Abstract
It is well known that females of “Jaera albifrons” species complex cannot be determined on the basis of morphological analysis. In this work, an attempt to separate females of three Jaera species on the basis of morphometric characters, the length of so-called “coupling zones” (CZ), was made. Coupling zones are those parts of the partners’ bodies that contact during copulation. Female CZ is a region between the posterior edge of the pleotelson and the border between the fourth and the fifth thoracic segments, where openings of dorsal vaginas are situated. Male CZ extends from the anterior edge of the cephalon to the first abdominal segment carrying the copulative organ. Absolute and relative lengths of CZ were used. The latter was calculated as ratio of absolute length of CZ to body length. Two settlements of Jaera in the White Sea were studied for 3 years. Species composition of these populations was quite different (the first one was dominated by J. ischiosetosa and the second by J. albifrons and, 1 year by J. praehirsuta). Males of different species were shown to be distinct in terms of absolute and relative length of CZ. Frequency distributions of absolute and relative CZ length of females differed significantly in the two settlements. The experiments based on male choice in mixed samples were organized. They revealed that females chosen by males of different species differ significantly by CZ parameters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
It had long been considered that only one species of the isopod genus Jaera inhabited northern Atlantic seas: J. albifrons Leach 1814 (= Jaera marina Fabricius 1780) (Sye 1887; Gaevskaya 1948; Kuznetsov 1964). However, this species was then shown to be subdivided into several races (Forsman 1944, 1949) that, in further investigations, were recognized as independent species (Bocquet 1953). It is now accepted that the so-called “Jaera albifrons” complex consists of five distinct species: J. albifrons Leach 1814, J. ischiosetosa Forsman 1949, J. praehirsuta Forsman 1949, J. posthirsuta Forsman 1949 and J. forsmani Bocquet 1950. The former three species are common in the shallow waters of the White Sea (Kulinich and Frolov 1970; Bek 1988; Kuzmin and Khaitov 2004).
Distinct ecological preferences of different Jaera species have been shown in a range of works (Forsman 1949; Bocquet 1953; Naylor and Haahtela 1966; Jones 1972b; De Grave and Holmes 1998). Furthermore, there is some evidence of genetic (Siegismund 2002; Carvalho and Piertney 1997) and karyological (Staiger and Bocquet 1956) distinction between these species.
Clear morphological differences between species of the “Jaera albifrons” complex may be found only in males. Species identification of males is based on the secondary sexual characters associated with certain parts of certain pereopods that are involved in precopulation overtures (Solignac 1972). Since females lack such characters, confident species identification on the basis of morphological features has been thought impossible (Bocquet 1953). Therefore, investigators either identify Jaera females on the basis of genetic markers (Solignac 1978) or simply leave the females out of the research, basing the analysis of species composition on males only (e.g., Jones and Naylor 1971; Sjöberg 1970).
It has been shown that interspecific hybrids in Jaera are extremely rare in the nature (Bocquet 1953; Bocquet and Solignac 1969; Solignac 1981). There are only single instances of introgression in very limited areas (Solignac 1969). Solignac (1978) has shown that the choice of a conspecific female by a male is based on genetically determined behavioral patterns, different in different species. Thus, ethological isolation of species may take place.
At the same time, ethological features cannot be used for taxonomic or ecological investigations. Therefore, the necessity of identifying females of different species calls for a search for morphological differences between them. In this work we attempt to solve this problem on the basis of statistical morphometric analysis.
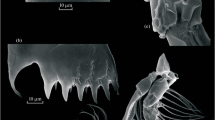
A prerequisite for this analysis is the existence of a typical behavioral pattern demonstrated by all the members of “Jaera albifrons” complex during copulation. In the beginning of the coupling, a male climbs up on the dorsal surface of a female and orientates itself in the position “head-to-tail” (Fig. 1). During copulation, certain body areas of the partners are in contact. In case of the male, the contact area extends from the anterior edge of the cephalon to the first abdominal segment carrying the copulative organ, while the female contact area is the part of the dorsal surface between the posterior edge of the pleotelson and the border between the fourth and the fifth thoracic segments, where the openings of dorsal vaginas are situated. Further, we call these body areas “coupling zones” (CZ) (Fig. 1). We assumed that discrepancy in the size of male and female coupling zones should hamper further copulation. Accordingly, our investigation focused on morphometric analysis of these zones.
