Abstract
The pattern of diel vertical migration and the trophic interactions of moon jelly (Aurelia sp.) were investigated in the sea lakes of Mljet Island (Adriatic Sea) where this scyphomedusa is present throughout the year. Water column characteristics, plankton and in situ behaviour of Aurelia were followed over several 24-h cycles (6–8 times during each cycle) from the surface to the bottom (44 m). Aurelia exhibited a consistent pattern of diel vertical migration. Most of the time Aurelia were located at the bottom of the thermocline layer at temperatures lower than 19°C. Aurelia migrated towards the surface at dusk when the majority was found within the thermocline or just above it. During the night the medusae sank into the deepest layers below 25 m. The main medusa food items inferred from stomach contents were small adult copepods like Oithona nana and Paracalanus parvus and copepodites of small calanoids and cyclopids. In addition, in situ feeding experiments indicated high clearance rates for nauplii and naked ciliates and clear response of bacterial populations pointing to indirect cascade effects of Aurelia on microbial in addition to classical food web.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In many areas worldwide there is strong evidence for changes in plankton communities, over recent decades (Hays et al. 2005), an increase in the frequency and intensity of jellyfish outbreaks is among most evident (Purcell et al. 2001; Dumont et al. 2004). Some studies have indicated a correlation between high jellyfish abundance and increased eutrophication (Papathanasiou et al. 1987) but Purcell et al. (1999) argued that the connections between jellyfish population size and eutrophication are difficult to make because of the lack of appropriate historical data. Arai (2001) concluded that increases in coelenterates could rarely be unequivocally linked to eutrophication. Long-term fluctuations in jellyfish abundance have been associated with climatic changes (Brodeur et al. 1999; Mills 2001; Molinero et al. 2005), which, in turn, mediated trophic regime shifts (Lynam et al. 2004). The decline of fish competitors due to over-fishing has also been suggested as factor favouring jellyfish and contributing to regime shift (Daskalov 2002; Sommer et al. 2002). When very numerous, jellyfish play an important trophic role (Mills 1995; CIESM 2001), if massive occurrences persist, ecosystem structure and functioning may change (Kideys 2002). Dramatic changes in zooplankton and a strong decline of the pelagic fishery have been attributed to predation and competition by pelagic coelenterates (Hay et al. 1990; Niermann 2004), although the significance of such interactions to either fish or jellyfish populations is still poorly understood (Purcell and Arai 2001).
Most studies of the trophic interactions of jellyfish and their impact concentrated on mesozooplankton. Despite some evidence of jellyfish preying on microzooplankton (Stoecker et al. 1987; Sullivan et al. 1994; Fukuda and Naganuma 2001) little is known about the cascading impact of jellyfish predation on the microbial food web, via indirect trophic linkages. Laboratory and in situ studies have demonstrated the influence of grazers of different size fractions (nano, micro and mesozooplankton) on the microbial food web (Azam et al. 1983; Calbet and Landry 1999). The heterotrophic part of the microbial food web appears to contain several trophic levels, representing a link to higher animals (Sherr and Sherr 1988; Wikner and Hagström 1988; Turk and Hagström 1997). Larger protists, copepods and other planktonic grazers are known to be the main predators on nanoflagellates, and these, in turn, are dominant bacteriovores (Stoecker and Capuzzo 1990; Verity 1991; Deibel and Lee 1992). Recent studies have described the ingestion of bacteria by the naupliar and juvenile copepodite stages of small copepod species, Cladocera, and pelagic tunicates (Turner et al. 1988; Deibel and Lee 1992; Bedo et al. 1993), which in turn may represent food items for jellyfish (Omori et al. 1995; Purcell and Sturdevant 2001; Barz and Hirche 2005). In any case, direct or indirect trophic interactions are likely to be central for understanding changes in jellyfish abundance as well as their effects on the marine ecosystem.
Among macroscopic jellyfish that recurrently appear in great densities, Aurelia is the most widespread. It forms large aggregations particularly in enclosed seas such as the Black Sea, the Baltic and the Seto Inland Sea, in protected coastal waters i.e. bays, fjords, estuaries and enclosed water bodies (Möller 1980; Hay et al. 1990; Hamner et al. 1994; Schneider and Behrends 1994; Omori et al. 1995; Ishii and Båmstedt 1998; CIESM 2001; Fukuda and Naganuma 2001; Kideys and Romanova 2001; Mutlu 2001; Xian et al. 2005). Swarms of Aurelia are more common in shallow cold and temperate regions with lower salinities; however, aggregations were also observed in warm to tropical euhaline waters (Hamner et al. 1982; Papathanassiou et al 1987; Avian and Rottini 1994; Dawson and Martin 2001). Despite many field and laboratory studies there are still controversies regarding the prey and feeding of Aurelia (Sullivan et al. 1994; Graham and Kroutil 2001) and consequently their effects on the plankton food web. Behavioural characteristics like the presence/absence of migration behaviour can also be expected to have important consequences for trophic interactions in environments where this species forms dense aggregations. There is evidence from field and laboratory results that Aurelia is flexible in its migration patterns (Mackie et al. 1981; Hamner et al. 1982, 1994), undertaking diurnal vertical migrations, no vertical migration and/or horizontal migrations in relation to sun orientation.
Our study of Aurelia’s vertical migration, feeding and the cascading effects of Aurelia predation were carried out in the Mediterranean euhaline enclosed water body, where dense swarms were observed (Benović et al. 2000). A seawater lake situated on the southern Adriatic island of Mljet has been part of a national park for more than 40 years; hosts a large Aurelia population with medusae presents throughout the year and thus provides an excellent study site without direct human impact. In particular, our study concentrates on Aurelia vertical migration in a stratified water column and the impact on microbial plankton that has been ignored in past studies of jellyfish trophic interactions.
Materials and methods
Study area
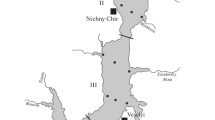
Veliko Jezero (Big Lake), a submerged karstic valley, is situated on the north-western side of Mljet, an offshore south Adriatic island that extends in a NW–SE direction (Fig. 1). Big Lake (BL) has a surface area of 1.45 km2 and a maximum depth of 46 m. Exchange between this enclosed marine lake and open Adriatic waters is established through a 1 km long, 10 m wide channel that has been artificially deepened to a depth of about 3.8 m (Ridžanović and Šimunović 1995). The whole north-western part of the island of Mljet including BL has been protected as a national park since 1960.
