Abstract
Laboratory experiments on ovigerous females of northern shrimp (Pandalus borealis) were used to assess the effects of temperature and food ration on female condition during incubation and examine how combined effects of temperature and female condition influenced egg survival, embryonic development, and larval characteristics. Ovigerous females were maintained at 2°C, 5°C, and 8°C and fed on a low (three times/week; 2–2.7% W/W) or high ration (five times/week at satiation). The increase in temperature accelerated the developmental time of the eggs but their survival at 8°C was reduced. Conversion efficiency of yolk reserves in developing embryos was significantly reduced at elevated temperatures and larvae hatching at 2°C and 5°C were significantly larger and heavier than those hatching at 8°C. The experimental design did not result in any effect of food ration on the energetic condition of females or on egg characteristics and their biochemical composition. However, lower energy reserves were observed for females held at 8°C.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The northern shrimp is largely distributed in the Northwest Atlantic from the Davis Strait to the Gulf of Maine (Bergström 2000). This species is a protandric hermaphrodite. Each individual matures and functions first as a male, passes through a transitional or intersexual phase, and becomes a female (Shumway et al. 1985). In the Gulf of St. Lawrence, ovigerous females are generally concentrated at 150- to 350-m depth (Simard and Savard 1990) where temperatures range from 2°C to 6°C (MPO 2001). However, in its southernmost limit in the Gulf of Maine, temperature can exceed 6°C and even reaches 9°C during some periods of the year (Apollonio et al. 1986; Koeller 2000).
Fluctuations in the abundance of northern shrimp, Pandalus borealis, have been observed in some populations such as in the Gulf of Alaska (Anderson 1999), Gulf of Maine (Apollonio et al. 1986), Barents Sea (Lysy and Dvinina 1991), and Scotian Shelf (Koeller 2000). Important declines in the landings of northern shrimp in Alaska and Gulf of Maine during the last half of the 1970s led to the closure of these fisheries for a few years (Apollonio et al. 1986; Anderson and Piatt 1999). These declines were attributed to an increased exploitation of stocks and/or responses to changes in environmental conditions.
Relationships between fluctuation in abundance and seawater temperature regime have been established for some populations (Apollonio et al. 1986; Lysy and Dvinina 1991; Anderson 1999; Anderson and Piatt 1999). In the Gulf of Alaska, declines in the abundance of several pandalid species occurred quickly following water column warming (Anderson 1999). It was hypothesized that the mechanism controlling long term variations in fecundity and, perhaps, changes in population size was water temperature (Apollonio et al. 1986). High fecundity in the mid-1960s and late 1970s corresponded with periods of low April–July offshore bottom water temperatures in the southwest Gulf of Maine while low fecundity in the late 1960s and early 1970s corresponded with high offshore bottom water temperatures at the same time of the year (Apollonio et al. 1986). In the Barents Sea, shrimp biomass was associated with the spring temperature occurring 2 or 3 years before (Lysy and Dvinina 1991). The highest level of biomass was observed when spring surface and bottom water temperatures were 1–3°C lower than in situations where the biomass was at its lowest level.
A marked effect of temperature on the length of the incubation period was observed in ovigerous females of northern shrimp (Stickney and Perkins 1977; Nunes 1984). Egg mortality, however, was not affected by temperature (Stickney and Perkins 1977). An inverse relationship was observed between fecundity and water temperature; fecundity of shrimp at 3°C and 6°C being greater than fecundity at 8–9°C (Nunes 1984). Based on morphometric characteristics of eggs and larvae, it was concluded that optimum temperature for reproductive processes was within a narrow range of temperature between 3°C and 6°C (Nunes 1984). Larger larval size and lower egg mortality were observed at these temperatures.