In the present work we have tested the following hypotheses that follow from our assumption:
-
1.
Males of different species should differ in morphometric parameters of CZ.
-
2.
In settlements with different species composition, a different frequency distribution of female CZ parameters should be observed.
-
3.
CZ of the females chosen as mates by males of different species should have different morphometric parameters.
-
4.
CZ parameters of males of a certain species should correlate with those of females of the same species.
Materials and methods
Samples and experiments
The investigations were carried out in the Kandalaksha Bay of the White Sea (Fig. 2). The samples were taken between late May 2003 to early June 2005 at two stations situated on the intertidal zone of the Youzhnaya Inlet (Ryashkov island, Kandalaksha Nature Reserve). The stations’ positions were the same every year.
The first station (St. 1) was situated in the mouth of a small stream in the upper part of the inlet. Submerged stones were sampled there and thoroughly rinsed in a bucket. The second station (St. 2) was in the middle of the eastern shore of the inlet. There, fucoids (Ascophyllum nodosum and Fucus vesiculosus) were sampled and rinsed. All the samples were taken a few days after the ice had melted. At the time of sampling, water salinity at these two stations was similar (∼5‰), though in August it is quite different.
Materials from each station were sorted. In 2003–2004, all animals from different stations were preserved separately in 70% ethanol (a general description of material is given in Table 1). In 2005, the scheme of collections was more complicated: two types of samples were taken.
Samples of the first type were the same as in 2003–2004. They were taken on 24 May 2005, 2 and 3 June 2005 (Table 1). However, the isopods sampled on 24 May 2005 were not preserved immediately but placed alive in plastic dishes. After several hours they were dead, although the technique of alive animals’ maintenance was fine-tuned in the previous years. The cause of their death is unclear.
According to our previous investigations (unpublished), dead Jaera individuals loose natural body proportions. Therefore, dead isopods from the first 2005 batch of samples were not measured, only counted. To correct the failure, new samples were taken (Table 1), where mass Jaera mortality was not observed.
Since the sampling period falls on the peak of sexual activity of Jaera in the region (our unpublished data), a sufficient number of coupling animals was easy to obtain in a short time. Therefore, in 2005, samples of the second kind were taken that provided material for “mixed samples” experiments (Table 1). After the samples from the two stations in a certain day were sorted, all alive animals were placed in one metallic enameled plate with a rugged bottom surface (90 cm2) filled with fresh seawater. The animals were kept in the laboratory at 7–13°C (water temperature near the shore was 8°C at that time).
In the beginning of the experiment, the plate was shaken in order to mix the isopods. After the water had settled down, we started to catch the animals that demonstrated the “head-to-tail” pattern. Each pair was preserved in separate test tubes with 70% ethanol. Collection was carried out for 40–60 min and after that the plate was shaken again. Catching proceeded as described for 24–48 h in each experiment (3–6 catch periods for each sample). The experiments were repeated three times (Table 1) and their results were pooled for the analysis. The animals that did not demonstrate precopulating behavior were preserved in ethanol and later identified and counted.
The species of each preserved male was determined according to the key given in Kussakin (1988). All animals were pasted on slides with the bakelite–phenolic glue (BF-6) diluted with ethanol (70% of glue, 30% of ethanol and several drops of glycerin per portion). Paired animals were pasted together. For each animal, the body length (L) and the length of CZ (Lczf for females and Lczm for males) were measured, according to the scheme given in Fig. 3. Binocular microscope at 24× magnification with an ocular-micrometer was used. These measurements are further referred to as “absolute measurements”. Besides the absolute value, we used the relative one. The relative CZ length (RLcz) was calculated as a ratio as Lcz to L.
Since the distribution of Lcz and RLcz in most of the samples was different from normal (Kolmogorov–Smirnov test, P < 0,01), we used nonparametric Mann–Whitney’s U test for statistical comparisons. The rank Spearman coefficient was used for correlation analysis. Standard deviations are given as an estimation of variation in all cases.