The environmental and biological characteristics of BL are mainly defined by lake bathymetry and restricted communication with the open sea through the channel. Tides are weak with a tidal range of <25 cm. The waters of BL are generally calm since BL is surrounded by shores with inclinations of 12°–32° towards the south and west, and 32°–55° towards north and east (Bognar and Čurić 1995). During the summer months a strong thermocline at a depth of around 12–20 m influences hydrographical, chemical and biological parameters (Vučetić 1957; Kršinić 1995). The water mass below this layer is characterized by stable conditions with low temperatures (10–12°C) and high salinity (37.5–38.5 psu) throughout the year. Higher values of nutrients and nanophytoplankton were measured in this layer compared to the surface (Benović et al. 2000). During late summer maximal surface temperatures are around 26°C but may reach 28°C. The salinity of the surface layer is also more variable (36.3–38.6 psu) compared to deeper layers. Phytoplankton structure and density are similar to that in neighbouring open Adriatic waters with a maximal abundance of microphytoplankton and nanophytoplankton of around 4.0 × 104 and 4.5 × 106 l−1, respectively (Jasprica et al. 1995). Jasprica (pers. comm.) identified 78 microphytoplankton species in lake, which is comparable to 83 species he found in neighbouring open Adriatic waters. In contrast, zooplankton in BL are characterised by lower abundances and a remarkably reduced number of species compared to neighbouring open Adriatic waters. For example: 22 species of copepods were found in BL versus 53 species at a location about 300 m from the opening of the Lake’s channel to open Adriatic waters. Abundances assessed using zooplankton net with 250-μm meshes was not that different (max. 581 in lake vs. 879 ind. m−3 outside, Lučić et al. 1995). Among copepods Oithona nana is the dominant species in BL with an annual average contribution to total mesozooplankton of 43% followed by Paracalanus parvus (26%) and Oithona similis (21%). Other Copepods (Calanus helgolandicus, Acartia clausi, Isias clavipes, Diaixis pygmaea) contributed less than 5% of total copepod abundance (Lučić, unpubl. data). Oithona nana dominates the surface and thermocline layers while Calanus helgolandicus, Diaixis pygmaea and Oithona similis are found in the deeper layer (>20 m). Other zooplankton groups that have been recorded in significant numbers throughout the year in BL are Oikopleura dioica, Sagita setosa, as well as Bivalvia and Gastropoda larvae. Copepod nauplia and ciliates are dominant microzooplankton groups that are most abundant in the surface 10 m (Kršinić 1995). An important component of the BL zooplankton that is absent in open Adriatic waters around offshore Mljet island, is the jellyfish Aurelia. This jellyfish is present in the shallow northern Adriatic and some semi-enclosed bays along eastern Adriatic. It has traditionally been termed A. aurita although molecular criteria used in recent studies questioned this designation (Schroth et al. 2002; Dawson 2003); therefore we prefer to use Aurelia sp. Abundance of Aurelia in BL may reach 10 ind. m−3 and up to a few hundred in swarms (Benović et al. 2000).
Environmental parameters, vertical distribution and stomach content of Aurelia sp.
The water column structure, plankton characteristics and vertical distribution of Aurelia, were studied in 2003 (4–18 July) and 2004 (16–23 May) in the two deepest areas of BL (Fig. 1, open circles a, b). The vertical distribution of temperature, salinity, and fluorescence were determined using a CTD fine-scale probe (University of Western Australia and a Sea-Tech inc. fluorometer). The vertical distribution of Aurelia was estimated using a Sony DCR-VX200E video camera enclosed in an underwater housing (Ikellite) equipped with two 100 W lights and a depth gauge. The dimension of the imaged area of the video camera was assessed according to Youngbluth and Båmstedt (2001) and was 2.3 m2. The camera was lowered at a speed of about 2 m min−1 until the bottom was visible. The depth of occurrence of medusae was recorded and the abundance of Aurelia was estimated in the laboratory. We used digital video playback and “frame by frame” mode to estimate Aurelia abundance and orientation. Afterwards medusae abundances were pooled in four separate layers according to CTD depth profiles: above thermocline, within thermocline, below thermocline and deep layer (below 25 m). In addition, the mean vertical position (MVP), i.e. weighted mean depth (Schabetsberger et al. 2000) of the Aurelia population in the water column was calculated using the method of Pearre (1973):
where
ni is the total number of Aurelia recorded over a depth range i (2 m), and
di is the mid-point of depth range i.
We also recorded radial orientation of medusae in the plane of image: medusae with aboral surface straight up and angled up (i.e. animals were swimming upwards either straight up or under an angle) were assigned to category up, individuals swimming straight down or angled down (aboral surface straight down and angled down) were assigned to category down, to last category horizontal were assigned individuals swimming in horizontal plane. Additional information on Aurelia’s vertical distribution was provided by divers who quantified the number of medusae in their field of view as none, rare, dense, and very dense. Medusae bell diameters were measured for Aurelia collected by divers during five video recordings. Individuals were spread flat on a glass plate and the bell diameter was recorded to the nearest 1 mm. Aurelia were collected individually by divers for stomach content analysis. Freshly collected animals were brought aboard the research boat where they were dissected immediately. Stomach, canals and gastric pouches we examined for prey organisms which were identified to genus or species level.
Aurelia feeding experiments
We carried out five in situ feeding experiments: 8 July, 10–11 July, 12–13 July 2003 and 18–19 May, 22 May 2004. Aurelia feeding experiments were carried out in large enclosures made of clear acrylic plastic with a volume of 110 l, and additionally, in polycarbonate enclosures with a volume of 8 l, which were all incubated in situ (Fig. 2). The natural plankton assemblage at ambient concentration was the food source for the enclosed Aurelia. On the basis of CTD and video camera casts we selected incubation depths for the large and small enclosures: the former were incubated within the thermocline layer (14 m in 2003, and 12 m in 2004) and the latter in the surface layer (5 m) and below the thermocline layers (25 m). Experiments in small enclosures were carried out only during 2003. Since no light/dark difference in the feeding rate was found (Bailey and Batty 1983) and in accordance with our observations of Aurelia migration towards the surface layers at dusk, we decided to start feeding experiments between 7 and 8 p.m.