Larval size and condition at hatching affect the ability of the larvae to begin exogenous feeding. During their development, embryos rely on yolk reserves for their nutritional needs. Growth during this period of yolk dependence is determinant for later survival (Braum 1967). Rate of yolk absorption and incubation time are temperature dependent and the conversion efficiency of yolk into tissue is affected by the temperature at which embryos are developing (Heming 1982). To date, no studies have examined the pattern of energy utilization during embryogenesis in northern shrimp and the corresponding characteristics of larvae at hatching. Rate of utilization of organic reserves during embryogenesis had been studied, however, for other shrimps species (Wehrtmann and Graeve 1998; Wehrtmann and Kattner 1998; Nates and McKenney 2000; Narciso and Morais 2001; Morais et al. 2002) and marine invertebrates such as lobsters (Pandian 1970a, 1970b; Sasaki et al. 1986; Attard and Hudon 1987) and crabs (Biesiot and Perry 1995; Petersen and Anger 1997; Gardner 2001). Few studies have examined the link between egg characteristics and characteristics of the larvae at hatching (Sasaki et al. 1986; Nates and McKenney 2000; Gimenez and Anger 2001).
During the incubation period, northern shrimp embryos are carried in a mass attached to the pleopods of the female. Parental care is provided to the embryos through pleopod movements, which bring oxygen to embryos (Baeza and Fernandez 2002). This behavior is probably essential for their normal development. Larger energy expenditures have been reported for some crab species with this increase in the activity of ovigerous females (Fernandez et al. 2000; Baeza and Fernandez 2002). Thus, the energetic condition of ovigerous females may play an important role in the development of the embryos. Major costs experienced by females during the incubation period are associated with maintenance and parental care since no molt occurs during that period. Energy reserves available for maintenance and activity are influenced by food availability and temperature. It has been hypothesized that northern shrimp were less tolerant of warmer temperature after becoming female (Apollonio et al. 1986) but no experiments in controlled conditions have confirmed this hypothesis. Moreover the effect of food availability on female condition, egg survival, and embryonic development has not been examined.
The objective of this study was to examine, under laboratory conditions, the combined effects of temperature and food ration on the energetic condition of ovigerous female of northern shrimp and how combined effects of female condition and temperature influence egg survival, embryonic development and larval characteristics. Experimental results on female condition, egg and larval production and characteristics were also compared to observations in the wild. Laboratory experiments were conducted at three temperatures and two food rations. Temperatures of 2°C, 5°C, and 8°C were selected as these represent temperatures where ovigerous females are usually found in their distributional area in the North Atlantic.
Materials and methods
Shrimp collection
Ovigerous females of northern shrimp (carapace length 20–30 mm) were captured using a rigid frame trawl (~150 m depth) in the St. Lawrence estuary near Rimouski, Québec in late October 2001 and April 2002. Following capture, shrimp were transported to the Maurice Lamontagne Institute and kept in 670-l rectangular tanks at a temperature of 5°C under natural photoperiod (latitude 49°45′N) and salinity (>28 psu). In late October 2001, a sample of 60 females was taken at random prior to the start of the experiments to determine female condition, and egg number and characteristics in field conditions at the beginning of the experiment. Three hundred and sixty shrimp also captured in October were then assigned at random to the tanks with the different experimental treatments. In the spring of 2002, 25 ovigerous females caught prior to the hatching period of larvae in the St. Lawrence estuary were isolated in tanks and followed during the hatching period to determine female condition, larval production and characteristics in the wild.
Experimental design
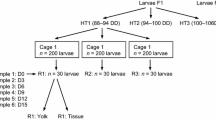
The laboratory experiment was conducted between November 2001 and July 2002. The experimental set-up was made up of three semi-open seawater systems, each system having two tanks with a capacity of 670 l (2.23 m2 area, 0.30 m depth). Each system had a head tank with a capacity of approximately 125 l, a sand filter and a heat pump to regulate water temperature. Seawater flow to each tank was of ~15 l min−1 and new seawater flow through each system was ~2.5 l min−1.
Temperature treatments of 2°C, 5°C, and 8°C were randomly assigned to each system and the two tanks of each system were divided into two equal parts with a partition. Each division was then randomly assigned to a low or high food ration. The three levels of temperature and two levels of food ration resulted in six experimental treatments, each one having two replicates. Thirty shrimp were placed in each experimental unit (division) and half of these were individually identified with a Visual Implant Tag (Northwest Marine Technology, Shaw Island Washington) fixed to the cephalothorax. Tagged specimens were used to monitor the variations in the energy content of the eggs during embryogenesis and untagged specimens, which were not manipulated during the experiment were used to evaluate egg and larval production and characteristics at hatching. During the acclimation period, shrimp were fed ad libitum with a natural diet consisting of equal parts of frozen pacific krill, capelin, shrimps, and euphausids. At the beginning of the experiment, shrimp in the low ration condition were fed three times a week with a limited amount of food while shrimp in the high ration condition were fed to satiation five times a week. Meals distributed to the different experimental groups were adjusted with shrimp biomass in each group. On a weekly basis, shrimp fed with the low ration ate a maximum of 2.0%, 2.3%, and 2.7% of their biomass per day, at 2°C, 5°C, and 8°C, respectively. These rates of consumption represented 32% of the amount of food eaten by shrimp fed on the high ration.