Results
Species composition
Males of three Jaera species were present at the two stations (Table 2). The settlement at St. 1 was mostly formed by J. ischiosetosa; J. albifrons males were not numerous and J. praehirsuta males were not observed at all. This pattern of species composition was stable during the 3 years of investigation. Species composition at St. 2 changed from year to year. In 2003–2004, the population was dominated by J. albifrons; males of J. ischiosetosa and J. praehirsuta were rare. In 2005, a dramatic increase in the proportion of J. praehirsuta males was observed (Table 2). Samples that were taken on 2 July 2005 at St. 2 included only J. praehirsuta males (Table 1), but materials collected earlier (taken on 24 May 2005) showed the presence of all three species at this station.
Body parameters of males
Since the sampling size for males of all three species was large enough only in 2005, only this material was used for comparisons of male body parameters.
Frequency distributions of Lczm (Fig. 4a) and RLczm (Fig. 4b) were quite different in males of the three species. Mean values of Lczm and RLczm and the results of statistical testing are given in Tables 3 and 4.
It is obvious that J. praehirsuta males are slightly larger than males of the other two species. Moreover, they have different body proportions, which is expressed in a relatively longer CZ. Body proportions of J. albifrons and J. ischiosetosa seem to be indistinguishable. However, absolute measurements are slightly larger in J. ischiosetosa than in J. albifrons.
Body proportions of females from populations with different species composition
Frequency distributions of RLczf (Fig. 5) across populations were significantly different every year (U test, P < 0.01 in all comparisons). On average, females from St. 1 have a larger relative CZ than females from St. 2. This pattern was stable throughout the investigation period. However, frequency distributions of absolute CZ length demonstrate certain changes from year to year (Fig. 6). At the same time, the differences in frequency distributions of Lcz across stations were also significant every year. In 2003–2004, the females from St. 2 had a significantly shorter CZ than those from St. 1. In 2005, a contrary pattern was observed: coupling zones of females from St. 2 were larger than those of females from St. 1.
Body parameters of paired animals in “mixed samples” experiments
In “mixed samples” experiments, males could choose partners from females of all the species present at the two stations. Noteworthy, according to the data presented in Table 2, J. praehirsuta and J. albifrons males used in these experiments are likely to come mostly from St. 2, while J. ischiosetosa males mostly come from St. 1.
Comparisons of absolute CZ length (Fig. 7a) display an obvious separation of Lczf-distribution of females from pairs with J. albifrons males. These females have a significantly shorter CZ than females from pairs with males of other species (Table 5). At the same time, the differences in absolute CZ length of females from pairs with J. ischiosetosa and J. praehirsuta males were not significant.
Females from pairs with J. ischiosetosa males have a significantly longer CZ in terms of relative length than females from pairs with J. albifrons and J. praehirsuta males (Table 6; Fig. 7b). The differences between RLczf of the latter two species were not significant.
Thus, in our experiments with “mixed samples”, males of J. ischiosetosa preferably formed pairs with females that had larger relative coupling zones. Males of J. praehirsuta chose females with the largest absolute CZ size. Males of J. albifrons preferred females with the shortest CZ in terms of both absolute and relative length.
Correlation of body parameters of males and females
The materials of “mixed samples” experiments were used for analysis of correlations between parameters of males and females in pairs (Table 7). Rank Spearman coefficients were not significant for all the species taken separately. However, analysis of all pairs (without separation according to the species of the male) displays a significant correlation between the absolute size of both the body and the CZ of paired males and females.
Discussion
The search for specific differences between females of “J. albifrons” complex has a long tradition. It was accepted that Jaera females had no unique morphological characters allowing species distinction (Forsman 1944; Bocquet 1953). At the same time, Solignac (1972) found significant ethological differences between species.
However, the above ethological differences do not have much value for female identification in most kinds of investigations. Therefore, several attempts to find structural characters that could be used for female identification were made. For example, Solignac (1978, 1981) tried to apply specific markers (color variations). However, this character is unsuitable for pilot investigations, when the researcher knows nothing about phenetic structure of populations in a certain area. Moreover, markers like these could not be found in several populations of the White and Barents seas (our unpublished data). All phenetic characters that were at first hypothesized to be specific were later encountered in populations with a different species composition. Molecular and cytogenetic methods are also useless for female identification because they only work post factum, even though they provide sound evidence of morphological or ecological variety at a species level. Thus, only morphological features of females may be applicable to identification.
