Abstract
The softshell clam, Mya arenaria, is a commercially important bivalve with wide latitudinal distribution in North America. Populations of clams with a history of repeated exposure to toxic Alexandrium spp. have developed a natural resistance to the paralytic shellfish toxins (PSTs) produced by these algae. An association between PST resistance in individual clams and a single mutation in the saxitoxin (STX) binding region of the α-subunit of the voltage-gated sodium (Na+) channel gene was previously identified. Here we establish that more than one mutation associated with nerve resistance to STX occurred at this locus. Both cDNA from mRNA and genomic DNA sequences from individual clams are identical demonstrating that both alleles are expressed simultaneously. In addition, one resistant allele per individual is sufficient to confer neural resistance to STX even though heterozygous individuals show an intermediate level of resistance to STX in in vitro nerve trunk assays.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Paralytic shellfish poisoning (PSP) results from the ingestion of toxic shellfish by humans. Bivalve molluscs, the main vectors of paralytic shellfish toxins (PSTs), acquire their toxicity by suspension-feeding on toxigenic dinoflagellates (Alexandrium spp. in North America). These toxins and the resulting PSP constitute a public health hazard and cause severe economic losses on both coasts of North America. PSP is also a global problem and the historical record indicates that its occurrence is expanding geographically (Hallegraeff 1993). Paralytic shellfish toxins are composed of more than 20 derivatives that differ in their potency and proportion among strains of Alexandrium spp. and shellfish tissues, including saxitoxin (STX), the most potent derivative (Anderson et al. 1994). These toxins act on the voltage-gated Na+ channels of nerve and muscle cells by altering the channel’s ability to control the passage of Na+ ions into the cell (Catterall 1992).
The softshell clam, Mya arenaria, is a bivalve species native to the Atlantic coast of North America, where it can form dense, commercially exploitable populations. Softshell clams have been shown to differ in their behavioral and physiological responses to toxic Alexandrium cells under laboratory conditions depending on their prior history of PST exposure in the field (MacQuarrie 2002; Bricelj et al. 2005). Thus, most clams from a population with no known history of PSP (Lawrencetown River estuary, Nova Scotia) showed reduced feeding rates on toxic cells, reduced toxin accumulation rates, lower survival and greater in vitro nerve sensitivity to STX compared to clams from a population that experiences recurrent PSP outbreaks (Lepreau Basin, Bay of Fundy) (MacQuarrie 2002; Bricelj et al. 2005).
Large scale latitudinal characterization of the resistance to PSTs of North American M. arenaria populations was provided by Bricelj et al. (2004) using a burrowing index, based on the observation that sensitive clams experience burrowing incapacitation following short-term exposure to toxic Alexandrium cells. This study correlated the resistance of softshell clam populations with the history of PSP outbreaks along the Atlantic coast, and showed that there was generally good agreement between the determination of resistance using the burrowing assay and that by the in vitro nerve assay.
A natural mutation responsible for conferring resistance to STX in individual M. arenaria was identified as a single amino acid change in the domain II (DII) pore region of the α-subunit of the voltage-gated Na+ channel (Bricelj et al. 2005). The mutation identified a transversion of an adenine (A) to a cytosine (C) resulting in an amino acid change at a position equivalent to E945 in rat (Goldin et al. 2000) from a glutamic acid (E) to an aspartic acid (D). This change causes a 1,000-fold decrease in affinity at the STX-binding site in the Na+ channel pore of resistant, but not sensitive, clams. Thus, a seemingly conservative point mutation leading to the change of a single amino acid was found to result in a dramatic decrease in the binding of STX and tetrodotoxin (TTX) to the Na+ channel. In the present study, we investigate the sequence of the pore region in DII of the Na+ channel from 39 individual clams originating from selected populations along both coasts of North America. These populations were previously characterized as being predominantly composed of resistant or sensitive individuals based on their burrowing behavior (Bricelj et al. 2004). However, because there were occasional discrepancies between the burrowing and the in vitro nerve trunk assays, it became important to confirm the nerve response from each individual used for gene sequence analysis in the present study. We therefore report the proportion of resistant and sensitive clams within each of the clam populations, as determined by the in vitro nerve assay. A 100% correlation was found in the paired data of the in vitro nerve trunk assay response and the genotype from the same individual clam, regardless of the source population. The DNA sequence data can be used to predict potential toxin load of an individual clam after a highly toxic Alexandrium spp. bloom.
Materials and methods
Source of clams
Mya arenaria were collected at coastal sites in Canada and the USA. Populations were selected based on their history of exposure to PSP in the natural environment and on previously characterized burrowing behavior upon laboratory exposure to toxic Alexandrium cells (Bricelj et al. 2004). These sites are identified as follows: Lawrencetown Estuary, Nova Scotia (LE); Lepreau Basin, Bay of Fundy, New Brunswick (BF); Havre-Aubert, Îles des Madeleine, Québec (HA); Essex, Massachusetts (EX); Orleans, Massachusetts (OR); Quartermaster Harbor, Washington (QMH), and Lummi Bay, off Bellingham, Washington (BEL) (Fig. 1). All clams were collected at a time of year when they contained no detectable PSTs in tissues, as verified by reverse phase high-performance liquid chromatography with fluorescence detection (HPLC-FD) using methods described by Oshima (1995) with minor modifications (MacQuarrie 2002). Following collection and transport, and prior to their use in nerve assays, clams were maintained for a minimum of 2 weeks in a recirculating, temperature controlled seawater system with the addition of non-toxic algae at the Marine Research Station of the Institute for Marine Biosciences of the National Research Council of Canada (IMB/NRC). NRC complies with regulations from the Canadian Council of Animal Care (CCAC).