Female condition
An initial sample of 60 females was sacrificed prior to the start of experiment. At the end of the experiment, all females submitted to the different temperatures and food rations were sacrificed after the hatching of the larvae. Female samples were used to determine the variations in size and energetic condition in the different experimental treatments. Various length and mass measurements were made and tissue samples were collected for each shrimp for the determination of their size, condition, and energy content. For each shrimp, length of cephalothorax (CL, ±0.01 mm) was measured and total mass (±0.001 g), and egg, hepatopancreas, muscle, and carcass (mostly carapace) masses were taken. Two condition indices were used to determine the nutritional condition of the shrimp: the condition factor (CF) and the hepatosomatic index (HSI). The CF (Fulton’s K) and HSI are largely used to determine fish condition (Bolger and Connolly 1989; Lambert and Dutil 1997b; Grant and Brown 1999). HSI has been used as an index of shrimp condition (Clarke 1982; Jeckel et al. 1990) as lipid reserves in decapod crustaceans are stored in the hepatopancreas (Galois 1987). Fulton’s K has been used as an index of condition for king crabs, Paralithodes platypus and Lithodes aequispina (Hawkes et al. 1986) but in general it is less frequently used for invertebrate species.
The CF was expressed with the formula:
where W is somatic mass (g) and CL the length of cephalothorax (mm). Somatic mass was calculated as the total mass of shrimp less the egg mass. The value of the exponent (2.6) was taken from the relationship between somatic mass and length of cephalothorax of the females. The HSI was calculated as:
where HW and W represent hepatopancreas and somatic mass (g), respectively.
Hepatopancreas, muscle, and carcass samples were taken to determine their water and energy content. Water content was determined by drying all tissue samples to constant mass (±0.001 g) at 65°C. Tissue energy contents were not analyzed for all samples. Experimental conditions resulted in a broad range of values in water content. Based on the relationships observed between water content and energy content in the different tissues of many fish species (Holdway and Beamish 1984; Hartman and Brandt 1995; Lambert and Dutil 1997a), it was hypothesized that the same type of relationships would be observed in shrimp tissues. Tissue subsamples (50–65 per tissue) covering the entire range of values in water content were used to determine the relationships between energy content and water content for each tissue. Energy content of tissues only analyzed for water content was obtained from these relationships. Energy content was determined by combusting tissue samples in an oxygen bomb calorimeter (Parr, model 1261). Benzoic acid with an energy equivalence of 26.453 kJ g−1 was used as a standard for the determination of energy content. Specific energy content was calculated as the energy content (kJ) per gram (g) of fresh tissue. Total energy content for each tissue was determined as the product between specific energy content and tissue mass. Somatic energy content for each female was calculated as the sum of total energy of muscle, hepatopancreas, and carcass.
Egg development time
Development time of the embryos was estimated as the number of days between the beginning of the experiment in November and the hatching period of larvae. Prior to hatching, ovigerous females were placed in individual circular tanks to determine the exact date of hatching. The date of hatching was determined as the date of the first day of regular hatching.
Egg mortality
Egg mortality during the incubation period was estimated as the difference between fecundity-at-size of ovigerous females sampled at the beginning of the experiment and the number of newly hatched larvae and remaining eggs of isolated females during hatching. Fecundity-at-size at the beginning of the experiment was estimated from the relationship between the length of cephalothorax of ovigerous females and their fecundity. Fecundity was estimated by counting two replicate samples of 100 eggs and drying both these subsamples and the remaining eggs. Fecundity was estimated from the mean dry mass of one egg, calculated with the subsamples, and the total brood dry mass (±0.001 g, Clarke 1993). Fecundity during hatching for isolated females was estimated by manually counting the number of newly hatched larvae and by determining the remaining number of eggs. Egg mortality was estimated with untagged ovigerous females only to eliminate possible egg mortality caused by the sampling of egg mass during embryogenesis in tagged females. Egg mortality rate during incubation was calculated as:
where Fi represents the observed fecundity at hatching for female of size i and EFi the estimated fecundity of a female of the same size calculated from the relationship between fecundity and length of cephalothorax at the beginning of the experiment.
