Abstract
A total of 12 feeding experiments were conducted in the northern Gulf of Aqaba during spring (March/April) and autumn (September/October) 2002 at the Marine Science Station (MSS) in Aqaba. Females of three species of clausocalanids were selected: Clausocalanus farrani, C. furcatus and Ctenocalanus vanus. Natural occurring particle (NOP) larger than 5 μm were investigated as food source. The ambient chlorophyll a concentration at sampling depth (∼70 m) ranged between 0.15 and 1.00 μg chl a l−1 and NOP concentrations ranged between 1.78 and 14.0 × 103 cells l−1 during the sampling periods. The division of particles into five size classes (5–10, 10–20, 20–50, 50–100 and >100 μm) revealed that most of the particles were found in the size classes below 50 μm (81–98%), while most of the natural occurring carbon (NOC) was concentrated in the size classes larger than 20 μm (70–95%). Ingestion rates were food density dependent rather than size dependent ranging between 0.02 and 1.65 × 103 NOP ind−1 day−1 and 0.01 and 0.41 μg NOC ind−1 day−1, respectively, equivalent to a body carbon (BC) uptake between 0.4 and 51.8% BC day−1. The share of the size classes to the total ingestion resembled in most cases the size class composition of the natural particle community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In subtropical and tropical pelagic environments small calanoid copepods often form a dominant zooplankton group (e.g. Hopcroft et al. 1998). The genus Clausocalanus of the family Clausocalanidae is one of the most dominant and widespread taxon within the small-sized calanoids (Frost and Fleminger 1968). They are found in high numbers, for example in the Caribbean (Webber and Roff 1995), the Sargasso Sea (Schulz 1986), the Mediterranean (Mazzocchi et al. 1997) and the Gulf of Aqaba (Almeida Prado-Por 1983, 1985). Despite its wide distribution, the ecology of Clausocalanus has received little attention in comparison to its neritic and temperate counterpart Pseudocalanus (e.g. Poulet 1973, 1974, 1976; Ohman 1990; Dagg et al. 1998).
Historically, small calanoid copepods were considered herbivorous which had to be revised after increasing evidence of omnivory was found (for review see Kleppel 1993). Protozoans, especially ciliates, are known to be an important food source to small calanoids (Kleppel et al. 1988; Stoecker and Mc Dowell Capuzzo 1990; Gifford 1991; Calbet and Landry 1999; Levinsen et al. 2000; Broglio et al. 2004). The importance of microzooplankton as a food source is extremely important in subtropical and tropical oligotrophic oceanic waters where most of the primary production is provided by pico-sized phytoplankton (<2 μm) (e.g. Campbell and Vaulot 1993; Lindell and Post 1995). This group is generally unavailable as a direct food source for mesozooplankton because of its small size. Nonetheless, the picoplankton is indirectly used by copepods via additional steps (protozoans) in the food chain. Feeding experiments with natural occurring particles (NOP) are more difficult to interpret compared to experiments using cultured phyto- or microzooplankton due to the diversity of particles, but certainly give a more realistic reflection on in situ copepod feeding. Feeding experiments of Pseudocalanus minutus with NOP have indicated that copepods seem to be rather unselective and opportunistic in what they ingest (e.g. Poulet 1973, 1974, 1976; Dagg and Grill 1980; Huntley 1981; Turner 1991). Also, it has been noted that the feeding behaviour alters with the seasonal changes in particle concentration (Poulet 1978; Dagg and Grill 1980; Kleppel et al. 1988). Hence, the copepods have to be able to adapt rapidly to changing natural particle composition.
The Gulf of Aqaba is a unique environment characterised by stronger seasonal fluctuations than other subtropical seas (Reiss and Hottinger 1984). Deep vertical mixing (down to more than 600 m) and repletion of nutrients in winter (December–March) is followed by summer stratification and depletion of nutrients in the euphotic zone. The changes in the mixing and stratification trigger a seasonal succession of the phytoplankton and microzooplankton community (Kimor and Golandsky 1977). During winter, diatoms and dinoflagellates are abundant due to the increased nutrient levels, but the main primary producers during summer and autumn are ultraplankton (<8 μm) which consist of Prochlorococcus, Synechococcus and small eucaryotic algae (Lindell and Post 1995; Sommer 2000). The calanoid copepod community of the Gulf of Aqaba is dominated by small-sized epipelagic species (Almeida Prado-Por 1990) in which the clausocalanids play a significant role. The Gulf of Aqaba is inhabited by the following clausocalanid species: Ctenocalanus vanus, Clausocalanus farrani and Clausocalanus furcatus (Almeida Prado-Por 1990) as well as Clausocalanus minor (this study). Only few investigations have included data of feeding rates for Clausocalanus spp. and Ctenocalanus vanus (e.g. Kleppel et al. 1988; Peterson et al. 1990; Broglio et al. 2004). The most detailed studies were conducted on Clausocalanus furcatus (Mazzocchi and Paffenhöfer 1998, 1999). No feeding data are available for Clausocalanus farrani so far.
Feeding studies are often conducted under laboratory conditions with high quality foods where the concentration of food particles can be increased to a saturated feeding level. This is not applicable to the natural pelagic system where the food source comprises many different types and sizes of particles. Therefore we chose incubation experiments to determine ingestion rates of copepods with natural particle concentrations and composition. This study presents the first results on the feeding ecology of females of Clausocalanus farrani, Clausocalanus furcatus and Ctenocalanus vanus on naturally occurring particles (NOP) in the Gulf of Aqaba during spring and autumn in 2002. It will be considered to what extent particle size selectivity, composition or concentration influence the feeding patterns of the selected species.
