Abstract
Observations from the purse seine fishery off northern Portugal are used to describe the early dynamics of sardine (Sardina pilchardus) stress reactions and identify likely stressors during the commercial fishing operation. Sardine blood and muscle were sampled from the onset of fishing (school identification and encircling) to the end of fish transfer onboard (90–120 min later). The evolution of haematocrit, haemoglobin, cortisol, glucose, ionic concentrations, ATP and its catabolites were modelled using linear mixed models as a function of time spent in the net, biological (sex, reproductive state and condition) and operational variables (catch, light level and phase of fishing operation). Significant linear trends with time were detected for most stress variables and mean concentrations after 2 h in the net were similar to literature values corresponding to acute stress reactions for teleosts. Biological variables were rarely significant and explained a small proportion of variation, while operational variables were never significant. For each stress variable, levels varied considerably between trips but the temporal evolution was common across trips. Random trip effects were uncorrelated among most biochemical variables, suggesting that distinct factors affected each stress variable during the sampled trips. However, the linear trend with time spent in the net observed for most stress variables indicates that the duration of the fishing operation is an important stressor in purse seine fishing due to the progressive water volume restriction, crowding and manipulation associated to the fishing method.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Responses to stressful events are well documented for several fish species, where unavoidable manipulation (capture, handling, netting, crowding or transport) and poor water quality under aquaculture conditions can affect commercial production or re-stocking programs. Stress is related to a cascade of physiological reactions that range from immediate hormonal responses to long-term changes in growth and reproductive potential (Mazeaud et al. 1977; Wood et al. 1983; Barton and Iwana 1991). In the attempt to regain homeostasis, responses are grouped into primary (hormonal), secondary (metabolic, hematological, hydromineral and structural) and tertiary (whole organism and population). Primary responses are characterized by the activation of the hypothalamo-pituitary interrenal axis and the release of catecholamines and cortisol into the bloodstream (Wendelaar Bonga 1997), whereas secondary responses arise from the action of these hormones at the blood and tissue level (e.g. hyperglycemia, increases of cardiac output, oxygen uptake, erythrocyte release and swell, rise in haematocrit and haemoglobin and disturbance of the hydromineral balance). Long-term (tertiary) reactions range from suppressed appetite, growth and reproduction to immunodeficiency and, eventually, death (Mazeaud et al. 1977; Bourke et al.1987; Barton and Iwana 1991; Schreck et al. 2001).
In several fisheries, concerns about the potential survival of released or escaping components of the fish catch have highlighted the importance to identify the main stressors, quantify the stress reactions and understand the consequences of stress caused during fishing (Pawson and Lockwood 1980; Swift 1983; Bourke et al. 1987; Chopin and Arimoto 1995; Olla et al. 1997, 1998; Davis et al. 2001). Studies are usually based on laboratory simulations of the fishing practice, where the additional effect of behaviour factors (e.g. swimming performance, predator avoidance) and environmental conditions (e.g. temperature, light intensity) can be tested experimentally (Olla et al. 1997, 1998; Davis et al. 2001; Davis 2005). Some studies question the correlation of stress variables with ensuing mortality (Davis et al. 2001; Davis 2002), although it is generally considered that physiological responses provide an accurate indicator of the magnitude of stressors experienced during the fishing process (Davis 2005).
Observations of fish stress reactions to commercial fishing are very scarce and problems usually arise in the determination of the duration and nature of the stressor related to the fishing operation (Chopin and Arimoto 1995; Manire et al. 2001; Davis 2002). In addition, uncontrolled additional stressors (e.g. changes in environmental conditions during fishing—Olla et al. 1998; Davis et al. 2001) can have a confounding effect in field studies, while the consequences of stress reactions to subsequent survival cannot be easily monitored (Davis 2002). Some of these problems seem to be less restrictive in purse seine fishing for small pelagics, where the operation takes place in the relatively stable upper part of the water column, the duration of fishing can be measured precisely for all fish and sampling can take place throughout the operation. In addition, purse seine fisheries provide an interesting case for fish stress studies since existing observations have demonstrated that the deliberate release of a component of the catch (slipping) is frequent (Lockwood et al. 1983; Misund and Beltestad 2000; Mitchell et al. 2002; Stratoudakis and Marçalo 2002) and potentially stressful events associated to the fishing operation have already been detected (Stratoudakis et al. 2003). Here, we use field samples from the Portuguese purse seine fishery to describe the early dynamics of stress variables in sardine (Sardina pilchardus) during commercial fishing operations. Linear mixed models are used to analyse stress variables as a function of the time elapsed since the original application of the fishing stressor (detection of the school and encircling), to identify biological and operational variables with a measurable impact on sardine stress reactions and to provide realistic estimates of mean levels of primary and secondary stress responses during and at the end of commercial fishing operations at sea.
