species (ANCOVA: slope F = 1.61, P = 0.21; intercept F = 0.20, P = 0.65).
There was little difference in H/W ratios between species (Table 1) but L. boschasina were more cylindrical than L. truncata (increased L/H), which in turn were less spherical than L. elliptica (McLachlan et al. 1995). There were no appreciable changes in either H/W or L/H ratios with shell length.
The relative foot length of L. elliptica was 1.5 and 1.75 times longer than that of L. truncata and L. boschasina, respectively (Table 2; ANOVA, F = 11.7, P < 0.01; Tukey test, T > 3.7, P < 0.01). There was no significant difference between the relative foot lengths of tropical species, so one regression was calculated to describe the change in foot length with shell length, Lf = 0.067L + 1.49 (R2 = 0.34, P < 0.05, df = 14). The foot size of L. elliptica increased with shell length, Lf = 0.16L + 2.75 (R2 = 0.72, P < 0.05, df = 6), but the difference between the relative foot size of tropical and Antarctic Laternula remained relatively constant with an estimated 2.1 times larger foot size for a 20 mm individual to 2.3 times for a 80 mm individual. Between a 20 and 80 mm individual the calculated relative foot size decreased by about 1.5 times for both tropical and Antarctic Laternula, respectively.
The burrowing cycle
A total of 80% of L. truncata (20 of 25) and 63% of L. boschasina (15 of 24) burrowed within 24 h of being placed on a suitable substratum. In most cases burrowing was initiated within minutes of being placed on the sand. The burrowing cycles in both L. truncata and L. boschasina started with foot probing and anchorage. This was followed by a series of foot extensions, formation of a pedal anchor and finally contraction, which drew the shell into the sediment. Once individuals were about half buried in the sediment, foot contraction cycles were interspersed by one or more siphon contractions which visibly raised the level of sand surrounding the animal, probably through water jetted into the sand from the anterior region of the animal. Siphon levering was used to aid initial foot anchorage in the largest L. truncata (L = 46.1 mm) in an identical fashion to that described for L. elliptica by Ansell and Rhodes (1997).
Time to complete shell burial
The time to complete shell burial (t) for L. truncata increased with shell length from 7 min for a 17 mm animal to 5.5 h for a 46 mm animal (Fig. 1). In contrast, there was no relationship between shell length and time to shell burial in L. boschasina (Fig. 1). The fastest individual (L = 11.3) buried completely in just 6 min whilst the slowest individual (L = 15.8) took 37 min to burrow. In most cases burrowing continued beyond shell burial until the siphon was also buried under the surface of the sand and the siphons fully extended.
Burrowing rate index
The BRI for L. truncata ranged between 1.1 and 32.9 and there was still a weakly significant reduction in BRI with shell length, indicating that reburial becomes increasingly less effective in large animals (Fig. 2). BRI for L. boschasina covered a similar range (3.8–20.4), but with fewer large animals observed there was no significant relationship with shell length.
Cycle time
The burrowing cycles of L. truncata and L. boschasina both follow the well described pattern for other deep burrowing bivalves (Ansell 1962; Ansell and Trueman 1967) including L. elliptica (Peck et al. 2004). The initial stage, pedal anchorage, can take longer than the first burrowing cycles, which increase in duration as more of the shell becomes buried in the sand. There is a clear transition from continuous burrowing cycles (early cycles, te) to those that are interspersed with siphon contractions (late cycles, tl). The duration (in seconds) of both early cycles (te = 0.58L + 14.21, r2 = 0.43, F = 12.3, P < 0.01, df = 17) and late cycles (tl = 2.87L − 20.52, r2 = 0.56, F = 20.3, P < 0.01, df = 17) increased linearly with shell length.
The number of burrowing cycles (Cn) required to complete shell burial increased logarithmically with shell length for L. truncata, lnCn = 0.05L + 2.32 (r2 = 0.47, F = 14.5, P < 0.01, df = 17; Table 3). The mean duration of burrowing cycles (Cd, in seconds) increased linearly with shell length for L. truncata (Cd = 0.036L − 0.34, r2 = 0.60, F = 23.7, P < 0.01, df = 17). There were no significant relationships with size for L. boschasina.
Q 10
Published BRI values for L. elliptica were all lower than those for similar-sized L. truncata, but were almost the same as values for L. boschasina (Table 4). Over the 28.2°C temperature difference between experiments, Q10 of BRI between L. elliptica and L. truncata was 1.2 for a 24.0 mm shell length animal and 1.1 for a 46.1 mm shell length animal. The BRI for an average sized L. boschasina (17.2 mm) was actually less than the estimated value for a L. elliptica of that size (Q10 = 1.0).