Methods
A total of 12 experiments were conducted in the northern Gulf of Aqaba in spring (February–April) and autumn (September–October) 2002. NOP were used as a food source to obtain information on the natural feeding preferences of the dominant calanoid copepod species Clausocalanus farrani, Clausocalanus furcatus and Ctenocalanus vanus. Incubation experiments were conducted within the facilities of the Marine Science Station (MSS) in Aqaba, Jordan.
Sampling
Sampling for the experiments took place in the open waters of the northern Gulf of Aqaba off the MSS (N29°27.868, E34°57.872) during daytime (Fig. 1; Table 1). Water samples were collected with 10 l Niskin water samplers at ∼70 m depth, which was within the chlorophyll a maximum layer (Levanon-Spanier et al. 1979; A. Cornils, unpublished data). A CTD was run every time to determine the ambient water temperature (0–100 m). The copepods were obtained with a 200 μm mesh Nansen net equipped with a flow meter towed vertically from 100 m depth to the surface. The clausocalanid copepods are found in the upper 100 m throughout most of the year (A. Cornils and V. Farstey, unpublished data). Two net hauls were taken for each experiment. The first haul was preserved in 4% buffered formalin for quantitative analysis. The content of the second was carefully transferred into a large bucket and brought to the laboratory for the experiments.
Experiments
Females of Clausocalanus farrani, Clausocalanus furcatus and Ctenocalanus vanus were separated from the net sample, transferred into 250 ml beakers of screened seawater (200 μm sieve to remove larger zooplankton) and kept there for 1–2 h to acclimatisation. Only free swimming specimens with intact antennae were selected for the experiments. For each species three experimental bottles and three control bottles (no copepod specimens) were prepared. Nalgene bottles (2.7 l), previously rinsed with 10% HCl, were filled with the 200 μm screened sea-water samples. Nutrients (NO3, PO4, SiO3) and vitamins were added to prevent limitation of phytoplankton growth. We incubated 20–25 pre-adjusted copepod specimens per experimental bottle for 8–24 h. In the first round of experiments (March/April), incubation took place on a plankton wheel revolving at 1 rpm in dim light during day hours and total darkness at night. During the hot season (September/October) a shaded water-bath with a flowing sea-water was necessary to ensure constant temperature during the experiments. Bottles of the latter experiments were turned every few hours in order to prevent settlement. The temperature during the experiments was maintained within 2°C of the ambient conditions (March/April 21, 22°C and September/October 24, 25.5°C).
Particle concentration was determined at the start and the end of the experiments. Two litres subsamples were filtered onto Whatman GF/F filter paper and frozen for chlorophyll a measurements. Single 500 ml subsamples were fixed with Lugol’s iodine solution for NOP counts. Copepods were removed after the experiment, screened for mortality and transferred to pre-weighed tin caps for CN-measurements.
Analysis
The GF/F filters for chlorophyll a analysis were transferred to 10 ml of 90% acetone, homogenised and extracted for 24 h. Subsequently, the fluorescence was measured (without acidification) with a spectrofluorometer (SFM 25 BIO-TEK KONTRON Instruments). The 500 ml subsamples for NOP counts were placed in cylinders to reduced the sample volume. Particle settlement was allowed over a period of 48 h. Afterwards, the volume of the sample was reduced to 100 ml by taking out the excessive water from the surface with a syringe and the sample was transferred to an 100 ml Utermöhl sedimentation chamber (Utermöhl 1958). After 24 h of particle settlement the sedimentation cylinder was removed and one half of the Utermöhl chamber was counted for all naturally occurring particles at a magnification of 400×; using an inverted microscope (Zeiss Axiovert 35). The number of NOP was then calculated for 1 l. Determination of taxa was done according to Massuti and Margalef (1950), Brandt and Apstein (1964), Drebes (1974), Pankow (1990) and Tomas (1993, 1995). Individual cells were identified, measured (spherical diameter), grouped into five taxa: Ciliates, Dinoflagellates, Diatoms, Flagellates and others. All cells were also divided into five size classes: 5–10, 10–20, 20–50, 50–100 and >100 μm (Table 2) according to Poulet (1978), Cowles (1979) and Uye and Kasahara (1983). The classification of size classes was made under the assumption that copepods in oligotrophic regions are non-selective feeders (Poulet 1974, 1976). First results of feeding experiments in the Gulf of Aqaba with small copepods (4.36 ± 1.02 μg C ind−1) showed higher clearance rates for particles larger than 10 μm (Sommer et al. 2002). Particles with a spherical diameter of less than 5 μm were therefore neglected since they could not be counted quantitatively with inverted light microscopy and have been claimed to be inefficiently fed on by copepods. Boyd (1976) stated that Clausocalanus arcuicornis retains only 50% of particles smaller than 8 μm, but virtually all particles above 20 μm. Carbon content (μg l−1) estimates were made according to Smetacek (1975) referring to similar genera of the same size. The derived natural occurring carbon (NOC) will only approximate the real carbon content, because the conversion factors for carbon were taken from another ecosystem.
For each experiment, the growth rate, the grazing coefficient, the mean cell concentration, the filtration rate and the ingestion rate were calculated according to the equations of Frost (1972). Selectivity was investigated according to Chesson (1983). The carbon content (body carbon) of the incubated copepod specimens was measured with a CHN-Analyser (EuroEA3000). The tin caps with the copepods were dried for 24 h, weighed and then combusted in the CHN-Analyser. The results were used to calculate the percentage of body carbon uptake per day.