Egg and larval sampling
Egg samples were collected from each tagged female on three occasions during the experiment. Samples were taken at the beginning of the experiment, in early January, and early March. At each sampling period, a minimum of 40 eggs were removed from the accessible surface of the egg mass. All egg samples were manipulated on crushed ice. Twenty eggs were immediately frozen and stored at –80°C for further determination of their biochemical composition. The rest of the sample was used for egg volume determination. Following measurements, eggs were immediately frozen and stored at –80°C for further determination of egg wet and dry mass.
Samples of larvae were collected from tagged and untagged females during hatching. All females were isolated before hatching in small circular tanks (80 l) maintained at the same experimental temperatures. During hatching, two samples of 20 emergent larvae were collected. One sample was used for morphometric measurements of the larvae and the other was immediately frozen and then stored at –80°C for further determination of the biochemical composition of the larvae.
Egg and larval morphometric analysis
Images of eggs and larvae were digitized using a video camera (Spot Insight ver. 3.2) mounted on a stereomicroscope (Wild Heerbrugg) and connected to a frame grabber. Morphometric measurements were realized using image analysis software (Image-Pro Plus, ver. 4.1.1.2). Egg length and width were measured and used to determine egg volume assuming an ellipsoid form of the egg. The volume was given by 4/3πr1r2r3, the value of r1 was taken as half the length and r2 and r3 as half the width. Wet and dry mass of eggs (±0.001 mg) were determined on a sample of 10 eggs. Dry mass of eggs was determined by drying the same sample of eggs 24 h at 65°C. Total length (TL), cephalothorax length (mm), and wet and dry mass of 20 individual larvae (±0.001 mg) produced by each female were measured. Dry mass of larvae was determined by drying larvae 24 h at 65°C.
Biochemical composition of eggs and larvae
Micro quantification methods were used to determine the biochemical composition of eggs and larvae (Holland and Gabbott 1971; Meyer and Walther 1988). Samples of eggs and larvae were homogenized in 0.9% NaCl solution and aliquots of aqueous homogenate were used for the estimation of total proteins, total lipids, and neutral lipids. Frozen eggs and larvae were manipulated on crushed ice and homogenized samples were immediately frozen in liquid nitrogen and stored at –80°C until they were used for protein and lipid analyses.
Protein content was determined with the bicinchroninic acid assay kit (TPRO-562, Sigma Aldrich) as described by Sibert et al. (2004). The extraction procedure for the total lipids was based on the protocol of Bligh and Dyer (1959). A 200-μl aliquot of homogenate was added to 750 μl of a mixture of methanol and chloroform (2:1, v/v) and proportional volumes of chloroform and water were added according to the Bligh and Dyer (1959) protocol. Following separation, the quantification of lipids was done gravimetrically. Three portions of 100 μl of the purified extract were removed with a 100-μl Hamilton syringe and transferred to small pre-weighted (0.001 mg) cups (aluminium micro weighing dishes, 11 mm diameter × 6 mm height). The solvent was evaporated at room temperature for 30 min and the lipids were weighed in triplicate. Two blanks were analyzed with each set of samples.
The extraction procedure for the neutral lipids was the same as in total lipids. However, 25 μl of nonadecane was added to homogenate and used as an internal standard. The lipid fraction was removed with a 500-μl Hamilton syringe and transferred to a clean tube and the solvent was evaporated under a nitrogen stream. The lipid was resuspended in 25 μl of solvent before 3 μl was spotted on a SIII-Chromarod for quantification using flame ionization detection with a model MK-III Iatroscan (Iatron, Japan). The rods were developed as described by Ouellet et al. (1992). Absence of DAG, FFA and C-Ester in samples indicates the low degradation of the lipids during handling and preservation.