The experimental set-up for in situ feeding experiments with Aurelia: an underwater photo of a large (110 l) enclosure (top) and a schematic presentation (bottom) of all enclosures. Squares on the schema indicate 110 l enclosures incubated at thermocline depth while circles represent 8 l nalgene bottles incubated above (5 m) and below (25 m) the thermocline. Enclosures marked with M contained 15 medusae (large enclosures) or 1 Aurelia (nalgene bottles); C indicates enclosures containing natural plankton assemblages only
Two large enclosures were lowered open (the top and bottom were removable) to a pre-selected depth where the bottoms were then sealed. Both enclosures thus contained plankton at that depth. Divers collected medusae individually close to the incubation depth and placed them gently in one enclosure while the second served as control and contained the natural plankton assemblage without medusae. After putting 15 Aurelia in one enclosure the tops of both were also sealed. In addition, plankton was pumped from 5 and 25 m depths into four 8 l Nalgene bottles; into two of these we added 1 Aurelia while two served as controls. These experimental bottles, one with Aurelia and one control, were incubated at depths above the thermocline (5 m) and below the thermocline (25 m) (see Fig. 2 for the experimental set-up schema and underwater photo of the large enclosures). Feeding experiments lasted for 6–7 h and changes in the abundance of the enclosed plankton assemblage were compared. Bell diameter of Aurelia used in feeding experiments varied between 7.5 and 10.1 (8.4 ± 0.7) cm for July 2003 experiments and 6.2 and 12.6 (9.2 ± 2.1) cm for May 2004 experiments. After recovering Aurelia, samples were taken for bacteria (50 ml), phytoplankton (1 l), and microzooplankton (5 l) from all enclosures. In addition, mesozooplankton samples were collected from the two large enclosures filtering all remaining water, after other samples were withdrawn, through 125 μm mesh. Clearance (F) for specific taxa was calculated (Båmstedt et al. 2000) from the equation:
where
C0 and Ct = number of prey organisms at time 0 and t, respectively,
t = incubation time (hours),
V = volume of the experimental bottle,
n = number of medusae.
Plankton collection and analyses
Plankton samples were collected at the beginning of each feeding experiment. Samples of phytoplankton, bacterioplankton, and microzooplankton were taken with a 5-l Niskin bottle at several depths according to the physical structure of the water column: above the thermocline (0.5, 5 m), in the middle of the thermocline layer (14 and 10 m in July 2003 and May 2004, respectively) and below the thermocline (25 m). Mesozooplankton were collected by vertical tows (a Nansen net equipped with a closing system, 125 μm mesh, 54 cm diameter) at depth intervals of 40–20, 19–10, 9–0 m as rough approximations of below thermocline, thermocline and above thermocline layers.
Formaldehyde-fixed samples of microzooplankton and mesozooplankton were analysed according to standard procedures (Postel et al. 2000). Seawater samples for heterotrophic bacteria counts were fixed with formalin (2% final concentration) and stained with 4′6-diamidino-2-phenylindole (DAPI) then filtered on 0.2 μm black polycarbonate filters (Poretics) (Porter and Feig 1980). Bacterial abundance was converted into carbon biomass using 19.8 fg C cell−1 as the conversion factor (Lee et al. 1987). Autofluorescent cyanobacteria were counted in green excitation light (Takahashi et al. 1985). Bacterial protein synthesis rates were measured using 3H-leucine by applying the centrifugation method described by Smith and Azam (1992). For each determination, three replicates containing seawater only were incubated with 3H-leucine (final concentration 20 nM) for 2 h. Incubation was stopped by adding concentrated trichloracetic acid (TCA) to a final concentration of 5%. In addition, two replicates were treated with TCA (final concentration of 5%) before the addition of 3H-leucine, and served as blanks. After incubation, all samples were centrifuged, aspirated, washed, and, after the addition of a scintillation cocktail (Ultima Gold, Packard), were counted in a scintillation counter (TR2500, Packard). Incorporation rates of 3H-leucine were converted to bacterial carbon production according to Simon and Azam (1989).
Feeding of Oithona nana on radio labelled bacteria
Feeding experiments and stomach content analyses indicated adults and copepodites of Oithona nana as important food items of Aurelia. Expanding upon this, during experiment in 2004 we tested the possibility of a direct trophic loop between these small copepods and bacteria using a radiolabeled culture of the natural bacterial assemblage from the lake to feed Oithona nana.
The work procedure was similar to that used by Turk and Hagström (1997) and described in detail by Båmstedt et al. (2000). The detailed experimental schema is shown in Fig. 3. The culture of bacteria for radiolabeling was prepared with 340 ml of seawater from BL filtered through 0.6 μm filters (Poretics membrane filters, Osmonics) to remove all bacteriovores. Bacteria were inoculated with 3H-thymidine (specific activity 82 Ci mmol−1) and then incubated in the dark at room temperature for 24 h. When the concentration of bacteria reached a density of approx. 10 × 108 l−1 the culture was divided into five subsamples and inoculated with 15 individuals of Oithona nana in 50 ml tubes (sterile Brand tubes). Replicate samples of the labelled bacteria were taken for initial measurement of isotope activity (expressed as disintegrations per minute, dpm). Oithona nana was isolated from natural plankton samples by the filtration of seawater through a plankton net with a 1 mm mesh size to remove larger organisms. In the filtrate, Oithona nana were picked out individually under a stereomicroscope (Olympus SZX9) and washed in prefiltered seawater (through 0.22 μm) three times successively. They were starved in particle and organism free seawater (0.22 μm filtered seawater) for 2 h before the experiment with the labelled bacteria. Fifteen individuals of Oithona nana were added to each tube containing labelled bacteria in triplicates, and incubated for 1 h in situ (in the sea at 2 m depth). As a blank, two subsamples (50 ml) with bacteria only were incubated under the same conditions and for the same incubation time. Subsamples were taken at the beginning and at the end of the incubation period for bacterial counts and isotope activity analysis. Seawater (5 ml) from each incubated tube was filtered through 0.22 μm pore size Poretics filters in triplicates. Each filter was well washed three times with trichloracetic acid (TCA) to a final concentration of 100%. After incubation, Oithona nana was collected on 10 μm Poretics filters rinsed abundantly three times with filtered seawater to wash off any remaining label and finally washed three times with 100% TCA. The number of individuals trapped on the filter was controlled under the stereomicroscope. In the laboratory, after addition of scintillation cocktail (Ultima Gold, Packard), samples were counted in a scintillation counter (TR2500, Packard) and the results expressed as dpm (disintegrations per minute).
Results
Environmental conditions and plankton vertical distribution
During both periods of our study the water column was stratified with the thermocline depth between the 10–15 and 9–14 m in July 2003 and May 2004, respectively. Surface temperatures were above 25°C in July 2003 and about 20°C in May 2004 while bottom temperatures were close to 10°C in both years (Fig. 4). In May 2004 a slight halocline was also noticeable at the same depth as the thermocline, while in July 2004 salinity was rather homogeneous throughout the water column.