Fatty acid and stable isotope analysis
For the fatty acid analysis a varies number of females of Clausocalanus farrani, Clausocalanus furcatus and Ctenocalanus vanus were obtained from the net samples in autumn 2002 and 2003 (see sampling method). The sorted females were immediately stored in glass vials at −80°C and before the analysis pooled in groups of 300 females. Lipids were extracted using chloroform:methanol (2:1) with 0.01% butylhydroxytoluene added as antioxidant. For the gas–liquid chromatographic analyses of the fatty acids and alcohol compositions, aliquots of the extracted samples were taken. Methyl esters of fatty acids and free fatty alcohols were obtained by transesterification with 3% sulphuric acid in methanol for 4 h at 80°C. After their extraction with hexane the composition was analysed with a gas–liquid chromatograph (HRGC 5300) using temperature programming according to the method of Kattner and Fricke (1986). Fatty acids and free alcohols were identified with known standards. For analytical details refer to Hagen (2000), Kattner and Fricke (1986), and Kattner et al. (1994).
For the stable isotope analysis 100 females were taken from the net sample in autumn 2002 (sampling described above) and stored on 25 mm GFF filter paper at −80°C. Stable isotope analysis and concentration measurements of nitrogen and carbon were performed simultaneously with a THERMO/Finnigan MAT Delta plus isotope ratio mass spectrometer, coupled to a THERMO NA 2500 elemental analyzer via a THERMO/Finnigan Conflo II-interface. Stable isotope ratios are given in the conventional delta notation (δ13C/δ15N) relative to atmospheric nitrogen (Mariotti et al. 1984) and PDB (PeeDee Belemnite standard). Standard deviation for repeated measurements of lab standard material (peptone) is better than 0.15‰ for nitrogen and carbon, respectively. Standard deviations of concentration measurements of replicates of our lab standard are <3% of the concentration analysed.
Results
Ambient conditions
In spring 2002, the chlorophyll a concentration at the sampling depth of 70 m varied between 0.26 and 1.00 μg l−1 in a random fashion, whereas in autumn 2002 it decreased from 0.42 to 0.15 μg l−1 within the study period (Table 1). The NOP concentration was highest during March (07–26) and on April 16, varying between 2.92 and 14.0 × 103 cells l−1, while in autumn NOP concentrations ranged between 1.78 and 6.56 × 103 cells l−1 with highest values recorded on September 30. The estimated natural occurring carbon (NOC) concentration varied in spring 2002 between 0.86 and 2.47 μg C l−1, and in autumn 2002 between 1.28 and 1.67 μg C l−1 (Table 1).
The natural sea-water samples consisted mainly of ciliates, diatoms, dinoflagellates and flagellates, varying throughout the experimental periods (Fig. 2). Highest number of cells were found for dinoflagellates (698–7,704 cells l−1) and for diatoms (124–8,496 cells l−1), followed by ciliates (72–2,160 cells l−1) and flagellates (118–2,652 cells l−1). The remaining particles consisted of silicoflagellates, coccolithophorids, foraminiferas, radiolarians and nauplii. In total, they contributed 96–2,820 cells l−1 to the NOP concentrations. In terms of biomass, most of the carbon was found within the ciliates (0.11–1.88 μg C l−1), diatoms (0.11–1.67 μg C l−1) and dinoflagellates (0.01–1.03 μg C l−1) (Fig. 2b). The carbon biomass of flagellates varied between (0.005–0.11 μg C l−1). Comparing the two sampling seasons, spring and autumn 2002, significant differences were only found for the abundance of ciliates (ANOVA posthoc test, P < 0.05) and flagellates (P < 0.01).
Particles smaller than 50 μm were most abundant (0.3–5.7 × 103 cells l−1; Fig. 3) and comprised 80.7–97.5% of the total abundance of particles. Particles greater than 50 μm occurred only in small concentrations between 0.1 and 1.6 × 103 cells l−1. The largest particles (>100 μm) were rarely found in the samples (on average 1.8%). Therefore, these particles were not included in the calculation of ingestion. With regard to the NOC concentrations, the size classes 20–50 and 50–100 μm made up most (70–95% or 0.07–1.66 μg C l−1) of the natural particle biomass larger than 5 μm (Fig. 3). Differences between the sampling seasons were not significant for the individual size classes (ANOVA posthoc test; P > 0.05).
Feeding experiments
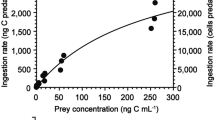
The ingestion rates of the clausocalanids in the Gulf of Aqaba varied between 0.02 and 1.65 × 103 cells ind−1 day−1 and showed no significant differences between the three species Ctenocalanus vanus, Clausocalanus farrani and Clausocalanus furcatus (ANOVA posthoc test; P > 0.05). The relationship between the ingestion rates and the food concentrations was significant only for Clausocalanus farrani and the NOP concentration (Fig. 4; Michaelis–Menten correlation; r2 = 0.813, P < 0.05), but neither for Clausocalanus furcatus nor Ctenocalanus vanus (ANOVA posthoc test; P > 0.05). Overall, Clausocalanus furcatus showed lower ingestion rates than the other two species, except for autumn where Clausocalanus farrani did not appear to feed much, but its ingestion rates in spring were comparable with those conducted in autumn at a similar food concentration.