A large number of eggs was necessary to determine glycogen content. Thus, it was not possible to determine the glycogen content of eggs for tagged shrimp during embryogenesis. An additional sample of 25 ovigerous females was obtained in October 2002 and used to determine a mean value of glycogen content for all egg samples. Glycogen was analyzed by the enzymatic method described by Carr and Neff (1984). Following hydrolysis of glycogen, glucose was determined with the Sigma Diagnostics glucose kit (no.115-A) and used to determine glycogen content.
Energy contents of eggs and larvae were determined by the energy equivalence of protein and total lipid contents. Energy equivalents of 23.64 kJ g−1 for protein and 39.54 kJ g−1 for lipid were applied.
Field samples
The initial sample of females collected at the beginning of the experiment in November 2001 was used to determine female condition and fecundity in the field after spawning. Another sample of females was obtained from commercial fisheries in the spring of 2002 to determine the fecundity of females before hatching and the mortality rate of eggs during the incubation period in the wild. Finally, a sample of 25 live ovigerous females was captured in the spring of 2002. These females were kept in individual tanks and monitored until the hatching of larvae. They were used to determine female condition and energy content at hatching and characteristics of hatching larvae in the wild. Methods used for the analyses were the same as described above.
Data analysis
The experiment had a split-plot nested design. Temperature was the first fixed factor, with three levels, and tanks, which were randomly assigned, were nested in temperature. Food ration was the second fixed factor with two levels and there were two replicates (tanks) for each combination of temperature and ration. Sampling time (November, January, and March) was a third factor for variables with repeated measures and in these cases, design was a split-split-plot nested design. The variables analyzed for female shrimp were size, mass, condition (CF and HSI), and energy content (somatic energy content and tissue energy contents). The same statistical design was used to analyze egg mortality rates, egg and larval characteristics and biochemical composition.
Variables were analyzed with a two-way analysis of variance (ANOVA) or with a three-way analysis of variance for repeated measures analyses using SAS software (GLM procedure and MIXED procedure, SAS, Cary, N.C.). The error term used to test temperature was tank (temperature) where df=3. The error term used to test food ration and its interaction with temperature was ration × tank (temperature) where df=3, and the error term used to test time and its interaction with temperature and/or ration was time × ration × tank (temperature), df=12. A probability level of P<0.05 was considered significant. Multiple comparison analyses were performed by Scheffe’s test to control experiment-wise error rate. In cases where homogeneity and/or normality of variance were not respected, the analysis of variance was performed on rank-transformed data. Z-tests adjusted with the Bonferroni correction were performed to compare data from the initial sampling and the end of the experiment and between experimental treatments and field data.
No statistical analyses were performed on the triacylglycerol (TAG) content of the larvae since the concentration was either negligible or not detected.
Results
Incubation time and female condition
Significant differences in hatching dates were observed between the temperature treatments (ANOVA P<0.0001). Mean hatching date varied from 10 March at 8°C to 9 June at 2°C. Mean incubation time calculated from the beginning of the experiment (7 November) was 123±10, 162±10, and 214±14 days at 8°C, 5°C, and 2°C, respectively. For 50% of the females, hatching date was within a 12- to 14-day interval around the median date of hatching. No significant effect of food ration on incubation time was detected.
The CF of the females decreased significantly (Z-test P<0.0001) during the experiment (Table 1). However, the decreased in mean mass was significant only for females on the high ration at 2°C and 5°C (Z-test P<0.003). HSI increased significantly (Z-test P<0.0001) from a mean value of 4.03 at the beginning of the experiment to values between 4.79 and 4.90 at 2°C and 5°C, while HSI for females held at 8°C did not change (Z-test P>0.77) during the experiment. At the end of the experimental period during hatching of larvae, HSI was significantly different between temperatures (ANOVA P<0.003); females maintained at 2°C and 5°C having higher mean HSI than females held at 8°C (Scheffe’s test P<0.005). Temperature and ration had no effect on CF (ANOVA P>0.19). No effect of ration level was detected on mean female condition but in each treatment, individual values of HSI were variable. For example, HSI varied between 1.5% and 7.5% in females on the high ration at 8°C.