Phytoplankton biomass was low in the layer above the thermocline as indicated by fluorescence. With decreasing temperature oxygen concentrations increased with depth but in the deep layers oxygen diminished. In July 2003 the average concentration of chlorophyll a in the surface layer (5 m) was 0.15 (0.1–0.42) μg Chl a l−1, at the thermocline layer 0.31 (0.24–0.5) μg Chl a l−1 and below the thermocline layer (25 m) 0.23 (0.1–0.35) μg Chl a l−1. Similar chlorophyll a concentrations but with slightly higher values in the above thermocline layer were also measured in 2004 (0.1–0.5 μg Chl a l−1, Flander Putrle, unpubl. data). The abundance of cyanobacteria varied from 1.9 to 4.6 × 107 l−1 with the maximal values within the thermocline.
The abundance of heterotrophic bacteria varied between 5.3 and 8.9 × 108 cells l−1, showing a slight increase with the depth in July 2003. The abundance was slightly lower in May 2004 with highest values (6.9 × 108 cells l−1) in the surface layer (Fig. 5, top). Bacterial carbon production measured by the 3H-leucine method varied from 0.3 to 2.8 μg C l−1 d−1 and was the lowest in deep layers in both years (Fig. 5, bottom).
Both, microzooplankton and mesozooplankton were more abundant in the upper water column (above and within the thermocline layers) either in July 2003 (Fig. 6, top) or May 2004 (Fig. 6, bottom). Microzooplankton was dominated by small nauplii and naked ciliates (Fig. 6, lines); both were more abundant in July compared to May. The most abundant mesozooplankton organisms, particularly in the upper part of the water column were two small copepods (adults and copepodites, since 125 μm mesh was used for sampling) Oithona nana and Paracalanus parvus (Fig. 6, bars), the former being most numerous within thermocline layer in July and the latter within the same layer in May. In the surface layer, the pteropod Limacina trochiformis was also moderately abundant, while in the layer below 20 m Oithona similis was numerous.
Abundance of the dominant mesozooplankton (Paracalanus parvus adults + copepodites, white bars; Oithona nana adults + copepodites, grey bars) and microzooplankton (naked ciliates, dashed line, solid circles; nauplia, solid line, open circles) organisms in layers above (9 m-surface), within (19–10 m) and below (40–20 m) the thermocline at the beginning of the feeding experiments on 12 July 2003 (top) and 18 May 2004 (bottom)
Vertical distribution of Aurelia
An overview of CTD casts and video profiles at different hours during both experimental periods (2003, 2004) is given in Table 1. The total number of Aurelia recorded per profile was highest at dusk and lowest during the night but variability was high and indicated some horizontal dispersion over the diel cycle. Additional semi-quantitative data from divers’ observations were obtained for the surface 25 m: four dives at dusk, and four dives during the day. During our observations medusae were swimming continuously with only a few exceptions.
The results obtained in 2003 (July) and 2004 (May) differed slightly although a general pattern was clear. During the night all medusae were located in deep layers below 20 m and the mean population depth (MVP) of Aurelia for all night recordings in both years was 28.6 ± 4.5 m. However, MVP was shallower in May (25.7 ± 3.3 m) compared to July (33.2 ± 2.0 m, Table 2). Orientation of Aurelia during the night recordings was variable without a clear prevailing direction. As with the night observations, daytime MVP in May were also at shallower depths, while at dusk MVP was similar for both years (Table 2). The proportion of the Aurelia population recorded in different layers over the diel cycle for the July 2003 observations is shown in Fig. 7. The peak in medusa abundance occurred in the sub-thermocline layer most of the time (44 ± 5% at dusk, 55 ± 10% during day and 56 ± 8% early morning) but a larger proportion of Aurelia were recorded in the thermocline layer at dusk and at that time only did Aurelia also appear in the above thermocline layer.
In both years a very clear migration of Aurelia towards the surface was registered at dusk with the large part of the Aurelia population located within the thermocline layer and a significant part recorded at depths shallower than 10 m. On two occasions in May 2004 we observed a swarming of Aurelia at the very surface for a short period. No such surface swarming of medusae was observed in July 2003 when surface temperatures exceeded 26°C. We followed one surfacing of Aurelia from 1730 to 2015 hours (local time) on 21 May 2004 when sunset was at about 1930 hours (the location where medusae surfaced was in shadow after this time), with repeated video recordings of vertical profiles from the surface to the bottom. At 1730 hours we recorded a total 150 Aurelia distributed from above the thermocline (18 individuals), within the thermocline (60 individuals) and just below the thermocline at a depth of 14–18 m (66 individuals). Only 6 animals were seen deeper than 22 m. Individuals within and above the thermocline layer were predominantly oriented upwards (64%); for about 28%, orientation was not clear from the video since the Aurelia were so numerous. The remaining 8% were oriented horizontally. Individuals in deeper layers were oriented upwards, downwards and horizontally in similar proportions. At 1830 hours about 70% of the medusae were recorded in the surface 15 m and all were oriented upwards; they surfaced shortly to form a surface swarm at about 1850–1900 hours. About 1 h later no Aurelia were observed shallower than 10 m and the highest density was at 12–14 m (bottom of the thermocline). Also, a significantly smaller total number (44) of Aurelia per profile was observed indicating that there was also some horizontal displacement of medusae. Divers observed this event from 1850 to 2010 hours and were located at 5 and 10 m depths. At 1855 hours, in a dense patch at a depth of 5 m, 77% of Aurelia were oriented upwards; at 1930 hours, at the same depth (5 m), essentially all individuals were observed to be oriented downwards. At a depth of 10 m more than 60% were oriented downwards between 1940 and 2010 hours. These data indicate rather rapid upward and downward swimming and migration greater than 10 m in 1 h.
In addition to the described event we collected eight data sets (video recordings) on vertical distribution and orientation of Aurelia between 1730–2130 hours. In both years Aurelia were most numerous in the layers within and above the thermocline (72 ± 19%) and the remaining medusae were observed just below the thermocline (15–16 m). More animals were recorded in the layers closer to the surface in May when temperatures in the upper 10 m were slightly less than 20°C, than in July when we measured temperatures over 26°C in surface 10 m (Fig. 4).
Feeding of Aurelia
The average size (bell diameter) of Aurelia analysed for gut content and used in enclosure feeding experiments was 7.4 ± 2.6 cm (n = 86) in 2003 and 9.9 ± 2.3 (n = 42) in 2004.