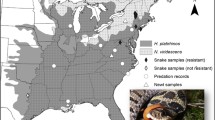
Map showing Mya arenaria collection sites on the Pacific (left panel) and Atlantic (right panel) coast of North America in relation to their history of paralytic shellfish poisoning (PSP). Pie charts show the proportion of individual clams that are sensitive (filled) or resistant (open) to PSP toxins based on results from the in vitro nerve assay (see Materials and Methods)
Neurophysiology
Individual M. arenaria of shell length (SL) 38–72 mm were dissected [for details of the species’ anatomy, see Fig. 22 (Vles 1909)]. The cerebrovisceral connective (CVC) was isolated posterior to the cerebral ganglion, and anterior to the visceral ganglion. Silk suture threads were tied at each end and the CVC removed to a dish of cold, aerated physiological saline solution (PSS) containing (in mM) 428 NaCl, 10 KCl, 20 MgCl2·6H20, 10 CaCl2·H20, buffered to pH 7.4 with 50 mM Tris–HCl. Air was bubbled through the stock solution, which was held on ice. The CVC is a major nerve trunk, devoid of ganglion cells and synapses. In the specimens used, the dissected CVC was 1.3–2.0 cm long, 0.2 mm in diameter, and ∼0.3 mg in wet weight. The connective can be used immediately or stored in PSS, in a covered Petri dish, at 4°C, overnight or longer before testing.
Stock standard solutions of STX ∼3.48 mM in 0.1 M acetic acid (HAc) were obtained from M. Quilliam, IMB/NRC, who determined the concentration of free base in the stock solutions by HPLC against a Certified Reference Materials Program (CRMP) standard. These solutions are stable for months at refrigerator temperature. For tests, the stock was diluted in PSS to achieve the appropriate STX concentrations. At STX concentrations of 10−4 g ml−1 (334 μM), the HAc concentration was 0.01 M and the pH 5.63. Diluting HAc alone in PSS to pH 5.6 did not block nerve conduction but further acidification caused partial and eventually complete block (reversible). Tests reported here were at STX concentrations ≤334 μM except for two tests, where the acid was neutralized and higher STX concentrations were tested (up to 668 μM).
The plexiglas (lucite) nerve chamber used for the nerve assay was divided into three parts: a middle (test) chamber 0.25 ml in volume (length, L = 2 mm; width, W = 20 mm; depth, D = 6.25 mm) and two end chambers of 1.0 ml volume (L = 10 mm; W = 20 mm; D = 5 mm each), one for stimulating the CVC and the other for recording of the response (for detailed examples of the response recording, see Twarog and Yamaguchi 1974). The CVC was draped over the dividing bridges so that it lay in all three chambers. Between the end and middle chambers, thin columns of Vaseline were laid down using a hypodermic syringe and #22 needle. The CVC was embedded in this water repellent, electrically insulating material, thus ensuring that test fluid in the middle chamber did not leak to the end chambers and that these were electrically isolated from one another. The ends of the CVC were drawn into snug-fitting suction electrodes. The middle chamber was grounded. The stimulating electrode led to a Grass SIU and thence to an Grass S48 stimulator. The recording electrode led to a Grass P3 amplifier and a cathode ray oscilloscope. In six subsequent experiments (HA population) the oscilloscope was replaced with a computerized recording system, a Grass PolyVIEW (ver. 2.5) data acquisition system, that allowed continuous recording of the response over the duration of the test.
The CVC was mounted in the chamber, in aerated PSS at 15°C with the cerebral end pulled into the stimulating electrode and the visceral end in the recording electrode. Square wave pulses (5 ms duration, 0.5 pulse s−1) were applied at increasing voltages from 0.1 to 10 V until the response was maximal. Following recording of the response, stimulation was shut off, to be resumed at 20 min intervals until the recorded responses were stable. This sometimes required 1–2 h, during which time the CVC was washed at intervals by emptying the chambers and refilling with fresh PSS.
A control response was recorded and immediately thereafter the PSS in the test chamber was replaced with STX in concentrations increasing sequentially from 17 to 334 μM (10−6 to 10−4 g ml−1). If the response was 100% blocked, the STX was immediately washed off. Otherwise, the response was recorded at 3 and 5 min. The test and end chambers were washed at least twice with fresh PSS and recovery monitored for at least 3 min before shutting off stimulation. Recovery was complete within ∼5 to 15 min, even following full block. After 20 min another control response was recorded. Tests continued until the concentration was determined at which 100% block occurred, or when the STX concentration reached 334 μM (10−4 g ml−1). In the rare case of failure to recover the experiment was discarded.
Sequencing of the M. arenaria Na+ channel
The cerebral and visceral ganglia were dissected from M. arenaria and stored as described by Bricelj et al. (2005). Tissues from each individual M. arenaria were homogenized in a micro tissue grinder containing 800 μl Trizol reagent (Invitrogen, Carlsbad, CA, USA) and 200 μg linear acrylamide (Ambion, Austin, TX USA). Both DNA and RNA were extracted following the manufacturer’s instructions for small samples. The RNA pellet was washed with 70% ethanol, air-dried and resolubilized in 30 μl RNAsecure® (Ambion), heated to 60°C for 10 min, then stored at −80°C. The DNA pellet was washed in 70% ethanol, air dried, resuspended in sterile distilled water and stored at −20°C.