Material and methods
The study took place between May and July 2002 onboard commercial purse seine vessels operating off the port of Figueira da Foz in northern Portugal. Sampled trips were short (total duration up to 12 h), took place within half a degree of latitude from the home port (39°46′–40°43′N) and usually consisted of a single setting of the net before or around sunrise. Fishing followed the typical purse-seine operation (Stratoudakis and Marçalo 2002), involving the rapid deployment of a long net (up to 800 m long and 150 m deep) around marks of pelagic fish identified by the electronic fish-finding equipment, the closure of the bottom of the net (trapping the fish in a purse) and the drying-up of the net (gradually reducing the volume of the purse until the density of the fish becomes sufficiently high to start the transport onboard). Once a target school was detected and the net was set, fishing operations usually lasted about 2 h (90–160 min). Hauling time was usually around 1 h, while the transfer period varied depending on catch size (Fig. 1). Catch and slipping estimates in each trip were obtained by visual evaluation provided by the skipper (Stratoudakis and Marçalo 2002). A trained observer followed 19 fishing trips and collected blood and muscle samples from fish individually collected from the encircled area. In nine trips no fish were caught (failure to detect sufficiently dense schools, gear failures, bad weather, etc.), so fish samples were only collected in ten fishing trips (Table 1). One set was sampled in each trip, although in two trips the net was set a second time before returning to port.
Diagram showing sequence of events during purse seining operations and sampling. Average operation time (minutes) and standard deviations for the ten trips are indicated in parenthesis. Increasing sampling intensity is indicated at the top of the diagram as net volume decrease facilitated the capture of fish
Fish with similar swimming performance (regular movements and presenting no signs of lethargic behaviour) were individually collected with a hand net from the encircled area from the start of hauling to the end of fish transfer aboard. Overall, 174 fish were collected in the 10 trips, ranging from 7 to 24 per trip. Time of sampling ranged from 30 to 138 min after net deployment, covering approximately equally the phase of hauling and fish transfer. Blood was taken immediately after capture by caudal vein puncture with a hypodermic needle. Depending on fish size, 400–700 μl of blood were retrieved and immediately transferred to heparinized tubes. Blood samples were kept at 0°C for roughly 24 h for subsequent haematological analysis. Fish tissue (3–5 g of muscle from the dorsal area) was also collected from some individuals (total of 72 sardines), cleaned from skin and scales and immediately frozen in liquid nitrogen for adenine nucleotide determination. There was insufficient time to sample both blood and muscle from all individuals, so muscle sampling was restricted to a few fish (3–4 fish) at the beginning and at the end of the sampling period. Handling to obtain both blood and muscle samples never exceeded 3 min per fish. Sampled fish were frozen for subsequent biological analysis. In the laboratory, fish were thawed, measured and weighed. Standard biological parameters such as sex, maturity state, fat index, gutted weight and gonad weight were also obtained. Fish condition was estimated as the ratio of total weight over the cube of total length (×1000) and the gonadosomatic index (GSI) estimated as the ratio of gonad weight over gutted weight (×100). Table 2 summarises the sampling effort and the biological measurements in each trip.
Within a day from collection, blood samples were processed at the Veterinary Medicine Laboratory of the Technical University of Lisbon. Blood samples were initially homogenized at room temperature for 15 min for haematocrit (Hct) and haemoglobin (Hb) analysis. Hct values (%) were obtained by centrifuging blood samples for 5 min at 5,000 rpm in microhaematocrit tubes and determined using a microcapillary reader. Whole blood Hb (g/dl) was measured using a Boehringer Mannhein Reflotron II chemistry set analyzer. The mean cell haemoglobin concentration (MCHC) was calculated using the formula Hb/(Hct/100). Plasma from the blood samples was separated by centrifugation at 2,500 rpm for 10 min and frozen at −20°C for subsequent analysis of glucose, ionic concentration (sodium, potassium and chloride) and cortisol. Glucose was measured using a Boehringer Mannhein Reflotron II chemistry set analyzer. Glucose consumption by blood cells was inhibited with blood refrigeration prior to analysis, although subsequent analyses showed that the 24 h between sampling and processing resulted in a reduction of glucose levels up to 10% (A. Marçalo, personal observations). Sodium, chloride and potassium concentration were determined in a Vet Lyte electrolyte analyzer (IDeXX Laboratories, Inc., USA). Plasma cortisol was assayed in duplicate and measured by a validated solid RIA assay, without extraction, using a commercially available kit (Coat-A-Count Cortisol kit, Diagnostic Product Corporation, Los Angeles, CA, USA). The intra-assay coefficient of variation of all samples was 5.1%, calculated according to Rodbard (1974).