Ingestion rates of the females of Clausocalanus farrani, Clausocalanus furcatus and Ctenocalanus vanus as a function of average cell concentration for a chlorophyll a, b NOP, c NOC and d % body carbon. Numbers display Michaelis–Menten correlations between cell concentrations and ingestion rates (significance levels of 0.05); IR Ingesti on rates, open circle spring 2002, filled triangle autumn 2002
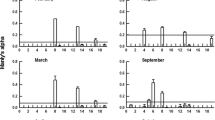
In Fig. 5 the contribution of the prey taxa to the clausocalanid diet as a relation of their relative availability is presented. In spite of the variance of the data, the clausocalanids fed on all abundant prey taxa (ciliates, diatoms, dinoflagellates, flagellates), with slight differences in their dietary preferences (Fig. 5a): Clausocalanus farrani and C. furcatus ingested preferably Ciliates and Dinoflagellates (i.e. most values were above the 1:1 line). No preference could be detected for diatoms and flagellates. For C. farrani, the percentages of the ingestion rates showed a scattered response (i.e. equal number of values on both sides of the 1:1 line). For C. furcatus, diatoms and flagellates were not selectively fed on (i.e. all values were below the 1:1 line). Ctenocalanus vanus fed preferably on diatoms and dinoflagellates, while the percentages of ingestion for ciliates and flagellates were scattered around the 1:1 line. Hence, the feeding of C. vanus revealed slight difference to the two Clausocalanus species. The remaining cells including coccolithophorids and silicoflagellates seem to be also on the prey list of the clausocalanids.
The relative ingestion of organic carbon showed a widespread response found for the abundant ciliates, diatoms and dinoflagellates, whereas flagellates were positively selected (Fig. 5b).
The prey contribution in terms of size classes revealed a scattered response both in the uptake of natural occurring particles and organic carbon, except for the smallest size class of 5–10 μm where most values for all three species were situated below the 1:1 line (Fig. 6). In terms of particles the major part of the ingestion took place in the size classes below 50 μm (Fig. 6a). For carbon the picture is reversed as the main carbon uptake was found for the particles larger than 20 μm (Fig. 6b).
Figures 5 and 6 both revealed, that the females of Ctenocalanus vanus, Clausocalanus farrani and Clausocalanus furcatus fed mainly on the abundant food particles in the water column. Selectivity indices, calculated according to Chesson (1983), revealed no preference of food type. Therefore, it can be assumed that the diet of the selected females is diverse and depends primarily on food abundance rather than food type.
Statistical tests (ANOVA posthoc test; P > 0.05) between the long (24 h) and the short (8–12 h) experiments as well as between experiments incubated on the plankton wheel and in the water tank showed no significant differences. Therefore, methodological bias in the ingestion rate results can be ruled out.
Fatty acid composition
The composition of the fatty acids showed no significant differences between Ctenocalanus vanus, Clausocalanus farrani and Clausocalanus furcatus (Table 2).
The results of the fatty acid analysis provided further evidence for the feeding pattern of the clausocalanids. High proportions of 16:0, 20:5(n − 3), 22:6(n − 3) and to lesser extent 18:0 and 18:1(n − 9) fatty acid showed the dominance of membrane lipids (phospholipids). Another evidence for the dominance of membrane lipids is the low amount of storage lipids, which are dominated by short-chained alcohols. In fact, only short-chained fatty alcohols were found for the three species (14:0, 16:0, 18:0). Prey type selection could not be detected within the three species, as no specific biomarkers were found.
δ15N and δ13C
The investigated females of three abundant clausocalanid copepods in the Gulf of Aqaba had similar shapes, sizes (prosoma length: 0.74–0.81 mm; A. Cornils et al., submitted) and weights. The carbon content of Ctenocalanus vanus was 4.12 ± 0.44 μg C ind−1, close to Clausocalanus farrani with 4.20 ± 0.99 μg C ind−1. Clausocalanus furcatus was the lightest with 3.69 ± 0.43 μg C ind−1.
The stable isotopes δ15N and δ13C were analysed for all three species (females) in autumn 2002 showing a similar range for all species with two exception (Fig. 7). The δ15N ranged between 0.7 and 5.3‰ for Ctenocalanus vanus, from 1.35 to 3.63‰ for Clausocalanus farrani and between 0.1 and 3.5‰ for Clausocalanus furcatus. The δ13C varied for Ctenocalanus vanus between −22.0 and −21.2‰, for Clausocalanus farrani between −23.0 and −20.6‰ and for Clausocalanus furcatus between −23.4 and −20.7‰. The δ15N and δ13C signatures overlapped greatly between the three species (Fig. 7a). The C/N ratio of the three species varied between 5 and 8 (Fig. 7b). For Clausocalanus farrani and Clausocalanus furcatus there seemed to be a negative linear relationship between δ13C and the C/N ratio. However, this relationship was only significant for Clausocalanus farrani (linear regression: r2 = 0.78; P < 0.05). The δ13C of Ctenocalanus vanus did not change with increasing C/N ratio.
Discussion
The present study strengthens the so far limited data base on clausocalanid feeding and provides further evidence that the subtropical species Clausocalanus spp. and Ctenocalanus vanus are well adapted to their oligotrophic environment. To the best of our knowledge, the analysis of the fatty acid composition and the proportions of δ15N and δ13C of clausocalanids are the first so far published. Only females were selected for the study, since the males of the clausocalanids have reduced mouthparts (Frost and Fleminger 1968; Heron and Bowman 1971) and are therefore unlikely to feed. Clausocalanids are epipelagic copepods and thus mainly found in the euphotic zone (e.g. Almeida Prado-Por 1990; Hure and Scotto di Carlo 1970), where the primary producers and consequently most of the heterotrophic protists are located (Kimor and Golandsky 1977).