To insure that the resultant cDNA from mRNA sequence truly represents only expressed genes (mRNA) it is important to remove all genomic DNA from the RNA prior to first strand synthesis. DNA was removed from the RNA using DNAse I from bovine pancreas (Molecular Probes) following the manufacturer’s instructions. DNAse was inactivated prior to first strand synthesis by heating for 10 min at 75°C. First strand cDNA was produced from isolated DNAase treated RNA using SuperScript II reverse transcriptase (Invitrogen) and random hexamer primers following the manufacturer’s instructions. To remove the RNA template, 1 μl RNAse H (Invitrogen) was added to each 20 μl reverse transcriptase reaction and incubated for 20 min at 37°C after first strand synthesis was complete. A set of controls that did not contain reverse transcriptase during first strand synthesis was carried out on DNA-free RNA to insure that there was no carry over genomic DNA contamination. The resultant cDNA was stored at −20°C.
Mya arenaria specific DII α-subunit Na+ channel fragments were generated from both cDNA and mRNA and genomic DNA extracted from each of the 36 individual M. arenaria. PCR amplification using M. arenaria specific primers oBTG-099F (5′ TTC GGG GAT GAT ATT CCA AG 3′) and oBTG-100R (5′ CCA CCA GAT TTC CTA TAA AG 3′) resulted in a 172 bp amplicon. The PCR reactions were carried out in 50 μl volumes with PfuUltraTM hotstart PCR master mix (Stratagene, La Jolla CA, USA) in a PTC-100 thermalcycler (MJ Research) with the following program: 94°C for 5 min; 35 cycles of (94°C for 30 s, 50°C for 30 s and 72°C for 1 min); ending with an extension at 72°C for 10 min. DNA from PCR reactions was immediately isolated by separation on a 1.8% NuSieve GTG low-melt agarose gel (Cambrex Bio Science Rockland, Rockland, ME, USA) in 1× TAE buffer (40 mM Tris–acetate, 1 mM EDTA) with ethidium bromide/UV visualization, and the DNA was excised. The DNA was then extracted from the agarose using a Qiagen MinElute kit (Qiagen, Valencia, CA, USA) following the manufacturer’s directions. The quantity of PCR amplicon recovered was determined with a PicoFluor hand held fluorometer (Turner Designs, Sunnyvale, CA USA) using Pico Green (Molecular Probes) fluorescent chemistry reagents according to the manufacturer’s directions.
The purified amplicon was used as a double-stranded sequencing template with oBTG-099F and oBTG-100R primers in sequencing reactions. Both strands were individually sequenced using Big Dye Terminator Cycle Sequencing in an ABI 377 autosequencer (Applied BioSystems, Foster City, CA, USA). Alignment of DNA sequences was accomplished using the software program Sequencer, Mac version 4.1 (Gene Codes Corp., Ann Arbor, MI, USA). The predicted amino acid sequence of M. arenaria was aligned using Clustal W (Thompson et al. 1994). Mutations (both homozygous and heterozygous) were identified as single nucleotide polymorphisms (SNPs) using the program Sequencher and confirmed with the web-base program SeqDoC (Crowe 2005).
Results
The nerve response
With extracellular recording, the nerve response is a compound action potential, made up of many superimposed action potentials from hundreds of individual axons in the nerve trunk, each conducting at a unique rate and contributing differently to the total voltage (Twarog et al. 1972; Twarog and Yamaguchi 1974). The “shape” and latency of the compound action potential are good indices of the number and kind of nerve fibers firing. The amplitude of any one peak does not indicate the total number of responding nerve fibers, thus, unlike microelectrode recording, it is not a direct measure of block. Partial block involves changes in shape and latency and overall amplitude decreases as some, but not all, fibers are blocked (Twarog and Yamaguchi 1974). Latency increases because the large, fast nerve fibers are most sensitive; they block at lower STX concentrations than the smaller slow fibers. At 100% block of all fibers, the action potential is abolished. Twarog and Yamaguchi (1974) show partial and full block of compound action potentials in bivalve species including M. arenaria. Bivalve species differ by up to four orders of magnitude in nerve sensitivity to STX. In the assays reported in the present study, clams which displayed full block of the CVC action potential at ≤33 μM (≤10−5g ml−1) were ranked as sensitive (S), while those which required ≥334 μM for full block were considered resistant (R) (Table 1).
Results from the in vitro nerve trunk assay show that three out of the seven populations tested were predominantly composed of clams with nerves resistant to STX (Fig. 1): Lepreau Basin, NB (91% resistant, n = 11); Essex, MA (75% resistant, n = 12); and Orleans, MA (83% resistant, n = 12). The other four populations tested were dominated by sensitive individuals: Havre-Aubert, QC (100% sensitive, n = 6); Lawrencetown, NS (69% sensitive, n = 13); Lummi Bay, Bellingham, WA (92% sensitive, n = 12; and Quartermaster Hbr., WA (92% sensitive, n = 12). Both resistant and sensitive clams from each of the populations were selected for molecular analysis, and detailed nerve assay results for those individuals are included in Table 1. On the Atlantic coast predominantly resistant populations occurred at the three sites which also had a documented history of exposure to toxic blooms, providing good agreement between the history of PSP and nerve resistance to STX. However, on the Pacific coast both sites were predominantly comprised of sensitive clams despite their documented [but more recent (see Discussion)] exposure to PSTs, in overall agreement with results obtained using the burrowing index (Bricelj et al. 2004).