All muscle samples were stored in liquid nitrogen onboard and maintained at −80°C until laboratory analysis (on average 1 month after collection). Extracts for analysis were prepared by blending 3–5 g of sardine muscle with 25 ml of 0.6 M perchloric acid at 0°C for 1 min in a Polytron PT3000 homogenizer according to Ryder (1985). They were analyzed using a simple reverse-phase separation with a commercially available column (Hewlett Packard LiChrosorb RP-18, 10 μm, 200×4.6 mm2) and a Hewlett Packard 1050 Series liquid chromatograph equipped with a multiple wavelength UV detector. Nucleotides were identified by comparing retention time with prepared standards as recommended by Ryder (1985). Samples were analyzed in duplicate and the means calculated.
The early dynamics of biochemical stress variables in sardine during purse seine fishing were investigated using linear mixed models (LMMs). LMMs provide a general framework for modeling unbalanced nested data (variable number of fish sampled at variables times within trips) that can incorporate information from continuous and categorical explanatory variables, while capturing the randomness inherent in field data by the concept of the random effect (see Pinheiro and Bates (2000) for statistical principles, Lai and Hesler (2004) for a fisheries application). Model selection for each biochemical variable was as follows. First a ‘full’ model was fitted with the fixed component set to the main effects of time (since net deployment), biological variables (maturity, sex and fat) and operational variables [log (catch), light level and phase of fishing process]. Trip was included as a random effect, with the mean response in each trip (for any given values of the fixed explanatory variables) assumed to be distributed normally with zero mean and constant variance. The within-trip errors were assumed to be independent of the random effects and also distributed normally with zero mean and constant variance (for nucleotide adenosine variables, 1–2 outliers where removed to approximate the distributional assumptions). The model was then simplified by stepwise elimination of the main effect with the lowest (non-significant) value of the Wald test statistic (in the results, the Wald statistic is reported as the equivalent F-statistic on the appropriate degrees of freedom). More complex models were also considered but gave no additional significant results. In particular, there was no evidence of any interaction between the time effect and the biological or operational variables or that the time effect varied between trips.
Results
The sampled trips took place over a range of environmental and operational conditions that are typical of purse seine fishing off Portugal (Table 1) and similar to those reported in southern Australia (Mitchell et al. 2002). Purse seining for small pelagics usually takes place at the inner continental shelf (20–60 m depth), where abrupt pressure and temperature differences are unlikely to have an impact on fish survival. Sea surface temperatures revealed that the variability within the 3 months was small (average = 15.7±0.4°C, range from 15.1 to 16.1°C) and its effect on stress in the fish should be negligible. Although there is a preference for net setting around sunrise (when distinct pelagic schools start to re-emerge after the night dispersal), the entire fishing operation can take place either at night or during daylight. Wave height can vary considerably among trips (<0.5 to >2 m of swell) and may affect stress reactions during hauling and fish transfer by increasing the probability of fish collision with the net walls or cause mutual abrasion. Of the ten sets sampled, six were on moderate echo-sounder marks and only two on dense marks, resulting in catches that varied from 1 to 17 tones. However, in three sets many fish escaped during the closing of the net. Depending on the catch composition and the daily landing limit, slipping varied from practically nothing to 7 tones per set. Only variables that changed within the trip and could be measured at each sampling time (presence/absence of light and phase of the fishing operation) were considered for modeling, together with the log (catch) in each trip.