Gut content analyses of Aurelia indicated 27 ± 6 prey items per medusa (bell diameter from 4.9 to 11.4 cm). The dominant prey were small copepods (Paracalanus parvus, Oithona nana) and copepodites that on average constituted 72% of food items; among these Paracalanus parvus adults and copepodites contributed 24%, Oithona nana adults and copepodites accounted for 21% and unidentified copepodites were 27% followed by the larvae of Gastropoda, Bivalvia, Cirripedia (17%) nauplii (8%) and Appendicularia (2%). Other prey items (less than 1%) included Cladocera and Limacina.
In situ feeding experiments using plankton at natural densities also indicated high clearance rates for small calanoid and cyclopoid copepods, copepodites and nauplii (Table 3; Fig. 8, top). In addition, naked ciliates were found to be an important prey of Aurelia. Clearance rates were the highest for Oithona nana, adults and copepodites, followed by nauplii and by Paracalanus parvus adults and copepodites, naked ciliates were also important prey items. Feeding data obtained for surface (5 m) and deep (25 m) layers are merely indicative because only the experiment done on 12 July 2003 included additional enclosures at depths of 5 and 25 m. Nevertheless, results point to strong temperature dependence but may also reflect a higher natural density of zooplankton, particularly of nauplia and ciliates in the surface layer. Neither stomach content analysis nor feeding experiments indicated any non-migrating organisms that are found in deeper layers, like the copepods Diaixis pygmoea, Oithona similis, Calanus helgolandicus (Lučić, pers. obs.), as prey items of Aurelia.
In addition to the direct effect on micro- and mesozooplankton prey, Aurelia also had an important indirect impact on microbial plankton as evidenced by cyanobacteria and heterotrophic bacterial biomass, as well as heterotrophic bacterial production during the enclosure experiments (Fig. 8, middle, bottom). At the end of incubations cyanobacteria and heterotrophic bacteria abundances were steadily lower in enclosures without jellyfish i.e. with micro- and mesozooplankton only (control, Fig. 8). The difference between control and enclosures containing jellyfish was the largest in treatments incubated in the thermocline layer (Table 4). Moreover, bacterial carbon production rates were invariably lower in enclosures without Aurelia (Fig. 8, bottom), as were P/B ratios (Table 4). These results suggest an indirect, yet consistent, trophic linkage at the base of the food webs either through consumption of bacteriovores or by release of DOM.
Microscopic observations of large clumps of bacteria in the Mljet lake (Turk pers. obs.) prompted us to test the possibility that one important prey for Aurelia, the small cyclopoid Oithona nana (about 0.5–0.7 mm body length), could ingest bacterioplankton clumps and thus reduce the number of trophic levels between bacteria and medusae. Radiolabeled bacteria were offered to Oithona at levels comparable to natural abundances (8 × 105 ml−1) in the lake. When counting bacteria incubated for 24 h with labelled 3H-thymidine that were subsequently used in feeding experiments with Oithona nana (abundance at t0, Table 5 and Fig. 9) we observed that bacteria formed large chains. Fifteen Oithona nana were incubated in 50 ml tubes containing on average 8.5 ± 4.6 × 105 radiolabeled bacterial cells ml-1, which was equivalent to 25.1 ± 3.2 dpm ml−1. After 1 h incubation bacterial abundance decreased by 3.4 ± 0.8 × 105 ml−1 compared to control (Table 5) indicating measurable ingestion. Since no other bacterial grazers could pass through the 0.6-μm filters we may assume that Oithona grazed bacteria. Ingestion of radiolabeled bacteria was also confirmed by radioactivity measured in Oithona (Fig. 9). These very preliminary data indicate that Oithona nana might be able to consume clumped bacteria.
Discussion
In contrast to most coastal areas where Aurelia medusae appear seasonally, in the Mljet lakes they occur throughout the year (Onofri pers. comm.). Moreover, being located within a protected national park where human activities are very limited makes the Mljet lakes very suitable for ecological studies. Our study concentrated on the spring-summer season, which is the typical season for Aurelia medusae outbursts in many other marine environments. Aurelia is one of the most studied scyphomedusa due to its widespread distribution, seasonally high abundances and consequent important ecological role. It is known to be an opportunistic predator feeding on zooplankton of a wide taxonomic and size range. Costello et al. (1998) classified Aurelia as a cruising predator entraining fluid during both bell contraction and relaxation (Dabiri et al. 2005). Prey capture success and ingestion depends on the relative sizes of medusa and prey, prey escape behaviour, as well as environmental factors such as temperature and prey availability (Sullivan et al. 1994; Olesen 1995; Schuman and Sullivan 2000; Graham and Kroutil 2001). Like other jellyfish, Aurelia is traditionally considered an important predator/competitor within a classical trophic chain (Purcell and Sturdevant 2001). A few studies indicate that this jellyfish may consume ciliates (Stoecker et al. 1987; Omori et al. 1995) and suggest a closer link to the microbial food web (Hansson and Norrman 1995; Fukuda and Naganuma 2001). A structuring impact of vertically migrating medusa Periphylla periphylla on microbial activity and composition was also observed by Riemann et al. (2005) in a Norwegian fjord dominated by this jellyfish.
Our data (gut content and in situ feeding experiments) indicate that Aurelia preyed upon adults and copepodites of small calanoids and cyclopoids, nauplii and naked ciliates, which were typically most abundant within and/or above the thermocline layer. It is difficult to compare our results on clearance rates with other similar studies because experimental conditions differ; however, obtained values are in the lower range of published estimates. This may be explained by oligotrophic nature of Mljet lakes but may partly be also due to depletion of prey during 6–7 h incubation. Larger calanoids or other zooplankton located in deep layers (>25 m) was not consumed although our vertical distribution analysis (Fig. 5) showed that practically the whole Aurelia population stayed in the deep layer over night. Aurelia were recorded at shallower depths in the early morning and during the day with the mean vertical position of the population in the sub-thermocline layer.
Similar vertical distribution was found by Mutlu (2001) who studied Aurelia in the inshore and offshore waters of the southern part of the Black sea: Aurelia was generally confined to depths up to 20–40 m, avoided warm surface waters (25–26°C) but were found within cold intermediate water at about 50 m towards midnight. Our results, video recordings and divers’ observations, indicate that Aurelia start to migrate towards the surface well before sunset, i.e. between 1700 and 1800 hours (local time) and the majority stayed in the upper layers (above and within the thermocline) until dark. After 2300 hours the main body of Aurelia population was in layers below 25 m in both years. All monitored vertical displacements, i.e., ascents and descents, were the result of active swimming.