Multiple variations at the 945 mutation site
Comparison of genomic DNA and cDNA from mRNA sequences of the Na+ channel DII pore region from M. arenaria revealed that each individual had identical chromatograph patterns of their cDNA from mRNA compared with their genomic DNA (an example of two individuals is shown in Table 2) and the genotype correlated with observed nerve trunk assay results (Table 1). Chromatogram peaks at the position of the SNP (mutation) for nucleotides (A, C or thymidine, T) were approximately equal height in heterozygous clams for both cDNA from mRNA and genomic DNA sequences. All individuals scored as sensitive (n = 19) in the nerve trunk assay carried only the wild type (WT) sequence of A (Table 1).
Alignment of individuals scored as resistant (n = 20) in the nerve trunk assay revealed four different genotypes (Table 1). The first genotype was comprised of homozygous individuals (n = 7) carrying the previously reported mutation of an A to a C in the third codon position, resulting in an amino acid substitution of glutamic acid (E), at position equivalent to 945 in rat, to aspartic acid (D) (Bricelj et al. 2005). The second genotype (n = 4 clams from Essex) revealed one allele with the A to C mutation and a second allele with an A to T mutation. All eleven of these clams were homozygous for the D/D predicted amino acid. Clams with the third genotype (n = 4) carried heterozygous alleles, one the wild type A and the other a C resulting in a predicted heterozygous amino acid alleles of D/E. The nucleotide substitution of a T was found in the fourth genotype (n = 5) resulting in the same amino acid substitution of E945 to D945, with heterozygous amino acid alleles of D/E.
The populations of clams used in this study were dominated by one phenotype (resistant, R or sensitive, S) as determined by the nerve trunk in vitro assay (Fig. 1) yet most locations had individual clams with the alternate phenotype. Therefore, it is likely that some individual clams may have inherited alleles associated with each of the two phenotypes. Additionally, many of the M. arenaria individuals that scored as resistant in the nerve assay were heterozygous at this locus: of the 20 resistant individuals tested, 13 had mixed alleles (Table 1).
An intermediate level of resistance was determined for individuals with mixed alleles leading to D/E (Table 1). Of the eleven clams homozygous for the D/D Na+ channel protein rated as resistant, eight did not block at 334 μM (10−4g ml−1) STX whereas three displayed a partial block at this STX concentration. Among the nine heterozygous individuals with the mixed (D/E) Na+ channel proteins, all showed partial or full block at 334 μM STX. The three resistant individuals from LE (T/A, D/E genotype) showed full block at only 167 μM (results not shown), again supporting intermediate resistance for heterozygotes.
Discussion
Spontaneous mutations and rearrangements of chromosomes are subcellular mechanisms that act to drive evolution. Although numerous models have been proposed for mutagenic processes, the factors that drive spontaneous mutations are unknown (for review see Maki 2002). Of the three primary types of spontaneous mutations, base substitutions, single base frame-shift and sequence substitutions, the former are likely the most common (Maki 2002). Not all nucleotides are equally mutable and it has been well established that the rate of substitutions also varies across sites (Yang 1996). Transitions from C to T occur in elevated frequency (Coulondre et al. 1978) and because transversions are less likely to spontaneously occur than transitions, it is possible that the two mutational events found in this study did not both involve the base A (e.g. A to C and A to T). The first event may have been a transversion of A to C and then a second transition event of C to T occurred. Support for this hypothesis is found by examination of populations with mixed alleles. There are four individuals with resistant phenotype in the EX population that have both C and T, two with A and C mutations, three with only C and none with A and T or with A and G, suggesting a sequence of mutational events rather than two separate transversion events. The two individuals with the resistant phenotype from the west coast (QMH 11 and BEL 1) both carry the mixed allele genotype. This small sample size is insufficient to draw conclusions about the potential temporal sequence of mutational events. In this case, there may have been only one A to T transversion event or the mutation was already in the population when it was imported.
Clams from the LE population with no history of PSP that displayed the rare, resistant phenotype (n = 3) had the T allele rather the C allele characteristic of the closest population with a recurrent history of PSP (Bay of Fundy). This, and the prevailing southwestward pattern of non-tidal surface circulation along the south shore of Nova Scotia (Anderson et al. 1994) suggest that gene flow from the LE to the BF populations is unlikely, and that more than one independent resistant mutation may have occurred in these populations. However, limited sampling size does not allow us to rule out the possibility of gene flow between these populations at the present time.