Table 2 shows that there was little variation in sardine size, but there was a change in mean condition along the study period. The mean total length of the 174 sampled sardines was 16.9 cm (range 14.7–19.5 cm), with a mean total weight of 39.6 g (range 24.0–74.1 g). There was no evidence of systematic variation in fish size within or between trips, although female sardine were larger/heavier than males. Despite the relatively large size of fish (which should correspond to a modal age of 2 years), sex could only be determined in 165 fish (77 males and 88 females). Sampling took place in late spring/summer, coinciding with the transition from the late spawning to feeding season. This transition is particularly evident in changes in the condition factor and gonadosomatic index through the study period; sardine condition factor is significantly lower in the first four trips (that took place in May) than in the remaining ones (F 1,8=12.1; P=0.008), while the converse is true for GSI (F 1,8=26.6; P<0.001). The biological transition from spawning to feeding is well depicted by the macroscopic data on maturity stage and fat content. Given that maturity and fat data can be easily summarized by 2-level categorical variables (maturity—active/inactive; fat—not visible/visible) these two variables were used as proxies for reproductive activity and condition respectively in the LMMs.
Reliable haematological data were not obtained in 11 of the 174 fish. Preliminary exploration also showed a potentially significant effect of sex, so data for the 9 unsexed fish were removed, reducing the total number of observations to 154 fish. Haematocrit (observations ranging between 21.9 and 51%) decreased significantly with time (F 1, 143=17.0; P<0.001). The estimated rate of decrease was 3.8% per hour, resulting in mean heamatocrit (over fish and trips) of 39.7% 1 h after the onset of fishing (Table 3; Fig. 2a). Haemoglobin concentration (observations ranging between 6.9 and 16.1 g/dl) also decreased significantly with time (F 1, 142=12.5; P<0.001). The estimated rate of decrease was 11.5 g/dl per hour, resulting to a mean haemoglobin concentration of 12.52 g/dl for male fish after 1 h in the net (Table 3; Fig. 2b). Females had slightly higher haemoglobin levels than males (F 1, 142=4.7; P=0.031). Neither time nor any of the other variables had any effect on MCHC. The decrease of both haematocrit and haemoglobin concentration over time and the temporal stability of MCHC indicate that the observed physiological changes most likely resulted from an increase in the volume of plasma over time and not from haemolysis under stress.
Observed (points) and fitted (lines) effect of time from onset of fishing on the haematocrit (left) and haemoglobin (right) of sardine in the purse seine net (filled circles females; open circles males). Sex was marginally significant in the haemoglobin model (solid line fitted effect for females, broken line fitted effect for males)
Observed (points) and fitted (lines) effect of time from onset of fishing on glucose (left) and cortisol (right) levels in the plasma of sardine blood. Fish with larger fat deposits (open circles) had a significantly higher level of blood glucose than fish with lower fat deposits (solid line time effect for fat fish, broken line time effect for thinner fish)
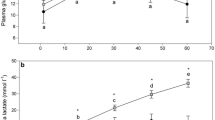
Glucose (observations ranging between 46.7 and 192 mg/dl) and cortisol (observations ranging between 0.2 and 31.1 μg/dl) levels (n=174; data not available for one fish) both increased linearly with time (glucose: F 1,161=63.9, P<0.001; cortisol: F 1,162=59.8, P<0.001). Glucose levels were greater in fatter fish (F 1,161=11.7, P<0.001). Glucose mean concentration after 1 h in the net was 99.7 and 120.9 mg/dl for lean and fat fish respectively, with an estimated increase in both cases by 35.2 mg/dl per hour. Cortisol mean concentration was 8.9 μg/dl after 1 h in the net, increasing by a rate of 6.9 μg/dl per hour.
Of the three ionic concentrations measured in the blood plasma (sodium, Na+, potassium, K+ and chloride, Cl−) only Cl− (observations ranging between 119 and 200 mmol/l) was modelled (n=130, ionic concentrations not measured for 44 fish). This is because Na+ (observations ranging between 133 and 205 mmol/l) and K+ (observations ranging between 1.5 and 15 mmol/l) concentrations often exceeded the higher and lower limits of calibration respectively (Fig. 4c, d). Cl− concentration increased with time (F 1,119=14.4; P<0.001) by an estimated rate of 10 mmol/l per hour, reaching 144.3 mmol/l after an hour in the net (Fig. 4a, Table 3). No other variable had a significant effect on Cl−. The high correlation between Cl− and Na+ (positive, Fig. 4c) and Cl− and K+ (negative, Fig. 4d) suggest that Na+ and K+ increased and decreased respectively with time, although we cannot assess the statistical significance of this. Further, if we only consider ionic concentrations for Cl−<160 mmol/l (where saturation for Na+ seems to have occurred), ionic balance seems also to have changed over time (Fig. 4b). This is because the increase in Na+ was more rapid than the decrease in K+ and increase in Cl− combined, leading to an increase in the ratio of positively: negatively charged ions in the plasma with time spent in the net.