Papathanassiou et al. (1987) also observed diel vertical migration in the stratified water column in Saronikos Gulf, Greece. Aurelia were recorded at shallower depths in late afternoon and early morning but the authors ascribed the sinking of medusae to inactivation of bell pulsation. In contrast, we believe that swimming upwards and downwards enables Aurelia to prey actively when passing through the thermocline and the above-thermocline layers where nauplii and ciliates aggregate at dusk. During daylight hours we found the peak of Aurelia concentration in the sub-thermocline and, to lesser extent, thermocline layers, where small copepods and copepodites Paracalanus parvus and Oithona nana, were the most numerous. Such vertical distribution over the diel cycle would enable Aurelia to use available food resources most efficiently. When located in the deep layers at night Aurelia were also observed to be active. However, neither gut content nor feeding experiments indicated ingestion of larger copepods that dominated the deep (>25 m) layer. It is not clear why Aurelia would migrate to deeper layers overnight although residing in cold water may provide a metabolic advantage (Hays 2003). A number of factors may influence the migratory behaviour of zooplankton (Ohman 1990), among which, in particular light (Mackie et al. 1981; Hamner et al. 1994; Schuyler and Sullivan 1997), temperature conditions (Sparks et al. 2005) and increased reproduction success (Hamner et al. 1994) or a combination of factors seem to be important for jellyfish. Observed patterns of Aurelia migration behaviour in Mljet lakes indicates that, besides changes in light levels, other factors may be important. Predator avoidance did not seem to be a likely factor in the Mljet lakes since no obvious Aurelia predators were identified; optimal foraging behaviour (Graham et al. 2001) would thus be more probable.
The observed vertical migration also has important implications for Aurelia impact on the water column food web. Aurelia impact on the classical plankton food web has been well documented in the past and seems to be important also in the Mljet lakes. Predatory pressure by Aurelia is a likely explanation for the drastically reduced number of copepods in the lakes compared to the open Adriatic a short distance (300 m) from the lake channel. Moreover, we propose that Aurelia might have important effects on the microbial food web preying on naked ciliates as well as nauplii and copepodites. These results indicating microzooplankton as an additional food source to mesozooplankton are further supported by measurements of stable isotope ratios of carbon and nitrogen of Aurelia, mesozooplankton and microzooplankton which imply that Aurelia preyed upon both micro- and mesozooplankton (Malej et al. 2006).
It has been demonstrated that the nauplii and copepodites of several small copepods are capable of bacterivory (Turner and Tester 1992; Roff et al. 1995). Further, jellyfish could stimulate bacterial production and growth by providing a supplementary source of DOC (Hansson and Normann 1995). In addition, our preliminary experiment with radiolabeled bacteria indicates that the small cyclopoid copepod Oithona nana might also be able to ingest clumped bacteria reducing the number of trophic levels from bacteria to jellyfish. This finding is particularly relevant in the stratified water column and where small-sized food resources may be dominant.
In conclusion, our results, obtained during feeding experiments, elicited clear responses of bacterial populations indicative of indirect cascade effects of Aurelia on ‘microbial’, in addition to, ‘classical’ food webs. The observed impacts are very likely to be found in other stratified and/or oligotrophic environments where Aurelia is seasonally abundant.
References
Arai MN (2001) Pelagic coelenterates and eutrophication: a review. Hydrobiologia 451:69–87
Avian M, Rottini Sandrini (1994) History of scyphomedusae in the Adriatic Sea. Boll Soc Adriat Sci Nat Trieste1:5–12
Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Bailey KM, Batty RS (1983) A laboratory study of predation by Aurelia aurita on larval herring (Clupea harengus). Experimental observations compared with model prediction. Mar Biol 72:295–301
Båmstedt U, Gifford DJ, Irigoien X, Atkinson A, Roman M (2000) Feeding. In: Harris R, Wiebe P, Lenz J, Skjodal HR, Huntley M (eds) ICES zooplankton methodology manual. Academic, London, pp 297–399
Barlow RG, Mantoura RFC, Gough MA, Fileman TW (1993) Pigment signatures of the phytoplankton composition in the northeastern Atlantic during the 1990 bloom. Deep-Sea Res 40:459–477
Barz K, Hirche H-J (2005) Seasonal development of scyphozoa medusae and predatory impact of Aurelia aurita on the zooplankton community in the Bornholm Basin (central Baltic Sea). Mar Biol 147:465–476
Bedo AW, Acuna JL, Robins D, Harris RP (1993) Grazing in the micron and the sub-micron particle size range: the case of Oikopleura dioica (Appendicularia). Bull Mar Sci 53:2–14
Behrends G, Schneider G (1995) Impact of Aurelia aurita medusae (Cnidaria, Scyphozoa) on the standing stock and community composition of mesozooplankton in the Kiel Bight (western Baltic Sea). Mar Ecol Prog Ser 127:39–45
Benović A, Lučić D, Onofri V, Peharda M, Carić M, Jasprica N, Bobanović-Čolić S (2000) Ecological characteristics of the Mljet Island seawater lakes (Southern Adriatic Sea) with special reference to their resident populations of medusae. Sci Mar 64:197–206
Bognar A, Curić L (1995) Geomorphological characteristics of Island Mljet. In: Durbešić P, Benović A (eds) Natural characteristics and social valuation of the Mljet Island. Croatian Ecological Society, Zagreb, pp 73–84 (in Croatian)
Brodeur RD, Mills CE, Overland JE, Walters GE, Schumacher JD (1999) Evidence for a substantial increase in gelatinous zooplankton in the Bering sea, with possible link to climate change. Fish Oceanogr 8:296–306
Calbert A, Landry MR (1999) Mesozooplankton influences on the microbial food web: Direct and indirect trophic interactions in the oligotrophic open ocean. Limnol Oceanogr 44:1370–1380
CIESM (2001) Gelatinous zooplankton outbreaks: theory and practice. CIESM Workshop Series 14, Monaco