In most organisms with natural TTX or STX resistant Na+ channels, such as the puffer fish (Takifugu spp.), rat and human cardiac tissues, and the fruit fly (Drosophila melanogaster) containing the para mutation (Catterall 2000; Denac et al. 2000; Anderson et al. 2005b), mutations or variations have been reported in either the first or third domain (DI or DIII) of the outer pore region. Mutations generated in vitro developed for mapping STX and TTX binding sites have also identified amino acids primarily in DI and DII (Anderson 1987; Noda et al. 1989; Backx et al. 1992; Satin et al. 1992). In populations of the garter snake (Thamnophis sirtalis) Na+ channel mutations in the domain IV outer pore of skeletal muscle were found to be responsible for TTX resistance (Geffeney et al. 2005). No natural mutations have been reported for DII associated with STX or TTX binding other than that for M. arenaria (Bricelj et al. 2005). However, resistance to pyrethroid pesticides is also mediated though the Na+ channel and multiple sites associated with resistance have been identified (Soderlund and Knipple 2003). These pesticides primarily affect the Na+ channel by increasing Na+ ion permeability and thus exhausting the nervous system, leading to death of the organism (Narahashi 1992). Most of the mutations identified to date associated with pyrethroid resistance are in the DII region of the Na+ channel gene (Soderlund and Knipple 2003). The site for a mutation associated with pyrethroid resistance in the cattle tick (Boophilus microplus) (He et al. 1999) and the malaria vector mosquito (Anopheles gambiae) (Martinez-Torres et al. 1998) is the same amino acid as the mutation found in M. arenaria (Bricelj et al. 2005). At this site in all other organisms where the Na+ channel has been sequenced, the WT amino acid E is found. Both of the mutations conferring pyrethroid resistance are a substitution of the WT amino acid E for the uncharged polar amino acid glutamine (Q). Pyrethroid pesticides have been widely used since the 1970s because it was believed that they specifically target insects (Class Insecta) and cattle ticks (Class Arachnida), not other taxa. More recently a single point mutation was found in the crustacean Lepeophtheirus salmonis (sea louse) in a DII trans-membrane region (Fallang et al. 2005), just upstream from the DII Na+ channel pore where the M. arenaria mutation associated with PSP resistance was found. With reports of mutations in taxa other than the original target organisms (ticks and insects) questions have been raised about the safety of these pesticides in the environment.
Regulation of Na+ channel genes has been well studied in a number of systems and was found to be simple in M. arenaria, compared with other taxa. Because the cDNA, based on the expressed cellular RNA, and the genomic DNA patterns are identical in each of the clams in this study, the data reported in this study give no support for gene regulation at the transcriptional level in M. arenaria tissue, although this possibility cannot be ruled out without further investigation. In other systems Na+ channels comprise a multigene family, for example in rats, humans and other vertebrates (Trimmer and Agnew 1989). These vertebrate Na+ channels demonstrate tissue-specific expression, even within an organ such as the heart (Kallen et al. 1990). Expression of Na+ channel genes can also be regulated through activity-dependent control as reported for Drosophila motor neurons (Mee et al. 2004). RNA editing has been demonstrated as a mechanism for increasing diversity (and thereby potential resistance to toxins) for some invertebrate Na+ channels, including those from D. melanogaster (Hanrahan et al. 2000; Reenan 2001). In the present study, both alleles are co-expressed but only one copy of the mutation is required for the individual to show the PSP resistant phenotype. However, a lower resistance may be associated with heterozygotes, as suggested by the intermediate level of nerve resistance to STX shown by heterozygous individuals (Table 1). A larger sample size, as well as nerve assays at additional STX concentrations, for heterozygous compared to homozygous resistant individuals must be obtained in future studies to further define an intermediate phenotype. Our results to date suggest that the M. arenaria model described here may represent one of the simplest forms of Na+ channel resistance to STX, a point mutation.
It is likely that algal toxins in the environment were instrumental in the evolution of Na+ channel genes over a broad range of taxa as suggested by Anderson et al. (2005b). This hypothesis is supported by a survey of organisms that have STX or TTX insensitive Na+ channels, such as members of the phylum Cnidaria that are sedentary and cannot leave waters when toxic algal blooms occur (Anderson et al. 2005b). Within molluscs, it is known that some bivalves species show marked (greater than 1,000-fold) variation in their neural levels of sensitivity to STX and it was suggested that some of these species may have developed resistance to PSTs in areas where blooms are common (Twarog et al. 1972; Twarog and Yamaguchi 1974). It is now clear that algal toxins can act as a natural selection agent and shape the genetic population structure of M. arenaria, and may be involved in selection for PST resistance in populations of other bivalve species.
Populations of organisms with different genotypes (or gene expression) exposed to diverse environmental conditions are able to exploit changing conditions by virtue of selective advantage (increased fitness) and potentially exploit marginally suitable habitats or expand the range of the species overall. There are few well-studied examples of genetic mutations or regulation of genes known to be directly involved with adaptation to specific natural environmental stressor gradients, such as temperature, salinity, or in this study toxin levels. For example, the relationship between the lactate dehydrogenase-B enzyme (Ldh-B) and water temperature gradients along Atlantic North America was demonstrated in the killfish, Fundulus heteroclitus (Crawford and Powers 1992). Variants of the Ldh-B gene are differentially expressed in killfish populations based on sequence changes in the transcriptional start sites (Schulte et al. 2000). In mussels (Mytilus spp.) aminopeptidase-1 enzyme polymorphisms (Lap locus) were found to be associated with differential survival of immigrants when transported into areas with differing salinities resulting in significant mortality and higher stress for those individuals carrying the Lap94 allele in low salinity habitats (Koehn et al. 1980). The basis for natural selection against the Lap94 allele was attributed to higher nitrogen excretion rates leading to reduced growth in the fall (Hilbish and Koehn 1985). These studies illustrate that there is segregation of populations based on allelic frequencies of specific functional genes in relation to specific environmental stressors. Yet changing patterns of gene regulation do not reveal the entire story and the simple mechanism of gene mutation is once again being explored in links between environmental stress and physiology, as with the case of M. arenaria resistance to PSTs.