Variations in water content were observed in the different tissues of females between experimental treatments. These differences indicated significant changes in the energy content of tissues as strong relationships between water content and specific energy content were observed (Fig. 1). Each relationship presented strong coefficients of determination (0.84<r2<0.98). A significant increase (Z-test P<0.0001) in specific energy content was observed in all tissues as well as in specific somatic energy content (sum of all tissues) during the experiment (Table 1). Mean specific energy content of the carcass was influenced by temperature treatments (ANOVA P<0.002) but differences for the hepatopancreas and muscle were not large enough to be significant (ANOVA P=0.05). Multiple comparisons indicated significant differences in the specific energy content of the carcass between each temperature (Scheffe’s test P<0.03). Temperature also had a significant effect on the specific somatic energy content of the females (ANOVA P<0.001). Multiple comparisons procedures indicated significant differences (Scheffe’s test P<0.002) between each temperature, highest values being observed for females held at 5°C and lowest values for females held at 8°C. No statistical difference (ANOVA P>0.41) was detected in mean female somatic energy between temperatures. However, considering the relationship between individual values of somatic energy and length of cephalothorax for each temperature, significantly lower somatic energy was observed for females held at 8°C than at 5°C (ANCOVA P<0.04). Thus, female condition during the incubation period was higher in colder temperatures. In general, mass, condition and energy content values appear to be lower at 8°C. During hatching, HSI was higher for females held at 2°C and 5°C than at 8°C. Specific somatic energy content was also higher at 5°C than at 8°C.
Egg loss during incubation
Significant egg loss was observed during the incubation period. Mean egg loss for untagged females in the different experimental treatments varied between 40% and 75% (Table 2). A significant temperature × ration interaction was observed on the mortality rate of eggs during incubation (ANOVA P<0.04) but a multiple comparisons procedure did not show significant differences in egg mortality between the high and low ration for each temperature. Grouped data for each temperature indicated that mean egg loss was higher at 5°C and 8°C (64% and 63%, respectively) than at 2°C (50%). Temperature had a significant effect (ANOVA P<0.04) on egg mortality but a posteriori comparisons did not detect differences.
While a significant relationship was observed at the beginning of the experiment between fecundity and CL (P<0.0001, r2=0.59), the individual variability in egg mortality resulted in the absence of relationship between fecundity and CL at the time of hatching. Relative fecundity of the females decreased from 143 eggs/g of wet mass at the beginning of the experiment to values between 39 and 97 eggs/g of wet mass at hatching (Table 2).
Egg characteristics and biochemical composition
Mean egg volume, mass, and water content were significantly different (Scheffe’s P<0.0031) between November and March in all temperature treatments (Table 3). Total egg lipid content decreased significantly at 5°C and 8°C between November and March (Scheffe’s P<0.0027) and significant decreases in protein and TAG content of eggs were observed at 8°C between November and March (Scheffe’s P<0.001). No significant difference was observed in egg sterol content for all temperature treatments (Scheffe’s P>0.9683). A temperature × time interaction was observed in all egg characteristics and in biochemical composition during the experiment. The only exception was for egg dry mass where no interaction was observed.
In order to compare similar stages of development of the embryo between temperatures, egg characteristics and biochemical composition were examined in relation to relative time to hatching. The time of sampling was expressed as a proportion of the total development time, the end of development time being determined by the date of hatching of the larvae for each female.
Mean egg volume (0.47 mm3) and egg water content (435 ug/egg) were comparable in all temperature–ration treatments at the beginning of the experiment in November (Scheffe’s P>0.22). Differences in the slopes of the regressions between egg volume, egg water content, and relative time to hatching at the different temperatures (F=24.94 and F=32.38, respectively; P<0.0001) indicated significant differences in the rate of change in egg volume with developmental stage between temperatures (Fig. 2a, c). Thus, at half the development time, egg volume and egg water content for females held at 8°C were higher than at 2°C and 5°C (Table 4).
Variation in the egg volume (a), egg mass (b), and egg water content (c) during the embryonic development for females of northern shrimp held at 2°C (●), 5°C (Δ), and 8°C (■). Mean values are expressed in relation to relative time to hatching. Relative time to hatching represents the time of sampling expressed as a proportion of total development time. The equations of the regressions are presented in Table 4
Mean egg mass (728 ug) was comparable in all temperature treatments at the beginning of the experiment (Scheffe’s P>0.60). The relationship between egg mass and relative time to hatching at 2°C was not significant (Fig. 2b, Table 4). No difference was observed in the slope of the regressions between egg mass and relative time to hatching at 5°C and 8°C (F=3.18 P>0.08) indicating that changes in egg mass with time were similar between these temperatures (Fig. 2b, Table 4).