It is well known that males of different species are fairly different as to parameters of allometric growth (Bocquet 1953; Solignac et al. 1990). An analogous analysis for females shows only slight differences that are impossible to use (Boitard et al. 1982; Solignac et al. 1990). In a work of Boitard et al. (1982), analysis of principal components was based on the width of segments and length of some parts of pereopods, cephalon and pleotelson. Females of different species were shown to be separate from each other at the level of the third component. These differences were not as obvious as those of males because of greater variability in females (Boitard et al. 1982).
Our analysis of body parameters shows that males of different species have both different CZ size and different body proportions in relation to the CZ. It means that secondary sexual characters of males (upon which species diagnoses are based) are not the only ones that can be used for species separation. Absolute CZ length in males (in 2005) increases in the following series: J. albifrons–J. ischiosetosa–J. praehirsuta. An analogous pattern of male CZ length distribution across the species was shown in 2003 (Kuzmin and Khaitov 2004).
Presence of differences in absolute and relative CZ length between males leads us to expect differences like these between females. If this hypothesis is correct, a significant difference in female body parameters between groups of individuals of various species should be observed. We used two criteria for separation of females into groups with presumptively different species composition: ecological differences between populations and male choice experiments.
The pattern of species distribution observed in this investigation (in analysis of males) agrees with the results presented by Jones (1972a) who showed that J. ischiosetosa was the most euryhaline form of the “J. albifrons” complex. It may live on stones in freshwater streams crossing the intertidal zone at low tide, whereas the other species tend to inhabit more saline areas (Naylor and Haahtela 1966). In the investigation area, the majority of J. ischiosetosa individuals were found in the mouth of a stream, while individuals of other species are far from the stream’s influence.
Species composition at St. 1 was relatively stable from year to year. However, a dramatic increase in J. praehirsuta proportion was observed at St. 2 in 2005. This was probably associated with an unusually strong gale that destroyed the fucoid belt in the investigation area in the end of August 2004. Sampling at St. 2 in 2005 was much more difficult than in the previous years. The isopods were by far less numerous, so we had to process huge amounts of fucoids to procure the necessary material. It is possible that J. praehirsuta that partly replaced J. albifrons at this site arrived there from the low intertidal or the upper subtidal zone.
Jaera is known to form local assemblages (Piertney and Carvalho 1995a, b; Bek 1988). This may account for the fact that in one of the samples at St. 2 (2 June 2005, Table 1) males of J. praehirsuta were exclusively found, although samples were made at the same place by the same methods. Local assemblages of J. praehirsuta had never been found during previous investigations. However, the partial replacement of J. albifrons by J. praehirsuta in 2005 provided an additional possibility to test female population structure because changes in the number of males of a certain species should probably be accompanied by changes in the number of its females.
Results of female body parameters analysis confirm that the difference in distribution of males and temporary changes in species composition correlate with size-frequency distributions of females. Thus, at Sts. 1 and 2 both the size and the body proportions of females were significantly different. In 2003–2004, females demonstrated lesser values of absolute and relative CZ length at St. 2, where J. albifrons males were numerous, than at St. 1, where J. ischiosetosa males dominated.
In 2005, when J. praehirsuta expansion was observed, frequency distribution of absolute female CZ length at St. 2 shifted to the region of larger values. As follows from frequency distribution of absolute female CZ length (Fig. 6) in 2005, Jaera population at St. 2 was dominated by larger females than in previous years. At the same time, values of relative CZ length of females were smaller at St. 2 than at St. 1. Thus, in terms of RLcz, females in the population dominated by J. praehirsuta males differ from females in the population dominated by J. ischiosetosa males in the same way as the latter differ from females in the population dominated by J. albifrons males.
To sum up, Jaera females, on average, have a larger relative CZ length in populations where J. ischiosetosa males are numerous than in populations where J. albifrons or J. praehirsuta males are numerous. Values of absolute CZ length in females are lower in populations dominated by J. albifrons males than in populations dominated by J. praehirsuta males.