Costello JH, Klos E, Ford MD (1998) In situ time budgets of the scyphomedusae Aurelia aurita, Cyanea sp., Chrysaora quinquecirrha. J Plankton Res 20:383–391
Dabiri JO, Colin SP, Costello JH, Gharib M (2005) Flow patterns generated by oblate medusan jellyfish. Field measurements and laboratory analyses. J Exp Biol 208:1257–1265
Daskalov GM (2002) Overfishing drives a trophic cascade in the Black Sea. Mar Ecol Prog Ser 225:53–63
Dawson MN (2003) Macro-morphological variation among cryptic species of the moon jellyfish, Aurelia (Cnidaria: Scyphozoa). Mar Biol 143:369–380
Dawson GM, Martin LE (2001) Geographic variation and ecological adaptation in Aurelia (Scyphozoa, Semaeostomeae): some implications from molecular phylogenetics. Hydrobiologia 451:259–273
Diebel D, Lee SH (1992) Retention efficiency of sub-micrometer particles by the pharyngeal filter of the pelagic tunicates Oikopleura vanhoeffeni. Mar Ecol Prog Ser 81:25–30
Dumont H, Shiganova TA, Niermann U (2004) Aquatic Invasions in the Black, Caspian, and Mediterranean Seas. NATO Science Series 35. Kluwer, Dordrecht, pp 313
Fukuda Y, Naganuma T (2001) Potential dietary effects on the fatty acids composition of the common jellyfish Aurelia aurita. Mar Biol 138:1029–1035
Graham WM, Kroutil RM (2001) Size-based prey selectivity and dietary shifts in the jellyfish, Aurelia aurita. J Plankton Res 23:67–74
Graham WM, Pagès F, Hamner WM (2001) A physical context for gelatinous zooplankton aggregation: a review. Hydrobiologia 451:199–212
Hamner WM, Gilmer RW, Hamner PP (1982) The physical, chemical and biological characteristics of a stratified, saline, sulphide lake in Palau. Limnol Oceanogr 27:896–909
Hamner WM, Hamner PP, Strand SW (1994) Sun-compass migration by Aurelia aurita (Scyphozoa): population retention and reproduction in Saanich Inlet, British Columbia. Mar Biol 119:347–356
Hansson LJ, Norrman B (1995) Release of dissolved organic carbon (DOC) by the scyphozoan jellyfish Aurelia aurita and its potential influence on the production of planktik bacteria. Mar Biol 121:527–532
Hay SJ, Hislop JRG, Shanks AM (1990) North Sea scyphomedusae: summer distribution, estimated biomass and significancy particularly for 0-group gadoid fish. Neth J Sea Res 25:113–130
Hays GC (2003) A review of the adaptive significance and ecosystem consequences of zooplankton vertical migration. Hydrobiologia 503:163–170
Hays GC, Richardson AJ, Robinson C (2005) Climate change and marine plankton. Trends Ecol Evol 20(6):337–344
Ishii H, Båmstedt U (1998) Food regulation of growth and maturation in a natural population of Aurelia aurita(L.). J Plankton Res 20:805–816
Jasprica N, Viličić D, Carić M, Njire J (1995) Phytoplankton in the Malo and Veliko Jezero (Island Mljet, Southern Adriatic). In: Durbešić P, Benović A (eds) Natural characteristics and social valuation of the Mljet Island, Croatian Ecological Society, Zagreb, pp 454–463 (in Croatian)
Kideys A (2002) Fall and rise of the Black Sea ecosystem. Science 297:1482–1483
Kideys A, Romanova Z (2001) Distribution of gelatinous macrozooplankton in the southern Black Sea during 1996–1999. Mar Biol 139:535–547
Kršinić F (1995). The vertical distribution of zooplankton in Veliko Jezero on the island Mljet. In: Durbešić P, Benović A (eds) Natural characteristics and social valuation of the Mljet Island. Croatian Ecological Society, Zagreb, 475–481 (in Croatian)
Lee S, Fuhrman JA (1987) Relationships between biovolume and biomass of naturally derived marine bacterioplankton. Appl Environ Microbiol 53:1298–1303
Lučić D, Onofri V, Bender-Pojatina A (1995) Copepods of the coastal sea of the Mljet Island. In: Durbešić P, Benović A (eds) Natural characteristics and social valuation of the Mljet Island, Croatian Ecological Society, Zagreb, pp 499–509 (in Croatian)
Lynam CP, Hay SJ, Brierley AS (2004) Interannual variability in abundance of North Sea jellyfish and links to the North Atlantic Oscillation. Limnol Oceanogr 49:637–643
Mackie GO, Larson RJ, Larson KS (1981) Swimming and vertical migration of Aurelia aurita (L.) in a deep tank. Mar Behav Physiol 7:321–329
Malej A, Faganeli J, Pezdič J (1993) Stable isotope and biochemical fractionation in the marine pelagic food chain: the jellyfish Pelagia noctiluca and net zooplankton. Mar Biol 116:565–570
Malej A, Cermelj B, Lojen S, Milos C (2006) Elemental and stable isotope composition of bloom-forming Scyphomedusae from the Adriatic Sea. ASLO abstract book, Summer Meeting 2006, Victoria, p. 76
Mills CE (1995) Medusae, siphonophores, and ctenophores as planktivorous predators in changing global ecosystems. ICES J Mar Sci 52:575–581
Mills CE (2001) Jellyfish blooms: Are populations increasing globally in response to changing ocean conditions? Hydrobiologia 451:55–68
Molinero C, Ibanez F, Nival P (2005) North Atlantic climate and northwestern Mediterranean plankton variability. Limno Oceanogr 50(4):1213–1220
Möller H (1980) Population dynamics of Aurelia aurita medusae in Kiel Bight, Germany. Mar Biol 60:123–128
Mutlu E (2001) Distribution and abundance of moon jellyfish (Aurelia aurita) and its zooplankton food in the Black Sea. Mar Biol 138:329–339
Niermann U (2004) Mnemiopsis leidyi: distribution and effect on the Black Sea ecosystem during the first years of invasion in comparison with other gelatinous blooms. In: Dumont H, Shiganova TA, Niermann U (eds) Aquatic invasions in the Black, Caspian and Mediterranean Seas. Kluwer, Dordrecht pp 3–31
Ohman MD (1990) The demographic benefits of diel vertical migration by zooplankton. Ecol Monogr 60:257–281
Olesen NJ (1995) Clearance potential of jellyfish Aurelia aurita, and predation impact on zooplankton in a shallow cove. Mar Ecol Prog Ser 124:63–72
Omori M, Ishii H, Fujinaga A (1995) Life history strategy of Aurelia aurita (Cnidaria, Scyphomedusae) and its impact on the zooplankton community of Tokyo Bay. ICES J Mar Sci 52:597–603
Papathanassiou E, Panayotidis P, Anagnostaki K (1987) Notes on the biology and ecology of the jellyfish Aurelia aurita Lam. in Elefsis Bay (Saronikos Gulf, Greece). PSZNI Marine Ecol 8:49–58
Pearre S (1973) Vertical migration and feeding in Sagitta elegans. Ecology 54:300–314