Genetic diversity of wild clam populations throughout the western Atlantic has not been well studied. However, wild populations of the hard clam, M. mercenaria, from Massachusetts through Virginia, USA, show remarkable genetic uniformity using seven polymorphic enzyme loci (Dillon and Manzi 1987) and a study of M. arenaria populations throughout New England found low diversity in the ribosomal internal transcribed spacer (ITS) gene (Caporale et al. 1997). These studies suggest that there is rapid gene flow among these clam populations, likely through transport of planktonic larvae by currents. However, despite this rapid gene flow, the present study shows considerable differentiation in Atlantic M. arenaria populations with respect to resistance to PSTs.
Selective pressures exerted on clam populations by PSTs have not yet been specifically measured, but a survey of Alexandrium spp. bloom history coupled with the extent of PST exposure to various M. arenaria populations may provide clues to the rate of expansion of a STX resistant phenotype. On the Atlantic coast, red tides of toxic Alexandrium spp. are well documented from the estuary of the Gulf of St. Lawrence, Canada to southern New England, USA, with shellfish toxicity levels typically highest in northern waters (Anderson 1997). Expansion of blooms from northern to southern New England has been attributed to advection of cysts following storms in the Gulf of Maine during September 1972 (Mulligan 1974; Anderson et al. 2005a). Both Alexandrium spp. and M. arenaria are native throughout this region and M. arenaria has coevolved with PSTs.
Mya arenaria is a non-native species on the west coast where it was first reported in San Francisco Bay, California in 1874 (Shebley 1917). It may have been incidentally introduced from the east coast of North America with oysters (Crassostrea virginica) (Townsend 1893), and starting in 1876 and 1882 was intentionally planted in Grays Harbor, WA from the San Francisco Bay population (Palacios et al. 2000). Presumably, the population expanded northward from Grays Harbor into Puget Sound and the Strait of Georgia. A large and unexplained die-off of M. arenaria between 1895 and 1897 left only remnant populations throughout the area (Palacios et al. 2000). Although the original source of the Pacific M. arenaria population is unknown, it is possible that some clams imported into San Francisco Bay already carried the T allele. Therefore, the evolution of resistance to PSP in Pacific M. arenaria populations is confounded by uncertainties regarding historical introductions and is more difficult to trace than that of native Atlantic populations.
Historically PSP closures in central Puget Sound (QMH) were uncommon until relatively recently, i.e. the 1970s (Trainer et al. 2003), even though there is evidence of PSP events as early as 1793 on what is now the coast of British Columbia (Vancouver and Broughton 1802). This recent occurrence of PSP (and/or reduced intensity of red tides) may explain the dominance of sensitive clams in clam populations from the two west coast sampling sites. Overall, all populations of M. arenaria sampled in this study consisted of both resistant and sensitive individuals, with the exception of the HA, Québec population, which contained only sensitive clams, although this may simply reflect the small sample size used. Independent of where any individual clam was collected, the nerve assay phenotype and the genotype in this study matched.
During 2005, there were massive and economically destructive blooms of toxigenic Alexandrium fundyense in NW Atlantic USA that resulted in extensive coastal clam bed closures as well as the closure of 40,000 km2 of offshore federal waters (Anderson et al. 2005a). These blooms further extended the distribution of PSP in southern New England (Anderson et al. 2005a). This raises the potential threat of future expansion of PSP even farther southward to previously unaffected waters, such as coastal waters of Rhode Island, via germination and transport of cysts deposited during the 2005 bloom. Geographic expansion of PSP will provide additional opportunities to follow the prevalence and type of Na+ channel mutations responsible for development of toxin resistance in populations of M. arenaria, and thus allow prediction of their capacity for toxin accumulation.
Other questions remain to be answered about the evolution of PST resistance in M. arenaria. The rate at which toxic Alexandrium blooms can exert selective pressure on M. arenaria populations, depending on the timing and intensity of outbreaks, is not yet known. The susceptibility to PST-induced mortalities is greater in smaller softshell clams (∼4 mm SL) than in larger (35–42 mm) juveniles (Bricelj and MacQuarrie 2004). Therefore, Alexandrium blooms will likely exert variable selective pressure on different life history stages.
Mutations that confer a selective advantage to an organism under particular conditions may also have adverse effects. For example, garter snakes (T. sirtalis) from TTX-resistant populations in western USA are able to consume their dominant prey species, the rough-skinned newt (Taricha granulosa) that accumulates high TTX levels (Geffeney et al. 2002, 2005), but have a slower crawling rate (Brodie and Brodie 1999) making them more vulnerable to their own top predators. Is there a fitness trade-off that M. arenaria has made to enable it to resist algal toxins? Currently it is unclear how a geographic expansion of clams carrying the resistant gene into new populations will affect the food web in general, and what the rate of selection for resistance to PSTs is under varying bloom scenarios in M. arenaria populations.