Discussion
Similar proportions of L. truncata (80%), L. boschasina (63%) and L. elliptica (71%; Peck et al. 2004) reburied in sand at normal ambient temperatures, contrary to previous reports for tropical Laternula (L. truncata, Morton 1973, 1976; Laternula spengleri, Savazzi 1990) which were thought incapable of burrowing if removed from the substratum due to their small foot size. The mechanics of the burying cycle for the two tropical Laternula species were very similar to their Antarctic congener L. elliptica (Peck et al. 2004) as well as to other bivalve genera (Trueman and Ansell 1969).
The observations of water being forced from the shell into the surrounding sediment, visibly raising the sand surrounding partly buried animals, confirm that members of this genus are capable of using hydraulic burrowing as well as burying by means of a foot anchor. In bivalves contraction of the adductor muscle draws the shell valves together, this increases the pressure in the body cavity, causing ejection of fluid from the mantel cavity and body fluids into the pedal haemocoel (Trueman 1966). In Laternula the siphon remains outside the shell and so contraction of the siphon is also required to increase hydraulic pressure for burrowing. The ejection of mantel fluid acts to liquefy the sand adjacent to the shell and dilation of the foot allows for a firm pedal anchorage. An essential feature of bivalves, hydraulic burrowing, was suspected but could not be confirmed for L. elliptica (Peck et al. 2004).
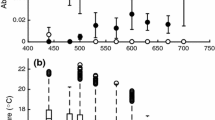
There was no, or only a small difference in BRI between Laternula species from Antarctica and the tropics at their environmental temperatures (Q10 = 1.0–1.2) which is in contrast to the general pattern for bivalves both within and across species. A within-genus comparison of Yoldia spp. measured Q10 values ranging from 2.8 to 10.4 (Peck et al. 2004). Q10 values for burrowing in Donax spp. ranged from 1.2 to 5.0 (McLachlan and Young 1982; Ansell 1983). In an extensive study of several unrelated bivalves covering a wide range of latitudes, there was a general negative relationship between burrowing rates and latitude and therefore temperature with Q10 values ranged from 2.9 to 6.4 (Fig. 3, redrawn from Peck et al. 2004). This indicates that BRI generally increases by as much and often more than would be expected assuming the usual effect of temperature on biological systems (Q10 of 2–3, Clarke 1983). The lack of this expected increase of BRI with latitude in Laternula suggests that burrowing is either perfectly compensated for temperature, or this activity is not directly dependent on the physiological rate of chemical reactions. Morphological or ecological constraints could be more important in determining burrowing rate than the direct physiological effect of temperature.
Burrowing rate index of Laternula elliptica, L. truncata and L. boschasina compared for a range of temperate and tropical sites (Figure extended from Peck et al. 2004). Points joined with a line indicate the range of values quoted for a species, where such a range exists. Data for Yoldia and Laternula species are labelled (Y. perp = Yoldia perprotracta)
Previously, temperature compensation of activity has only been shown for cruise swimming in fish (Johnston et al. 1998). No other activities have been found to be temperature compensated and activity of a range of Antarctic species is slower than for related or ecologically similar species from warmer environments (Peck et al. 2006). The compensation in cruise swimming is achieved by an increase in mitochondrial density in the red muscles of Antarctic fish that compensates for a temperature related reduction in mitochondrial function. For Laternula further research is required to investigate possible physiological compensation, e.g. mitochondrial densities, enzyme kinetics and muscle fibre densities, of burrowing activity, although pilot studies suggest that mitochondrial densities are not elevated in the foot muscle of the Antarctic L. elliptica (S.A.M. personal observation). Within species Q10’s of burrowing rate would also further our understanding of the mechanisms involved.
There is a large amount of variability in the global cross genera comparison of burrowing in bivalves (Peck et al. 2004). Factors such as sediment type (Alexander et al. 1993; Checa and Cadée 1997) and life history strategy (Peck et al. 2004) are likely to be responsible for much of this variation. However, these variables were minimised in this study by using a standard sediment type and by restricting comparisons to a single genus and to individuals of the same size. The greater muscle bulk of L. elliptica could be an example of morphological compensation to overcome the constraints of temperature on burrowing mechanics. The foot acts as the main muscle for burrowing and if foot volume changes isometrically with foot length, L. elliptica has 3.4–5.4 times more burrowing muscle mass than tropical species. Fewer, longer cycles would be expected in species with bigger relative foot size. Both tropical Laternula species used more than twice the number of burrowing cycles to bury completely in the substratum than L. elliptica. The greater part of each burrowing cycle consists of a lengthy recovery period between short bursts of muscular activity. Faster recovery rates and therefore faster cycling would be expected in species from warmer water (Peck et al. 2004); however, burrowing cycles were only approximately four times faster in tropical Laternula than in L. elliptica (Table 3). If temperature alone affected the recovery rates of each cycle then recovery rates would be expected to be at least eight times faster across the nearly 30°C temperature difference between environments. Rather than the burrowing rate of L. elliptica being up-regulated the burrowing rate of tropical Laternula may therefore be limited and the size of foot muscle constrained by as yet undetermined factors.