Comparisons with the few data available on feeding rates for either Clausocalanus spp. or Ctenocalanus spp. revealed that the ingestion rates of the present study correspond well with the results of experiments with natural occurring particles as well as with algal monocultures (Table 3). The low uptake rates for Clausocalanus furcatus (less than 4.5% BC day−1) compare well with laboratory findings of Mazzocchi and Paffenhöfer (1998) with dinoflagellates as food source (Table 3). However, the latter ratios increased with rising cell concentrations. Mazzocchi and Paffenhöfer (1999) reported that low ratios were found despite their observations of high swimming activity. They suggested that continuous movement might be energetically better than ‘stop-and-go’ movement. Broglio et al. (2004) found rates for Clausocalanus spp. between 10 and 40% BC day−1 in the NW Mediterranean, which is in the range of Clausocalanus farrani in this study (Table 3). Under natural conditions, the daily ration can vary over a wide range, which has also been shown for other small calanoid species (e.g. Centropages typicus, 4–70%; Dagg and Grill 1980).
Ctenocalanus vanus, Clausocalanusfarrani and Clausocalanusfurcatus belong to the most dominant calanoid copepod species in the northern Gulf of Aqaba (Almeida Prado-Por 1990; Cornils 2007). During both spring and autumn 2002 the uptake of body carbon was less than 40%. The investigated females showed no significant differences in the feeding pattern of ingestion between both seasons which might be due to the fact that the size class composition hardly changed. The total ingestion rates for chlorophyll a, NOP, NOC and %BC were rather connected with the mean food concentrations. However, this pattern was significant only for the NOP uptake of Clausocalanus farrani. The mean cell concentrations were too low to show a clear increase in ingestion. The impact of size classes on the ingestion rates of NOP and NOC revealed that the females fed mainly on small sized food items between 5 and 20 μm in terms of abundance while the main carbon uptake was found in the size classes larger than 20 μm. The small size classes were dominated by dinoflagellates and other “flagellates” while the larger size classes were dominated by diatoms and ciliates.
Gut content analysis of Clausocalanus spp. in the subtropical Atlantic at the Great Meteor Seamount revealed that the guts were filled with mainly unidentifiable particles, and histological pictures showed a good nutritional condition which is also an indication for the good adaptation of clausocalanids to low phytoplankton biomass (Fischer 2005). Feeding in the Gulf of Aqaba takes place predominantly on naked cells which includes small dinoflagellates, other flagellates and ciliates, as revealed in our study. Kleppel et al. (1988) investigated the gut pigments of Clausocalanus spp. and found mainly carotenoids of protozoans followed by carotenoids of dinoflagellates. Al-Najjar (2000) found mainly the pigment peridinin as a marker for dinoflagellates in the guts of small-sized calanoid copepods in the northern Gulf of Aqaba.
Poulet (1974) assumed from his results that some copepods are able to adapt fast to changes in the structure of food sources. This enables the copepods to switch between size classes. This pattern may also be important for the clausocalanids. They live in an oligotrophic environment where the primary producers in the food web are dominated by not directly available ultraplankton (Lindell and Post 1995) and where medium-sized phytoplankton is scarce (Sommer 2000). Therefore, the microzooplankton plays an important role as link between the small primary producers and the mesozooplankton (Gifford 1991; Calbet and Landry 1999). In addition, clausocalanids have short life spans with 4–5 generations each year (Gaudy 1972; Shmeleva and Kovalev 1974). Selective feeding would inhibit the development of a continuous population, but the clausocalanids are one of the most successful calanoid group in the subtropical and tropical regions (Frost and Fleminger 1968). Therefore, non-selectivity and omnivory are a good strategy in subtropical waters with low food concentrations. The fatty acid composition and the range of the δ15N and δ13C ratios indicate also non-selective feeding. Most of the detected fatty acids can be synthesized by the copepods themselves. The fatty acids used as biomarkers for specific prey types (e.g. diatoms, dinoflagellates) were not found in particularly high amounts, suggesting that the clausocalanids feed continuously, anindication is also the lack of storage lipids (Lee and Hirota 1973), and have a high turnover rate. The range of the δ15N and δ13C values indicates that the selected species feed on a similar spectrum of prey items. Generally, an enrichment of 3.4‰ in δ15N is suggested per trophic level (Ehleringer et al. 1986; Post 2002), but for zooplankton often a smaller step-size has been found (Fry and Quinones 1994). δ15N signatures for Clausocalanus furcatus and Ctenocalanus vanus cover a wider range, indicating a feeding strategy on more than one trophic level. While the δ15N values are comparable in their range to omnivor species from other, e.g. polar oceans, the δ13C values are distinctly higher (see for comparison: Schmidt et al. 2003), indicating a lower lipid content.