However, ecological separation of males does not necessarily mean that females are ecologically separated in the same way. Moreover, the differences observed between frequency distributions of body parameters may be explained otherwise (e.g., growth pattern may change under different conditions). To avoid this uncertainty, male choice experiments were performed.
In experiments with crossbred males (hybrids of J. albifrons and J. ischiosetosa), males that “contain” more “albifrons genes” chose more willingly J. albifrons females (Solignac 1978). Thus, male choice experiments may allow us to divide females into species groups, although this grouping will certainly have probabilistic nature because confuses in male choice are quite frequent (Solignac 1978).
Since in our “mixed samples” experiments significant differences were found between females chosen by males of different species, we may conclude that males of different species formed pairs non-randomly. Thus, values of relative CZ length of females chosen by J. albifrons and J. praehirsuta males were less than those of female chosen by J. ischiosetosa males. These results agree with those of RLczf frequency distribution analysis of females collected at different stations (see Fig. 5 and the above discussion). The values of absolute CZ length of females chosen by J. praehirsuta males were the largest. This fact corresponds with the results of frequency distribution analysis at two stations in 2005 (Fig. 6) and with the values of males’ body parameters (Table 3; Fig. 4). Analogously, females with a short CZ are chosen by J. albifrons males with the shortest CZ.
Summing up all the results, we may propose the following draft scheme of species differentiation for females. Individuals with a large relative CZ length (more than 0.49–0.50 or, in usual terms, more than a half of body length) may be considered as females of J. ischiosetosa. It should be noted that an experienced researcher can easily evaluate this ratio without measuring. Females with a short relative CZ (less than a half of the body length) may be identified as J. albifrons or J. praehirsuta. Separation of females of these species may be based on the absolute CZ length: females of J. praehirsuta are larger then those of J. albifrons (concrete values of CZ length may probably differ in different areas). The proposed scheme of female identification should be confirmed by an analysis of male species composition and verified further by molecular, genetic and karyological methods.
The last question to be discussed in the context of identification of Jaera females concerns sympatric coexistence of three species that are able to produce hybrids. Possible mechanisms of this coexistence were studied and reviewed by Solignac (1972, 1978, 1981). Ecological and ethological differences were shown to be pre-copulation factors, and hybrid inferiorities, a post-fertilization factor (Solignac 1981). Our results suggest one more pre-copulative isolation mechanism based on size incompatibility of coupling zones of males and females of different species. We found a certain correlation between absolute length of coupling zones of partners. It was negligible within one species but quite significant for all sets of pairs (Table 7). It means that males of different species tend to choose females of their own size class. This mechanism is certainly statistical and effective only in combination with other isolation forces.
Reference
Bek TA (1988) To the biology of Jaera albifrons group (Isopoda, Asellota) of the litoral of the White Sea (in Russian). Vestn Mosc Univ Ser Biol 16:32–36
Bocquet C (1953) Recherches sur le polymorphisme naturel des Jaera marina (Fabr.) (Isopodes Asellotes). Essai de systématique évolutive. Arch Zool Exp Gén 90:187–450
Bocquet C, Solignac M (1969) Étude morphologique des hybrides expériméntaux entre Jaera (albifrons) albifrons et Jaera (albifrons) praehirsuta. Arch Zool Exp Gén 110:435–452
Boitard M, Lefebvre J, Solignac M (1982) Analyse en composantes principales de la variabilité de taille de croissance et de conformation des espèces du complexe Jaera albifrons (Crustacés isopodes). Cah Biol Mar 23:115–142
Carvalho GR, Piertney SB (1997) Interspecific comparisons of the genetic population structure in members of the Jaera albifrons species complex. J Mar Biol Assoc UK 77:77–93