Top panels: Observed (points) and fitted (line) chloride concentration (left) and ionic balance (right) in the plasma over time spent in the net. Bottom panels chloride versus sodium (left) and chloride versus potassium (right) concentrations in the sampled fish. Lines in panels c and d correspond to smooth splines on 3 degrees of freedom
In the case of adenine nucleotides and related compounds (adenosine tri- bi- and mono-phosphate (ATP, ADP and AMP respectively); inosine monophoshate, IMP; inosine, HxR and hypoxanthine, Hx) muscle concentrations (μmol/g wet weight) were only measured in 74 fish. Concentrations were highest for IMP (observations ranging between 6 and 24.2 μmol/g) and lowest for Hx (observations ranging between 0 and 0.7 μmol/g) and HxR (observations ranging between 0 and 1.7 μmol/g). Marginally significant time effects (Table 3, Fig. 5) were detected for ATP (linear decrease with time—F 1,61=4.4; P=0.040) and ADP (linear decrease with time—F 1,61=4.78; P=0.033) and a highly significant time effect was found for HxR (linear increase with time—F 1,61=9.74; P=0.003). No other variables were significant, although in the case of inosine monophosphate only marginally so (linear increase with time—F 1,61=3.1; P=0.083).
To compare the rate of change with time among biochemical variables, the fitted slope (Table 3) was multiplied by 60 min (to represent an hourly change) and divided by the intercept (mean concentration after an hour spent in the net). Percent hourly change in concentration was lowest for chloride and haematological variables (<10%), intermediate for glucose, ATP and ADP (20–35%) and highest for cortisol and hypoxanthine (>75%). Table 3 also shows that the random standard deviation between trips ranged from less than half of the residual standard deviation between fish (haematological variables, cortisol, ATP and its catabolites) to approximately the same (glucose and chloride). Highly significant correlations (positive) of random trip effects were only found for haematocrit and heamoglobin and for ATP and ADP, while marginally significant correlations (negative) were also found for chloride with ATP and ADP.
Discussion
The present study demonstrates that purse seine fisheries can provide an interesting case study for improving the understanding of fish stress reactions to fishing. Purse seining off northern Portugal induced significant stress responses in sardines, with mean values at the end of the fishing operation similar to peak values reported in the literature after acute stress. Biological and operational variables had a minor impact on stress responses, while a highly significant linear trend with time spent in the net was observed for most variables. The lack of a significant catch effect (fixed) and the absence of significant between-trip variation in the time effect (random) indicate that time spent in the net is a more important operational variable than fish abundance (total catch in the net). Also, the lack of a significant operation phase effect (hauling or transferring) suggests that the observed pulse in nutrient release during the end of hauling (Stratoudakis et al. 2003) is not associated with a sudden increase in stress variables, while the lack of correlation between the random trip effects of groups of biochemical variables indicates that distinct (unaccounted) factors such as wave height, luminosity or handling during operations (all increasing vulnerability to abrasion and physical damage) may influence the mean concentrations (but not the temporal evolution) of each variable. Variables like change in sea water temperature and pressure that have been shown experimentally to be important stressors in other fisheries (Olla et al. 1998; Davis et al. 2001; Davis 2002) are less likely to be important in this study, since vertical or between-trip differences are less pronounced. Finally, deck exposure (Davis et al. 2001; Davis 2002) is a stressor that is usually not relevant in purse seine fisheries, where slipping occurs through the lowering of the head rope of the net in the water (Lockwood et al. 1983; Mitchell et al. 2002; Stratoudakis and Marçalo 2002).