References
Anderson PAV (1987) Properties and pharmacology of a TTX-insensitive Na+ current in neurons of the jellyfish Cyanea capillata. J Exp Biol 133:231–248
Anderson DM (1997) Bloom dynamics of toxic Alexandrium species in the northeast US Limnol Oceanogr 42:1009–1022
Anderson DM, Kulis DM, Doucette GJ, Gallagher JC, Balech E (1994) Biogeography of toxic dinoflagellates in the genus Alexandrium from the northeastern United States and Canada. Mar Biol 120:467–478
Anderson DM, Keafer BA, McGillicuddy DJ, Mickelson MJ, Keay KE, Libby SP, Manning JP, Mayo CA, Whittaker DK, Hickey JM, He R, Lynch DR, Smith KW (2005a) Initial observations of the 2005 Alexandrium fundyense bloom in southern New England: general patterns and mechanisms. Deep Sea Res II 52:2856–2876
Anderson PAV, Roberts-Misterly J, Greenberg RM (2005b) The evolution of voltage-gated sodium channels: were algal toxins involved? Harmful Algae 4:95–107
Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF (1992) Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science 257:248–251
Bricelj VM, MacQuarrie SP (2004) Can natural selection for resistance to paralytic shellfish poisoning toxins in softshell clam, Mya arenaria, populations occur during early life history stages? Harmful Algae 3:193–194
Bricelj VM, MacQuarrie SP, Twarog B, Trainer VL (2004) Characterization of sensitivity to PSP toxins in North American populations of the softshell clam Mya arenaria. In: Steidinger KA, Landsberg JH, Tomas CR, Vargo GA (eds) Harmful Algae 2002. Florida Fish and Wildlife Conservation Commission, Florida Institute of Oceanography and IOC of UNESCO, St. Petersberg, FL, pp 172–174
Bricelj VM, Connell L, Konoki K, MacQuarrie SP, Scheuer T, Catterall WA, Trainer VL (2005) Na+ channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature 434:763–767
Brodie ED III, Brodie ED Jr (1999) Costs of exploiting poisonous prey: evolutionary trade-offs in a predator–prey arms race. Evolution 52:626–631
Caporale DA, Beal BF, Roxby R, Van Beneden RJ (1997) Population structure of Mya arenaria along the New England coastline. Mol Mar Biol Biotechnol 6:33–39
Catterall WA (1992) Cellular and molecular biology of voltage-gated sodium channels. Physiol Rev 72:S15–S48
Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26:13–25
Coulondre C, Miller JH, Farabaugh PJ, Gilbert W (1978) Molecular basis of base substitution hotspots in Escherichia coli. Nature 274:775–780
Crawford DL, Powers DA (1992) Evolutionary adaptation to different thermal environments via transcriptional regulation. Mol Biol Evol 9:806–813
Crowe ML (2005) SeqDoC: rapid SNP and mutation detection by direct comparison of DNA sequence chromatograms. BMC Bioinformatics 6:133–144
Denac H, Mevissen M, Scholtysik G (2000) Structure, function and pharmacology of voltage-gated sodium channels. Naunyn Schmiedebergs Arch Pharmacol 362:453–479
Dillon RT Jr, Manzi JJ (1987) Hard clam, Mercenaria mercenaria, broodstocks: genetic drift and loss of rare alleles without reduction of heterozygosity. Aquaculture 60:99–105
Fallang A, Denholm I, Horsberg TE, Williamson MS (2005) Novel point mutation in the sodium channel gene of pyrethroid-resistant sea lice Lepeophtheirus salmonis (Crustacea: Copepoda). Dis Aquat Organ 65:129–136
Geffeney SL, Brodie ED Jr, Ruben PC, Brodie ED III (2002) Mechanisms of adaptation in a predator–prey arms race: TTX-resistant sodium channels. Science 297:1336–1339
Geffeney SL, Fujimoto E, Brodie ED III, Brodie ED Jr, Ruben PC (2005) Evolutionary diversification of TTX-resistant sodium channels in a predator–prey interaction. Nature (London) 434:759–763
Goldin AL, Barchi RL, Caldwell JH, Hofmann F, Howe JR, Hunter JC, Kallen RG, Mandel G, Meisler MH, Netter YB, Noda M, Tamkun MM, Waxman SG, Wood JN, Catterall WA (2000) Nomenclature of voltage gated sodium channels. Neuron 28:365–368
Hallegraeff GM (1993) A review of harmful algal blooms and their apparent global increase. Phycologia 32:79–99
Hanrahan CJ, Palladino MJ, Ganetzky B, Reenan RA (2000) RNA editing of the Drosophila para Na+ channel transcript: evolutionary conservation and developmental regulation. Genetics 155:1149–1160
He H, Chen AC, Davey RB, Ivie GW, George JE (1999) Identification of a point mutation in the para-type sodium channel gene from a pyrethroid-resistant cattle tick. Biochem Biophys Res Commun 261:558–561
Hilbish TJ, Koehn RK (1985) The physiological basis of natural selection at the Lap locus. Evolution 39:1302–1317
Kallen RG, Sheng Z-H, Yang J, Chen L, Rogart RB, Barchi RL (1990) Primary structure and expression of a sodium channel characteristic of denervated and immature rat skeletal muscle. Neuron 4:233–242
Koehn RK, Newell RIE, Immermann F (1980) Maintenance of an aminopeptidase allele frequency cline by natural selection. Proc Natl Acad Sci USA 77:5385–5389