All Laternula species are deep infaunal clams that inhabit stable, soft sediments (Morton 1976). All three species examined in this study experience long periods of inactivity with their siphons closed either during low tide or during seasonal inactivity (Brockington 2001). This ability to withstand long periods of hypoxia may predispose Laternula as a genus to a low activity lifestyle with the lowest BRI of any bivalve genus so far recorded. However, infrequent disturbance events remove animals from the sediment which would maintain the evolutionary pressure for burrowing capability. Iceberg scouring is one of the major forcing factors of shallow water communities in the Antarctic (Brown et al. 2004) and displaced animals need to rebury to avoid predation from nemertean worms (Parborlasia corrugatus) and starfish (Odontaster validus) at the surface (Brown et al. 2004). At low environmental temperatures the time frame for predation risk is also extended, as was found during a study of reburying of L. elliptica at the Palmer Archipelago (64.5°S, Zamorano et al. 1986). Over a 23 day period 60% of L. elliptica were able to re-bury into the sea bed with half of those animals that remained on the surface consumed by predators. This indicates that the burial rate recorded for L. elliptica in the laboratory, most burying within 24 h, is a realistic time frame for predator avoidance. If the burrowing rate of L. elliptica were slowed purely by the expected effect of temperature between it and the tropical species (Q10 = 2–3), then 2 h (L = 24 mm) to 216 days (L = 90 mm) would be required to complete the process, which would put them at a significantly enhanced risk. Predation pressure is also likely to be a major disturbance for tropical Laternula as they are forcibly removed by muricids (Tan and Oh 2002). Tropical muricid predators take between 6.2 and 48 h to drill and consume prey species (Harper and Peck, 2003) suggesting that the rate of burying reported here is sufficient to act as an escape response. Overall and most likely, a protected mode of life in marine sediments with occasional disturbance events may be linked to the very slow burrowing capacity in the genus Laternula. The constancy of burrowing rate between Laternula found in environments with a near 30°C difference in temperature is contrary to the general pattern across bivalve species. Over evolutionary time scales, compensation for the expected effect of temperature on burrowing rate was likely achieved not only through physiological and biochemical adaptation but also through morphological adjustments of performance capacity to match the requirements within each environment.
References
Alexander R, Stanton R, Dodd J (1993) Influence of sediment grain size on the burrowing of bivalves: correlation with distribution and stratigraphic persistence of selected neogene clams. Palaios 8:289–303
Ansell AD (1962) Observations on burrowing in the Veneridae (Eulamellibranchia). Biol Bull 123:521–530
Ansell AD (1983) Species of Donax from Hong Kong: morphology, distribution, behaviour and metabolism. In: Morton B, Dudgeon D (eds) Proceedings of the Second International Workshop on the Malacofauna of Hong Kong and Southern China, B. Hong Kong University Press, Hong Kong, pp 19–47
Ansell AD, Rhodes MC (1997) Unusual capabilities for surface movement in a normally deep-burrowed Antarctic bivalve. J Molluscan Stud 63:109–111
Ansell AD, Trueman ER (1967) Burrowing in Mercenaria mercenaria (L.) (Bivalvia, Veneridae). J Exp Biol 46:105–115
Brockington S (2001) The seasonal energetics of the Antarctic bivalve Laternula elliptica (King and Broderip) at Rothera Point, Adelaide Island. Polar Biol 24:523–530
Brown KM, Fraser KPP, Barnes DKA, Peck LS (2004) Links between the structure of an Antarctic shallow-water community and ice-scour frequency. Oecologia 141:121–129
Checa AG, Cadée GC (1997) Hydraulic burrowing in the bivalve Mya arenaria Linnaeus (Myoidea) and associated ligamental adaptations. J Molluscan Stud 63:157–171
Chou R, Lee HB (1997) Commercial fish farming in Singapore. Aquac Res 28:767–776
Clarke A (1983) Life in cold water: the physiological ecology of polar marine ectotherms. Oceanogr Mar Biol 21:341–453
Clarke A (2004) Is there a universal temperature dependence of metabolism? Funct Ecol 18:252–256