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Postel L, Fock H, Hagen W (2000) Biomass and abundance. In: Harris R, Wiebe P, Lenz J, Skjodal HR, Huntley M (eds) ICES Zooplankton Methodology Manual. Academic, London pp 83–192
Purcell JE, Malej A, Benović A (1999) Potential links of jellyfish to eutrophication and fisheries. In: Malone TC, Malej A, Harding LW, Smodlaka N, Turner ER (eds) Coastal and estuarine studies 55. American Geophysical Union, Washington, pp 241–263
Purcell JE, Arai MN (2001) Interactions of pelagic cnidarians and ctenophores with fish: a review. Hydrobiologia 451:27–44
Purcell JE, Graham WM, Dumont HJ (2001) Jellyfish blooms: ecological and societal importance. Development in hydrobiology 155. Kluwer, Dordrecht, pp 333
Purcell JE, Sturdevant MV (2001) Prey selection and dietary overlap among zooplanktivorous jellyfish and juvenile fishes in Prince William Sound, Alaska. Mar Ecol Prog Ser 210:67–83
Ridžanović J, Šimunović V (1995) Geographical Characteristics of the Island of Mljet. In: Durbešić P, Benović A (eds) Natural characteristics and social valuation of the Mljet Island, Croatian Ecological Society, Zagreb, pp 55–70 (in Croatian)
Riemann L, Titelman J, Griekspoor P. Båmstedt U (2005) Plankton community structure and bacterial community composition in a Norwegian fjord dominated by medusa Periphylla periphylla. http://www.sgmeet.com/aslo/santiago2005/search.asp
Roff JC, Turner JT, Webber MK, Hopcroft RR (1995) Bacterivory by tropical copepod nauplia: extent and possible significance. Aquat Microb Ecol 9:165–175
Schabetsberger R, Brodeur D, Ciannelli L, Napp JM, Swartzman GL (2000) Diel vertical migration and interaction of zooplankton and juvenile walleye Pollock (Theragra chalcogramma) at a frontal region near the Pribilof Islands, Bering Sea. ICES J mar Sci 57:1283–1295
Sherr E, Sherr B (1988) Role of microbes in pelagic food webs: a revised concept. Limnol Oceanogr 33:1225–1227
Schneider G, Behrends G (1994) Population dynamics and the trophic role of Aurelia aurita medusae in the Kiel Bight and western Baltic. ICES J Mar Sci 51:359–367
Schroth W, Jarms G, Streit B, Schierwater B (2002) Speciation and phylogeography in the cosmopolitan moon jelly, Aurelia sp. BioMed Cent Evol Biol 2:1–10 http://www.biomedcentral.com/1471–2148/2/1
Schuyler Q, Sullivan BK (1997) Light responses and diel migration of the scyphomedusa Chrysaora quinquecirrha in mesocosm. J Plankton Res 19:1417–1428
Simon M, Azam F (1989) Protein content and protein synthesis rates of planktonic marine bacteria. Mar Ecol Prog Ser 51:201–213
Smith DC, Azam F (1992) A simple, economical method for measuring bacterial protein synthesis rates in seawater using 3H-Leucine. Mar Microb Food Webs 6:107–114
Sommer U, Stibor H, Katechakis A, Sommer F, Hansen T (2002) Pelagic food web configurations at different levels of nutrient richness and their implications for the ratio fish production:primary production. Hydrobiologia 484:11–20
Sornes TA, Aksnes DL (2004) Predation efficiency in visual and tactile zooplanktivores. Limnol Oceanogr 49:69–75
Sparks C, Brierley A S, Buecher E, Boyer D, Axelsen B, Gibbons M J (2005) Submersible observations on the daytime vertical distribution of Aquorea ?forskalea off the west coast of southern Africa. J Mar Biol Assoc UK 85:519–522
Stoecker DK, Michaels AE, Davis LH (1987) Grazing by the jellyfish, Aurelia aurita, on microplankton. J Plankton Res 9:901–915
Stoecker DK, Capuzzo J M (1990) predation on protozoa: its importance to zooplankton. J Plankton Res 12:891–908
Suchman CL, Sullivan BK (2000) Effect of prey size on vulnerability of copepods to predation by the scyphomedusae Aurelia aurita and Cyanea sp. J Plankton Res 22:2289–2306
Sullivan BK, Garcia JR, Klein-MacPhee G (1994) Prey selection by the scyphomedusan predator Aurelia aurita. Mar Biol 121:335–341
Takahashi M, Kikuchi K, Hara Y (1985) Importance of picocyanobacteria biomass (unicellular, blue-green algae) in the phytoplankton population of the coastal waters of Japan. Mar Biol 89:63–69
Turk V, Hagström Å (1997) Seasonal distribution of nanoflagellates and bacterivory in the Gulf of Trieste (Northern Adriatic). Period biol 99:205–208
Turner JT, Tester PA, Ferguson (1988) The marine cladoceran Penilia avirostris and the ‘microbial loop’ of pelagic food web. Limnol Oceanogr 33:245–255
Turner JT, Tester PA (1992) Zooplankton feeding ecology: bacterivory by metazoan microzooplankton. J Exp Mar Biol Ecol 160:149–167
Verity PG (1991) Measurement and simulation of prey uptake by marine planktonic ciliates fed plastidic and nanoplankton. Limnol Oceanogr 36(4):729–750
Vučetić T (1957) Zooplankton investigation in the sea water lakes malo jezero and Veliko jezero on the Island of Mljet 1952–1953. Acta Adr 6:1–52
Wikner J, Hagström Å (1988) Evidence for a tightly coupled nanoplanktonic predator–prey link regulating the bacterivores in the marine environment. Mar Ecol Prog Ser 50:137–145
Xian W, Kang B, Liu R (2005) Jellfish blooms in the Yangtze Estuary. Science 307:41
Youngbluth MJ, Båmstedt U (2001) Distribution, abundance, behaviour and metabolism of Periphylla periphylla, a mesopelagic coronate medusa in a Norwegian fjord. Hydrobiologia 451:321–333
Acknowledgments
The Ministry of Higher Education, Science and Technology of the R Slovenia, the Ministry of Science, Education and Sport of the R Croatia funded this work. We also acknowledge the support of the US National Science Foundation (OCE-0116236). The authors thank J. Costello for his constructive criticism on the manuscript. We appreciate the valuable suggestions made by anonymous reviewers. We are grateful to the Mljet National Park authorities for the hospitality during field work, thanks also go to J. Forte, F. Kravos, T. Makovec, Č. Miloš, V. Onofri for their help in field work.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova.
Rights and permissions
About this article
Cite this article
Malej, A., Turk, V., Lučić, D. et al. Direct and indirect trophic interactions of Aurelia sp. (Scyphozoa) in a stratified marine environment (Mljet Lakes, Adriatic Sea). Mar Biol 151, 827–841 (2007). https://doi.org/10.1007/s00227-006-0503-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0503-1