The most significant and consistent systematic effect in this study was the linear trend in most stress variables related to the time spent in the net. Although this trend might be considered to reflect the time delay in attaining peak values after the application of a stressor at the onset of fishing, available evidence suggests that the stressor operates throughout the hauling and fish transfer process and therefore that the fish stress reaction is proportional to the duration of the fishing operation. This is most evident in cortisol, for which plasma concentration typically rises to peak levels within a few minutes after the application of an acute stressor (Wendelaar Bonga 1997). If capture itself (net encircling the fish school) was the only fishing stressor, then a linear model should not provide an adequate fit to the cortisol data (Fig. 3b) since peak values should be reached early within the fishing operation. The duration of the fishing operation has already been shown experimentally to be an important fish stressor (Olla et al. 1997), while observations in the Portuguese and Australian purse seine fisheries for sardine (Sardina pilchardus and Sardinops sagax respectively) indicate that extreme behavioural reactions (gulping for air, jumps out of the water, disoriented or lethargic swimming) become progressively more frequent up to the transfer of the fish onboard (Stratoudakis and Marçalo 2002; Mitchell et al. 2002). We therefore suggest that the duration of the fishing operation is an important stressor in purse seine fishing due to the progressive water volume restriction, crowding and manipulation associated to the typical operation of this fishing method.
Cortisol showed the highest rate of increase during the sampling period among the biochemical variables tested, reaching a mean concentration of 15.8 μg/dl after 2 h of confinement in the net. Glucose also increased with time (although at a lower rate), reaching 156.1 mg/dl after 2 h and being consistently higher in fatter fish. These results agree with the known patterns of cortisolemia and hyperglycemia associated to acute fish stress (Barton and Iwana 1991; Wendelaar Bonga 1997). For most teleosts, cortisol base levels are below 3–4 μg/dl with peak post-stress values up to 20 μg/dl or higher (Barton and Iwana 1991), while post-stress glucose peaks around 150–200 mg/dl or above are often reported (Barton and Iwana 1991; Olla et al. 1997; Davis et al. 2001; Manire et al. 2001). Experiments simulating the confinement of Atlantic mackerel (Scomber scombrus) during the drying-up of purse seine nets showed rapid post-stress increase in cortisol (>20 μg/dl) and significant concentration differences between moribund and healthy fish (Pawson and Lockwood 1980). However, a range of subsequent trials (including simulation of purse seine slipping) failed to demonstrate post-stress increase in cortisol and glucose or differences between moribund and healthy mackerel (Swift 1983). Although more recent experiments with other fish species have also failed to demonstrate a correlation between delayed discard mortality, cortisol and glucose (Olla et al. 1998; Davis et al. 2001; Davis 2002), it is worth noting that concentrations reported from the simulated slipping operation for mackerel (Swift 1983) are considerably lower to those observed for sardine in the field after 2 h of confinement in the purse seine net.
The analysis of adenine nucleotides and related compounds in sardine muscle showed a reduction in ATP and ADP concentration and a significant increase in inosine with time spent in the net. These results are in agreement with the known degradation sequence of muscle ATP during catabolic reactions resulting from fish exhaustion and stress (Wood 1991). At the onset of fishing, mean ATP concentration was 4.07 μmol/g, reducing by more than 50% within 2 h of confinement. These values confirm the suggestion of Mendes et al. (2001) that the low baseline ATP values (<1 μmol/g) reported for several teleost species immediately after trawl capture, were due to ATP breakdown resulting from stress reactions during fishing. More difficult to explain is the temporal stability (the linear increase observed was marginally non-significant) of inosine monophosphate (the main early product of ATP degradation in teleosts), although the highly significant increase of inosine confirms the standard degradation sequence.
Sardine haematocrit and haemoglobin significantly decreased with time spent in the net, with female fish having higher haemoglobin concentrations than males. The unexpected sex effect is likely due to an underlying relation of haematological parameters with fish weight (Nespolo and Rosenmann 2002), depicted by sex differences in weight distribution (female sardines were significantly heavier than males). Indeed, adding gonad-free weight as an explanatory variable in the LMMs, led to a significant (positive) relation with fish weight both for haematocrit and haemoglobin, turning the sex effect non-significant. The decrease of the two haematological parameters with time contradicts most existing literature, where haematocrit and haemoglobin usually rise during exposure to a stressor to increase oxygen transportation, facilitate acid/base regulation and reduce cardiac work costs (Swift 1982; Wood et al. 1983; Wells et al. 1986). In Atlantic mackerel, significantly higher haemoglobin concentration was found in stressed fish, with haemoconcentration being likely due to osmotic imbalance and dehydration resulting from injuries and skin loss (Swift 1982). In the only study demonstrating results similar to those found here for sardine, Bourke et al. (1987) demonstrated a considerable reduction in the haematocrit of skipjack tuna (Katsuwonus pelamis) in the first hours after capture, suggesting that extreme haemodilution (either due to increased drinking rate or, more likely, from inter- or intra-cellular tissue fluids) could be the major source of delayed tuna mortality.