Clarke A (2006) Temperature and the metabolic theory of ecology. Funct Ecol 20:405–412
Clarke A, Fraser KPP (2004) Why does metabolism scale with temperature? Funct Ecol 18:243–251
Hammond KA, Diamond J (1997) Maximal sustained energy budgets in humans and animals. Nature 386:457–462
Harper E, Peck LS (2003) Feeding characteristics and metabolic costs in the Antarctic muricid gastropod Trophon longstaffi. Polar Biol 26:208–217
Johnston IA, Calvo J, Guderley H, Fernandez D, Palmer L (1998) Latitudinal variation in the abundance and oxidative capacities of muscle mitochondria in perciform fishes. J Exp Biol 201:1–12
McLachlan A, Young N (1982) Effects of low temperature on the burrowing rates of four sandy beach molluscs. J Exp Mar Biol Ecol 65:275–284
McLachlan A, Jaramillo E, Defeo O, Dugan J, de Ruyck A, Coetzee P (1995) Adaptations of bivalves to different beach types. J Exp Mar Biol Ecol 187:147–160
Morton B (1973) The biology and functional morphology of Laternula truncata (Lamark 1818) (Bivalvia: Anomalodesmata: Pandoracea). Biol Bull 145:509–531
Morton B (1976) The structure, mode of operation and variation in form of the shell of the Laternulidae (Bivalvia: Anomalodesmata: Pandoracea). J Molluscan Stud 42:261–278
Peck LS (1998) Feeding, metabolism and metabolic scope in Antarctic marine ectotherms. In: Pörtner HO, Playle R (eds) Cold Ocean Physiology. Cambridge University Press, Cambridge, pp 365–390
Peck LS (2002) Ecophysiology of marine ectotherms: limits to life. Polar Biol 25:31–40
Peck LS (2005) Prospects for survival in the Southern Ocean: vulnerability of benthic species to temperature change. Antarct Sci 17:495–505
Peck LS, Ansell AD, Webb KE, Hepburn L, Burrows MT (2004) Movements and burrowing activity in the Antarctic bivalve molluscs Laternula elliptica and Yoldia eightsi. Polar Biol 27:357–367
Peck LS, Convey P, Barnes DKA (2006) Environmental constraints on life histories in Antarctic ecosystems: tempos, timings and predictability. Biol Rev 81:75–109
Pörtner HO (2001) Climate change and temperature dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner HO (2002) Climate variations and the physiological basis of temperature dependent biogeography: tradeoffs in muscle design and performance in polar ectotherms. J Exp Biol 205:2217–2254
Pörtner HO, Mark FC, Bock C (2004) Oxygen limited thermal tolerance in fish? Answers obtained by nuclear magnetic resonance techniques. Respir Physiol Neurobiol 141:243–260
Quayle DB (1949) Movements in Venerupis (=Paphia) pullastra (Montagu). Proc Malac Soc Lond 28:31–37
Savazzi E (1990) Shell biomechanics in the bivalve Laternula. Lethaia 23:93–101
Stanley SM (1970) Shell form and life habits in the Bivalvia (Mollusca). Geol Soc Am Mem 125:1–296
Tan KS, Oh TM (2002) Feeding habits of Chicoreus capucinus (Neogastropoda: Muricidae) in a Singapore mangrove. Boll Malacol Suppl 4:43–50
Trueman ER (1966) Bivalve mollusks: fluid dynamics of burrowing. Science 152:523–525
Trueman ER, Ansell AD (1969) The mechanisms of burrowing into soft substrata by marine animals. Ocenogr Mar Biol Ann Rev 7:315–366
Trueman ER, Brand AR, Davis P (1966) The dynamics of burrowing in some common littoral bivalves. Proc Malac Soc Lond 37:97–109
Yamahira K, Conover DO (2002) Intra-vs. interspecific latitudinal variation in growth: adaptation to temperature or seasonality? Ecology 83:1252–1262
Zamorano JH, Duarte WE, Moreno CA (1986) Predation upon Laternula elliptica (Bivalvia, Anatinidae): a field manipulation in South Bay Antarctica. Polar Biol 6:139–143
Acknowledgments
We thank the staff of Biodiversity Centre and Sungei Buloh Wetland Reserve, National Parks Board, Singapore for facilitating this project. S.A.M was funded by Antarctic Funding Initiative grant 2/34 awarded to L.S.P. The studies undertaken comply with the laws of UK and Singapore. Two anonymous reviewers provided comments which greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne.
Rights and permissions
About this article
Cite this article
Morley, S.A., Peck, L.S., Tan, K.S. et al. Slowest of the slow: latitudinal insensitivity of burrowing capacity in the bivalve Laternula . Mar Biol 151, 1823–1830 (2007). https://doi.org/10.1007/s00227-007-0610-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-007-0610-7