Non-selectivity under conditions of low food concentration has been reported by other authors (e.g. Poulet 1976, 1978; Cowles 1979; Huntley 1981) as well as omnivory (Paffenhöfer and Knowles 1980). Poulet (1976) claimed that the copepod Pseudocalanus minutus was feeding on the dominant food type at high food concentrations, whereas they feed on a broader size range at low food concentrations. Omnivory has been shown in several publications with selection for microzooplankton, for example ciliates (Kleppel et al. 1988; Batten et al. 2001; Halvorsen et al. 2001; Broglio et al. 2004). However, we found no indication of selection of ciliates in our investigation. Probably, non-living particles also play an important role as food source in regions with low living particle concentrations. The carbon–chlorophyll ratio in faecal pellets of Clausocalanus arcuicornis from Onagawa Bay (Japan) suggested that non-phytoplankton particles, presumably detritus, constitute a dominant fraction of particulate materials in their diet (Ayukai 1990). However, this pattern has not been investigated in this study.
The incubation of the experiments took place under different conditions and might therefore be susceptible to errors. One possible source of error was the duration of the experiments, which varied between 8 and 24 h. Gut content analysis of clausocalanids revealed a diel variation of feeding with high values at midnight and dawn (Mayzaud et al. 1984; Kleppel et al. 1988; Landry et al. 1994; Atkinson et al. 1996; Halvorsen et al. 2001; Fischer 2005). Another source of error in our NOP incubation experiments may be due to microzooplankton grazing. In oligotrophic pelagic systems the microzooplankton is known to play an important role inside the food web (Gifford 1991). Recent investigations of microzooplankton grazing in the Gulf of Aqaba showed that microzooplankton grazing rates were high, but declined with algal size (Sommer et al. 2002; N. Schaaf, unpublished data). They showed that the microzooplankton was responsible for the major loss of primary production. However, Nejstgaard et al. (2001a, b) offered a calculative solution for the problem of microzooplankton feeding in incubation experiments for mesozooplankton, conducting simultaneously grazing experiments of both. They included the potential loss of microzooplankton grazing in the copepod incubations. Due to the chosen densities of copepods in our experiments we assume that microzooplankton grazing did not interfere significantly with our findings.
In conclusion, clausocalanids seem to be omnivorous, non-selective feeders on the abundant particles, which fits the oligotrophic conditions of the Gulf of Aqaba with its dominance of ultraplankton. The overall results of this study suggest that the clausocalanids are highly adapted to subtropical, oligotrophic regions.
References
Al-Najjar T (2000) The seasonal dynamics and grazing control of phyto- and mesozooplankton in the northern Gulf of Aqaba. PhD thesis, University of Bremen, Germany, pp 121
Almeida Prado-Por MS (1983) The diversity and dynamics of Calanoida (Copepoda) in the northern Gulf of Elat (Aqaba), Red Sea. Oceanol Acta 6:139–145
Almeida Prado-Por MS (1985) Distribution of the calanoid Copepoda along the Gulf of Elat (Aqaba), Red Sea. Rapp Comm Int Mer Médit 29:249–252
Almeida Prado-Por MS (1990) A diel cycle of vertical distribution of the Calanoidea (Crustacea: Copepoda) in the northern Gulf of Aqaba (Elat). Bull Inst Océanogr Monaco 7:109–116
Atkinson A, Shreeve RS, Pakhomov EA, Priddle J, Blight SP, Ward P (1996) Zooplankton response to phytoplankton bloom near South Georgia, Antarctica. Mar Ecol Prog Ser 144:195–210
Ayukai T (1990) Fecal pellet production by two species of planktonic calanoid copepods fed on maturally occurring particles. Bull Plankton Soc Jpn 8:167–169
Batten SD, Fileman ES, Halvorsen E (2001) The contribution of microzooplankton to the diet of mesozooplankton in an upwelling filament off the north west coast of Spain. Progr Oceanogr 51:385–398
Boyd CM (1976) Selection of particle sizes by filter-feeding copepods: a plea for reason. Limnol Oceanogr 21:175–180
Brandt K, Apstein C (1964) Nordisches plankton. VII. Protozoa. Asher & Co, Amsterdam
Broglio E, Saiz E, Calbet A, Trepat I, Alcaraz M (2004) Trophic impact and prey selection by crustacean zooplankton on the microbial communities of an oligotrophic coastal area (NW Mediterranean Sea). Aquat Microb Ecol 35:65–78
Calbet A, Landry MR (1999) Mesozooplankton influences on the microbial food web: direct and indirect trophic interactions in the oligotrophic open ocean. Limnol Oceanogr 44:1370–1380
Campbell L, Vaulot D (1993) Photosynthetic picoplankton community structure in the subtropical North Pacific Ocean near Hawaii (station ALOHA). Deep-Sea Res 40:2043–2060
Chesson J (1983) The estimation and analysis of preference and its relationship to foraging models. Ecology 64:1297–1304