MacQuarrie SP (2002) Inter-and intra-population variability in behavioral and physiological responses of the softshell clam Mya arenaria, to the PSP toxin-producing dinoflagellate, Alexandrium tamarense. M.S. Thesis, Department of Biology, Dalhousie University, Halifax, Nova Scotia, Canada
Maki H (2002) Origins of spontaneous mutations: specificity and directionality of base-substitution, frameshift and sequence-substitution mutagenesis. Annu Rev Genet 36:279–303
Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL (1998) Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol Biol 7:179–184
Mee CJ, Pym ECG, Moffat KG, Baines RA (2004) Regulation of neuronal excitability through pumilio-dependent control of a sodium channel gene. J Neurosci 24:8695–8703
Mulligan HF (1974) Oceanographic factors associated with New England red tide blooms. In: LoCicero VR (ed) The first international conference on toxic dinoflagellate blooms. Massachusetts Science and Technology Foundation, Wakefield, pp 23–40
Narahashi T (1992) Overview of toxins and drugs as tools to study excitable membrane ion channels: II transmitter-activated channels. Methods Enzymol 207:643–658
Noda M, Suzuki H, Numa S, Stühmer W (1989) A single point mutation confers tetrodotoxin and saxitoxin insensitivity on the sodium channel II. FEBS Lett 259:213–216
Oshima Y (1995) Post-column derivatization HPLC methods for paralytic shellfish poisons. In: Hallegraeff GM, Anderson DM, Cembella AD (eds) Manual on Harmful Marine Microalgae, IOC Manuals and Guides. UNESCO 33:81–94
Palacios R, Armstrong DA, Orensanz J (2000) Fate and legacy of an invasion: extinct and extant populations of the soft-shelled clam (Mya arenaria) in Grays Harbor (Washington). Aquat Conserv Mar Freshw Ecosyst 10:279–303
Reenan RA (2001) The RNA world meets behavior: A->I (pre-mRNA editing in animals). Trends Genet 17:53–56
Satin J, Kyle JW, Chen M, Bell P, Cribbs LL, Fozzard HA, Rogart RB (1992) A mutant of TTX-resistant cardiac sodium channels with TTX-sensitive properties. Science 256:1202–1205
Schulte PM, Glemet HC, Fiebig AA, Powers DA (2000) Adaptive variation in lactate dehydrogenase-B gene expression: role of a stress-responsive regulatory element. Proc Natl Acad Sci USA 97:6597–6602
Shebley WH (1917) History of the introduction of food and game fishes into the waters of California. Calif Fish Game 3:3–12
Soderlund DM, Knipple DC (2003) The molecular biology of knockdown resistance to pyrethroid insecticides. Insect Biochem Mol Biol 33:563–569
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Townsend CW (1893) Report of observations respecting the oyster resources and oyster fisheries of the Pacific Coast of the United States. In: United States Commission of Fish and Fisheries (ed) Report of The Commissioner for 1889 to 1891. Government Printing Office 1893, pp 343–372
Trainer VL, Eberhart B-TL, Wekell JC, Adams NG, Hanson L, Cox F, Dowell J (2003) Paralytic shellfish toxins in puget sound, Washington State. J Shellfish Res 22:213–223
Trimmer JS, Agnew WS (1989) Molecular diversity in voltage sensitive Na+ channels. Annu Rev Physiol 51:401–418
Twarog BM, Yamaguchi H (1974) Resistance to paralytic shellfish toxins in bivalve molluscs. In: LoCicero VR (eds) Proceedings of the first international conference on toxic dinoflagellate blooms. Massachusetts Science and Technology Foundation, Wakefield, pp 381–393
Twarog BM, Hidaka T, Yamaguchi H (1972) Resistance to tetrodotoxin and saxitoxin in nerves of bivalve molluscs: a possible correlation with paralytic shellfish poisoning. Toxicon 10:273–278
Vancouver G, Broughton WR (1802) A narrative or journal of a voyage of discovery to the North Pacific Ocean and round the world [microform]: performed in the years 1791, 1792, 1793, 1794 and 1795 by Captain George Vancouver and Lieutenant Broughton. Smeeton, J (printer), London
Vles F (1909) Monographie Sommaire de la Mye (Mya arenaria Linne). Mem Soc Zool Fr 22:90–142 (in French, English translation by USFWS, 1954)
Yang Z (1996) Among-site rate variation and its impact on phylogenetic analysis. Trends Ecol Evol 11:367–372
Acknowledgments
We thank the many individuals that assisted in field sample collection: B. Myrand (MAPAQ, Québec), D. Whitaker, J. Kennedy, L. Savina, S. Cunningham, D. Farbes (Massachusetts DMF), G. Gillespie (DFO BC), N. Adams and B. Bill (NOAA NWFSC). We also thank anonymous reviewers for detailed comments that have led to improvement of the final version of this manuscript. This work was supported by a NOAA-ECOHAB #NA 17OP1451 grant to LBC, VMB and V. Trainer. This is IMB/NRC publication #42562.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. J. Thompson, St. John's.
Rights and permissions
About this article
Cite this article
Connell, L.B., MacQuarrie, S.P., Twarog, B.M. et al. Population differences in nerve resistance to paralytic shellfish toxins in softshell clam, Mya arenaria, associated with sodium channel mutations. Mar Biol 150, 1227–1236 (2007). https://doi.org/10.1007/s00227-006-0432-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0432-z