De Grave S, Holmes JMC (1998) The distribution of marine Isopoda (Crustacea) in Lough hyne. Biol Env Proc Roy Irish Acad 98B:23–30
Forsman B (1944) Beobachtungen über Jaera albifrons Leach an der schwedischen Westküste. Ark f Zool 35:1–33
Forsman B (1949) Weitere Studien über die Rassen von Jaera albifrons Leach. Zool Bidr 27:449–463
Gaevskaya NS (1948) The finder of fauna and flora of the northern seas of USSR (in Russian). Sov Nauka, Moscow
Jones MB (1972a) Effects of salinity on the survival of the Jaera albifrons Leach group of species (Crustacea: Isopoda). J Exp Mar Biol Ecol 9:231–237
Jones MB (1972b) Osmoregulation in the Jaera albifrons group of species (Isopoda, Asellota). J Mar Biol Assoc UK 52:419–427
Jones MB, Naylor E (1971) Breeding and bionomics of the British members of the Jaera albifrons group of species (Isopoda: Asellota). J Zool 165:183–199
Kulinich LY, Frolov YM (1970) Intraspecific variability of Jaera albifrons Leach, 1814 (Crustacea, Isopoda, Asellota) in the White Sea (in Russian). Tr Belomorsk Biol St Mosc Univ 3:60–68
Kussakin OG (1988) Marine and brackish water isopod crustaceans (Isopoda) of the cold and temperate waters of the Northern hemisphere (in Russian), vol 3, Part 1. Nauka, Leningrad
Kuzmin AA, Khaitov VM (2004) To the question about the species composition of isopods of the genus Jaera in the top of Kandalaksha Bay of the White Sea (in Russian). Vestn St-Peterbg Univ Ser 3 Biol 4:51–58
Kuznetsov VV (1964) The biology of mass and most common species of crustaceans in the Barents and White Seas (in Russian). Nauka, AS USSR, Zool. Inst., Moscow Leningrad
Naylor E, Haahtela I (1966) Habitat preferences and interspersion of species within the superspecies Jaera albifrons Leach (Crustacea: Isopoda). J Anim Ecol 35:209–216
Piertney SB, Carvalho GR (1995a) Detection of high levels of genetic relatedness in rock-populations of an intertidal isopod using DNA fingerprinting. J Mar Biol Assoc UK 75:967–976
Piertney SB, Carvalho GR (1995b) Microgeographic genetic differentiation in the intertidal isopod Jaera albifrons Leach. II. Temporal variation in allele frequencies. J Exp Mar Biol Ecol 188:277–288
Siegismund HR (2002) Disparity in population differentiation of sex-linked and autosomal variation in sibling species of the Jaera albifrons (Isopoda) complex. J Hered 93:432–438
Sjöberg B (1970) Population density, size, age, reproduction and microdistribution in the Jaera albifrons group (Isopoda). Oikos 21:241–247
Solignac M (1969) Hybridation introgressive dans la population complexe des Jaera albifrons de Luc-sur-Mer. Arch Zool Exp Gén 110:629–652
Solignac M (1972) Comparaison des comportements sexuels spécifiques dans la super-espèce Jaera albifrons (Isopodes Asellotes). C R Acad Sci Paris 274:2236–2239
Solignac M (1978) Genetics of ethological isolating mechanisms in the species complex Jaera albifrons (Crustacea, Isopoda). NATO Conf Series 2:637–664
Solignac M (1981) Isolating mechanisms and modalities of speciation in the Jaera albifrons species complex (Crustacea, Isopoda). Syst Zool 30:387–405
Solignac M, Cariou M-L, Wimitzky M (1990) Variability, specificity and evolution of growth gradients in the species complex Jaera albifrons (Isopoda: Asellota). Crustaceana 59:121–145
Staiger H, Bocquet C (1956) Les chromosomes de la super-espèce Jaera marina (Fabr.) et de quelques autres Janiridae (Isopodes Asellotes). Bull Biol Fr Belg 90:1–32
Sye CG (1887) Beiträge Anatomie und Histologie von Iaera marina. Inaug Diss Christ.-Albr.-Univ Kiel, Kiel
Acknowledgments
We are grateful to Natalia Lentsman (St.-Petersburg State University) for her brilliant linguistic assistance. We acknowledge Dr. Vitaly Bianki’s help during fieldwork. All experiments comply with the current laws of Russian Federation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe.
Rights and permissions
About this article
Cite this article
Khaitov, V.M., Kuzmin, A.A. & Terovskaya, E.V. Morphological differences between females of different Jaera species (Crustacea: Asellota: Isopoda) in the White Sea: a possible solution to an old problem. Mar Biol 150, 1205–1214 (2007). https://doi.org/10.1007/s00227-006-0450-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0450-x