Cornils A, Schnack-Schiel SB, Al-Najjar T, Badran MI, Rasheed M, Manasreh R, Richter C (2007) The seasonal cycle of the epipelagic mesozooplankton in the northern Gulf of Aqaba (Red Sea). J Mar Syst (in press)
Cowles TJ (1979) The feeding response of copepods from the Peru upwelling system: food size selection. J Mar Res 37:601–622
Dagg MJ, Frost BW, Newton J (1998) Diel vertical migration and feeding in adult female Calanus pacificus, Metridia lucens and Pseudocalanus newmani during a spring bloom in Dabob Bay, a fjord in Washington USA. J Mar Syst 15:503–509
Dagg MJ, Grill DW (1980) Natural feeding rates of Centropages typicus females in the New York Bight. Limnol Oceanogr 25:597–609
Drebes G (1974) Marines Phytoplankton. Eine Auswahl der Helgoländer Planktonalgen (Diatomeen, Peridineen). Georg Thieme, Stuttgart
Ehleringer JR, Rundel PW, Nagy KA (1986) Stable isotopes in physiological ecology and food web research. Trends Ecol Evol 1:42–45
Fischer L (2005) Der Einfluß der großen Meteorbank auf die Ernährungsbiologie und Verteilung dominanter Calanoida (Crustacea, Copepoda). Ber Polarforsch Meeresforsch 499:157
Frost BW (1972) Effects of size and concentration of food particles on the feeding behaviour of the marine planktonic copepod Calanus pacificus. Limnol Oceanogr 17:805–815
Frost BW, Fleminger A (1968) A revision of the genus Clausocalanus (Copepoda: Calanoida) with remarks on distributional patterns in diagnostic characters. Bull Scripps Inst Oceanogr Univ Cal 12:1–235
Fry B, Quinones RB (1994) Biomass spectra and stable isotope indicators of trophic level in zooplankton of the northwest Atlantic. Mar Ecol Prog Ser 112:201–204
Gaudy R (1972) Contribution a la connaissance du cycle biologique des copépodes du Golfe de Marseille 2. Étude du cycle biologique de quelques espéces caractéristique. Tethys 4:175–242
Gifford DJ (1991) The protozoen–metazoen trophic link in pelagic ecosystems. J Protozool 38:81–86
Hagen W (2000) Lipids. In: Harris R, Wiebe P, Lenz J, Skjoldal HR, Huntley M (eds) ICES zooplankton methodological manual. Academic, San Diego, pp 113–119
Halvorsen E, Hirst AG, Batten SD, Tande KS, Lampitt RS (2001) Diet and community grazing by copepods in an upwelled filament off the NW coast of Spain. Progr Oceanogr 51:399–421
Heron GA, Bowman TE (1971) Postnaupliar developmental stages of the copepod crustaceans Clausocalanus laticeps, C. brevipes and Ctenocalanus citer (Calanoida: Pseudocalanidae). In: LLano GA, Wallen JE (eds) Biology of the Antarctic Seas, 4. Ant Res Ser, Washington, pp 141–165
Hopcroft RR, Roff JC, Lombard D (1998) Production of tropical copepods in Kingston Harbour, Jamaica: the importance of small species. Mar Biol 130:593–604
Huntley M (1981) Nonselective, nonsaturated feeding by three calanid copepod species in the Labrador Sea. Limnol Oceanogr 26:831–842
Hure J, Scotto di Carlo B (1970) Distribuzione e frequenza delle specie del genere Clausocalanus Giesbrecht, 1888 (Copepoda: Calanoida) nel Golfo di Napoli e nell’Adriatico meridionale. Pubbl Staz Zool Napoli 38:289–304
Kattner G, Fricke HSG (1986) Simple gas-liquid chromatographic method for the simultaneous determination of fatty acids and alcohols in wax esters of marine organisms. J Chromatogr 361:263–268
Kattner G, Graeve M, Hagen W (1994) Ontogenetic and seasonal changes in lipid and fatty acid/alcohol composition of dominant Antarctic copepods Calanus propinquus, Calanoides acutus and Rhincalanus gigas. Mar Biol 118:637–644
Kimor B, Golandsky B (1977) Microplankton of the Gulf of Elat: Aspects of seasonal and bathymetric distribution. Mar Biol 42:55–67
Kleppel GS (1993) On the diets of calanoid copepods. Mar Ecol Prog Ser 99:183–195
Kleppel GS, Frazel D, Pieper RE, Holliday DV (1988) Natural diets of zooplankton off southern California. Mar Ecol Prog Ser 49:231–241
Landry MR, Peterson WK, Fagerness VL (1994) Mesozooplankton grazing in the Southern California Bight. I. Population abundances and gut pigment contents. Mar Ecol Prog Ser 115:55–71
Lee RF, Hirota J (1973) Wax esters in tropical zooplankton and nekton and the geographical distribution of wax esters in marine copepods. Limnol Oceanogr 18:227–239
Levanon-Spanier I, Padan E, Reiss Z (1979) Primary production in a desert—enclosed sea—the Gulf of Elat (Aqaba), Red Sea. Deep-Sea Res 26:673–685
Levinsen H, Turner JT, Nielsen TG, Hansen BW (2000) On the trophic coupling between protists and copepods in arctic marine ecosystems. Mar Ecol Prog Ser 204:65–77
Lindell D, Post AF (1995) Ultraphytoplankton succession is triggered by deep winter mixing in the Gulf of Aqaba (Eilat), Red Sea. Limnol Oceanogr 40:1130–1141
Lozan JL, Kausch H (1998) Angewandte Statistik für Naturwissenschaftler. Pareys Studientexte 74. Parey Buchverlag, Berlin. 2. Aufl., 287pp
Mariotti A (1984) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303:685–687
Massuti M, Margalef R (1950) Introduccion al estudio del Plancton marino. Patronata Juan de la Cierva de Investigacion tecnica (C. S. DE I. C.)