Ionic concentrations in sardine plasma changed with time spent in the net, either increasing (sodium and chloride) or decreasing (potassium), although measurement limitations only permitted the modeling of chloride. The relation with time spent in the net was highly significant, although the rate of chloride change was much lower than that of cortisol, adenosine nucleotides and glucose and only similar to that of haematocrit and haemoglobin. Marked ionic and fluid volume disturbances are known to be caused by exhaustive exercise and stress (Wood et al. 1983; Bourke et al. 1987; Wood 1991), but common patterns of stress response are difficult to establish as gas exchange, hydromineral control, acid/base balance and nitrogen metabolism are all closely linked through processes mainly located in the gills (Wendelaar Bonga 1997). Pawson and Lockwood (1980) suggested that the increase in sodium concentration observed in stressed mackerel was caused by dehydration due to skin damage and scale loss and was related to subsequent mortality. Bourke et al. (1987) also reported osmotic dysfunctions for skipjack tuna in the first hours post-capture, with the transient increase in serum osmolarity being mainly due to increase in chloride, potassium and lactate. The simultaneous increase in blood osmolarity and volume led the authors to suggest that this could result from fish swallowing and absorbing seawater and salts from the gut faster than the ion-exchange mechanism in the gills can excrete the added salt load. Although the hypothesis was not supported by early weight loss in skipjack tuna, the above mechanism seems plausible for the ionic and haematological early dynamics observed in sardine.
Overall, the physiological stress variables of sardine monitored during commercial purse seine fishing off northern Portugal seem to fall into two categories. Cortisol, glucose and adenine nucleotide dynamics agree with reported adaptive stress reactions for many teleosts. Mean concentrations at the end of fishing provided clear evidence of an acute stressor resulting from the fishing practice over the few hours, starting from the identification, chase and encircling of the target school and progressing with the gradual reduction of water volume and increase in crowding and manipulation. On the other hand, maladaptive responses such as changes in plasma ionic concentrations and haematological variables provided results that are less in line with existing stress literature. These variables have been less frequently monitored and there are indications that they can be related to fish death resulting from post-capture stress (Pawson and Lockwood 1980; Wood et al. 1983; Bourke et al. 1987). The hypothesis that excessive drinking and assimilation of salts by sardines can cause extreme hydromineral disturbances during purse seining and affect subsequent survival needs to be further explored. The field results obtained in this study can be used in future laboratory experiments to guarantee that the simulated fishing impact is realistic. Laboratory experiments can then be used to explore the links between magnitude of stressor, stress reactions and subsequent fish survival and to evaluate behavioral impairments related to the stress caused by purse seine fishing (Olla et al. 1997; Ryer 2004; Davis 2005).
References
Barton BA, Iwana GK (1991) Physiological changes in fish from stress in aquaculture with emphasis on the response and effects of corticosteroids. Annu Rev Fish Dis 1:3–26
Bourke RE, Brock J, Nakamura RM (1987) A study of delayed capture mortality syndrome in skipjack tuna, Katsuwonus pelanis (L). J Fish Dis 10:275–287
Chopin FS, Arimoto T (1995) The condition of fish escaping from fishing gears-a review. Fish Res 21:315–327
Davis MW (2002) Key principles for understanding fish bycatch discard mortality. Can J Fish Aquat Sci 59:1834–1843
Davis MW (2005) Behaviour impairment in captured and released sablefish: ecological consequences and possible substitute measures for delayed discard mortality. J Fish Biol 66:254–265
Davis MW, Olla BL, Schreck CB (2001) Stress induced by hooking, net towing, elevated sea water temperature and air in sablefish: lack of concordance between mortality and physiological measures of stress. J Fish Biol 58:1–15
Lai HL, Hesler T (2004) Linear mixed-effect models for weight-length relationships. Fish Res 70:377–387
Lockwood SJ, Pawson MG, Eaton DR (1983) The effects of crowding on mackerel (Scomber scombrus L.)—physical condition and mortality. Fish Res 2:129–147