Mayzaud O, Mayzaud P, Bigne C, de la Grohan P (1984) Diel changes in the particulate environment, feeding activity and digestive enzyme concentration in neritic zooplankton. J Exp Mar Biol Ecol 84:15–35
Mazzocchi MG, Paffenhöffer GA (1998) First observations on the biology of Clausocalanus furcatus (Copepoda, Calanoida). J Plankton Res 20:331–342
Mazzocchi MG, Paffenhöfer GA (1999) Swimming and feeding behaviour of the planktonic copepod Clausocalanus furcatus. J Plankton Res 21:1501–1518
Mazzocchi MG, Christou ED, Fragopolou N, Siokou-Frangou I (1997) Mesozooplankton distribution from Sicily to Cyprus (Eastern Mediterranean): I. General aspects. Oceanol Acta 20:521–535
Nejstgaard JC, Hygum BH, Naustvoll LJ, Båmstedt U (2001a) Zooplankton growth, diet and reproductive success compared in simultaneous diatom- and flagellate-microzooplankton-dominated plankton blooms. Mar Ecol Prog Ser 221:77–91
Nejstgaard JC, Naustvoll LJ, Sazhin A (2001b) Correcting for underestimation of microzooplankton grazing in bottle incubation experiments with mesozooplankton. Mar Ecol Prog Ser 221:59–75
Ohman MD (1990) The demographic benefits of diel vertical migration by zooplankton. Ecol Monogr 60:257–281
Pankow H (1990) Ostseealgenflora. Gustav Fischer, Jena
Paffenhöfer GA, Knowles SC (1980) Omnivorousness in marine planktonic copepods. J Plankton Res 2:355–365
Peterson WT, Painting SJ, Hutchings L (1990) Diel variations in gut pigment content, diel vertical migration and estimates of grazing impact for copepods in the southern Benguela upwelling region in October. J Plankton Res 12:259–281
Post DM (2002) Using stable isotopes to estimate trophic position: models, methods and assumptions. Ecology 83:703–718
Poulet SA (1973) Grazing of Pseudocalanus minutus on naturally occurring particulate matter. Limnol Oceanogr 18:564–573
Poulet SA (1974) Seasonal grazing of Pseudocalanus minutus on particles. Mar Biol 25:109–123
Poulet SA (1976) Feeding of Pseudocalanus minutus on living and non-living particles. Mar Biol 34:117–125
Poulet SA (1978) Comparison between five coexisting species of marine copepods feeding on naturally particulate matter. Limnol Oceanogr 23:1126–1143
Reiss Z, Hottinger L (1984) The Gulf of Aqaba. ecological micropaleontology (ecologial studies 50). Springer, Berlin Heidelberg New York
Schmidt K, Atkinson A, Stübing D, McClelland JW, Montoya JP, Voss M (2003) Trophic relationships among Southern Ocean copepods and krill: some uses and limitations of a stable isotope approach. Limnol Oceanogr 48:277–289
Schulz K (1986) Aspects of calanoid copepod distribution in the upper 200 m of the central and southern Sargasso Sea in spring 1979. Syllogeus 58:459–466
Shmeleva AA, Kovalev AV (1974) Cycles biologique des copepodes (Crustacea) de la mer Adriatique. Bolletino di Pesca Piscicoltura e Idrobiologia 29:49–70
Smetacek V (1975) Die Sukzession des Phytoplanktons in der westlichen Kieler Bucht. PhD Thesis, University of Kiel, Germany
Sommer U. (2000) Scarcity of medium-sized phytoplankton in the northern Red Sea explained by strong bottom-up and weak top-down control. Mar Ecol Prog Ser 197:19–25
Sommer U, Berninger UG, Böttger-Schnack R, Cornils A, Hagen W, Hansen T, Al-Najjar T, Post AF, Schnack-Schiel SB, Stibor H, Stübing D, Wickham S (2002) Grazing during early spring in the Gulf of Aqaba and the northern Red Sea. Mar Ecol Prog Ser 239:251–261
Stoecker DK, McDowell Capuzzo J (1990) Predation on Protozoa: its importance to zooplankton. J Plankton Res 23:891–908
Tomas CR (1993) Marine phytoplankton—a guide to naked flagellates and coccolithophorids. Academic, San Diego
Tomas CR (1995) Identifying marine diatoms and dinoflagellates. Academic, San Diego
Turner JT (1991) Zooplankton feeding ecology: do co-occuring copepods compete for the same food? Rev Aquat Sci 5:101–195
Utermöhl H (1958) Zur Vervollkommnung der quantitativen Phytoplanktonmethodik. Mitt int Ver theor angew Limnol 9:1–38
Uye SI, Kasahara S (1983) Grazing of various developmental stages of Pseudodiaptomus marinus (Copepoda: Calanoida) on natural occurring particles. Bull Plankton Soc Jpn 30:147–158
Webber MK, Roff JC (1995) Annual structure of the copepod community and its associated pelagic environment off Discovery Bay, Jamaica. Mar Biol 123:467–479
Acknowledgments
We thank the director of the Marine Science Station (MSS) in Aqaba, Dr. M. Badran, and the staff for their kind welcome and their help in sampling and analysis, especially Ehab Eid, Maysara Emor, Khaled Al-Tarabeen, Hassan Abu-Taleb and the late Said H. Sirhan. Further we thank Eva-Maria Zetsche and Christian Undeutsch for their assistence in conducting the experiments, Janna Peters for the help in fatty acid analysis, and Ute Jacob for help in the interpretations of the stable isotope data. We also thank two anonymous referees for their comments and suggestions.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe.
Rights and permissions
About this article
Cite this article
Cornils, A., Schnack-Schiel, S.B., Böer, M. et al. Feeding of Clausocalanids (Calanoida, Copepoda) on naturally occurring particles in the northern Gulf of Aqaba (Red Sea). Mar Biol 151, 1261–1274 (2007). https://doi.org/10.1007/s00227-006-0569-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0569-9