Manire C, Hueter R, Hull E, Spieler R (2001) Serological changes associated with gill-net capture and restraint in three species of sharks. Trans Am Fish Soc 130:1038–1048
Mazeaud MM, Mazeaud F, Donaldson EM (1977) Primary and secondary effects of stress in fish: Some new data with a general review. Trans Am Fish Soc 106:201–212
Mendes R, Quinta R, Nunes ML (2001) Changes in baseline levels of nucleotides during ice storage of fish and crustaceans from the Portuguese coast. Eur Food Res Technol 212:141–146
Misund OA, Beltestad AK (2000) Survival of mackerel and saithe that escape through sorting grids in purse seines. Fish Res 48:31–41
Mitchell RW, Blight SJ, Gaughan DJ, Wright IW (2002) Does the mortality of released Sardinops sagax increase if rolled over the headline of a purse seine net? Fish Res 57:279–285
Nespolo RF, Rosenmann M (2002) Intraspecific allometry of haematological parameters in Basilichthys australis. J Fish Biol 60:1358–1362
Olla BL, Davis MW, Schreck CB (1997) Effects of simulated trawling on sablefish and walleye Pollock: the role of light intensity, net velocity and towing duration. J Fish Biol 50:1181–1194
Olla BL, Davis MW, Schreck CB (1998) Temperature magnified post capture mortality in adult sablefish after simulated trawling. J Fish Biol 53:743–751
Pawson MG, Lockwood SJ (1980) Mortality of mackerel following physical stress and its probably cause. Rapp P-v Réun Cons Int Explor Mer 177:439–443
Pinheiro JC, Bates DM (2000) Mixed-effects models in S and S-PLUS. Springer, Berlin Heidelberg New York
Rodbard D (1974) Statistical quality control and routine data processing for radioimmunoassay and immunoradiometric assays. Clin Chem 20:1255–1270
Ryder JM (1985) Determination of adenosine triphosphate and its breakdown products in fish muscle by high-performance liquid chromatography. J Agri Food Chem 33:678–680
Ryer CH (2004) Laboratory evidence for behavioural impairment of fish escaping trawls: a review. ICES J Mar Sci 61:1157–1164
Schreck CB, Contreras-Sanchez W, Fitzpatrick MS (2001) Effects of stress on fish reproduction, gamete quality, and progeny. Aquaculture 197:3–24
Stratoudakis Y, Marçalo A (2002) Sardine slipping during purse-seining off northern Portugal. ICES J Mar Sci 59:1256–1262
Stratoudakis Y, Marçalo A, Vale C, Falcão M (2003) Changes in seawater nutrient concentrations during purse seine fishing for sardine Sardina pilchardus off northern Portugal. Mar Ecol Prog Ser 265:235–242
Swift DJ (1982) The blood haemoglobin concentration of the Atlantic mackerel (Scomber scombrus L.). Comp Biochem Phys A 73(2):229–232
Swift DJ (1983) Blood component value changes in the Atlantic mackerel (Scomber scombrus L.) subjected to capture, handling and confinement. Comp Biochem Phys A 76(4):795–802
Wells RMG, McIntyre RH, Morgan AK, Davie PS (1986) Physiological stress responses in big gamefish after capture: observations on plasma chemistry and blood factors. Comp Biochem Phys A 84A(3):565–571
Wendelaar Bonga SE (1997) The stress response in fish. Phys Rev 7:591–625
Wood CM (1991) Acid–base and ion balance, metabolism, and their interactions, after exhaustive exercise in fish. J exp Biol 160:285–208
Wood CM, Turner JD, Graham MS (1983) Why do fish die after severe exercise? J Fish Biol 22:189–201
Acknowledgements
We thank the Producer Organization CENTROLITORAL at Figueira da Foz and its chairman A. M. Lé for facilitating shipboard observations and sampling, together with the owners, skippers and crew members of the purse seine vessels Atleta, Além Mar, Nau dos Corvos, Beira Azul, and Titó. Three anonymous referees provided pertinent and helpful comments that allowed to improve the original manuscript. AM is grateful to Marta Costa, Rogério Mendes, Francisco Ruano and Alexandre Morais for advice in the laboratory. The work was part of the IPIMAR Programme PELAGICOS, funded by the Portuguese Ministry of Science (MLE 013/2000) and affiliated to the GLOBEC/SPACC initiative. Ana Marçalo was partly supported by a scholarship of the Portuguese Ministry of Foreign Affairs. Live fish handling in this study complied with the existing Portuguese laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Marçalo, A., Mateus, L., Correia, J.H.D. et al. Sardine (Sardina pilchardus) stress reactions to purse seine fishing. Mar Biol 149, 1509–1518 (2006). https://doi.org/10.1007/s00227-006-0277-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0277-5