No significant difference (Scheffe’s test P>0.95) in mean egg protein content (211 ug/egg ) and in mean total egg lipid content (84 ug/egg) was detected between the temperature and food ration treatments at the beginning of the experiment in November (Fig. 3a, b). Mean TAG and sterol content of the eggs represented 39% and 3% of the total lipids, respectively (Fig. 3c, d). Since no other neutral lipids were detected in the analysis, most of the remaining lipids were represented by polar lipid classes such as phospholipids. These lipid classes, however, were not analyzed in this experiment. No traces of DAG, FFA, and C-Ester were detected in egg samples, indicating the low degradation of lipids during handling and preservation. Mean glycogen content of the eggs in November was 2.2±0.3 mg/g (i.e. 1.6 ug/egg). The glycogen content of the eggs in January and March was not determined because of the low concentration and large number of eggs necessary for the analysis.
Variation in proteins (a), total lipids (b), TAG (c), and sterol content (d) in the eggs during the embryonic development of northern shrimp at 2°C (●), 5°C (Δ), and 8°C (■). Mean concentrations are expressed in relation to relative time to hatching. Relative time to hatching represents the time of sampling expressed as a proportion of total development time. The equations of the regressions are presented in Table 4
Rates of proteins and TAG depletion in the eggs during embryonic development increased with temperature (Fig. 3). Comparisons of the significant regressions between proteins and TAG content in relation to relative time to hatching indicated significant differences (P<0.003) in the slope of the regressions; steeper negatives slopes were observed at the higher temperature (Table 4). Thus, when half the development time had elapsed, protein and TAG contents of the eggs were higher at 2°C than at 8°C. The slopes of the regressions for total lipid content were not different (F=1.11 P>0.33) indicating similar rates of change in total lipid content with developmental stage between temperatures.
Larval characteristics and biochemical composition at hatching
Larval characteristics were significantly influenced by the temperature during the incubation. A negative gradient in the morphological characteristics (CL, TL, dry, and wet mass) of the larvae was observed in relation to temperature (Table 5). Data presented are pooled by temperature since no effect of food ration was detected (ANOVA P>0.75). Mean CL of hatching larvae at 2°C was significantly larger (Scheffe’s test P<0.02) than at 5°C and 8°C. TL of the larvae was similar at 2°C and 5°C but significantly larger than at 8°C (Scheffe’s test P<0.01). Mean wet mass of larvae hatching at 2°C was significantly higher than at 8°C (Scheffe’s test P<0.02). However, no significant effect of temperature was observed on the dry mass of larvae at hatching (ANOVA P>0.31).
Temperature had a significant effect on protein (ANOVA P<0.01) and sterol (ANOVA P<0.02) content of the larvae at hatching. Mean protein content of larvae was significantly lower in larvae hatching at 8°C than at 2°C and 5°C (Scheffe’s test P<0.0074). Total lipid content of the larvae at hatching was not different between temperatures (ANOVA P>0.06), while sterol content was significantly lower (Scheffe’s test P<0.02) in larvae hatching at 2°C than at 8°C. No DAG, FFA, or C-Ester was detected.
Not unexpectedly, food ration—which did not influence female condition and energy reserves—had no effect on larval characteristics and biochemical composition. However, within each temperature treatment, some relations were detected between lipid reserves of the females and characteristics of the larvae. Relationships were observed between TL, dry mass and energy content of the larvae and HSI, an index of lipid reserves of the females (Fig. 4). No relationships were observed with the water, protein, and total lipid content of the larvae.
Linear relationships between total length (a), dry mass (b), and energy content (c) of larvae and hepatosomatic index of female northern shrimp. Regressions for total length of the larvae are presented for each temperature 2°C (●), 5°C (Δ), and 8°C (■). Temperature treatments were not analyzed separately for dry mass and energy content of the larvae
Regressions between TL of larvae and HSI of the females were significant at 2°C and 8°C (P<0.04) indicating that females with higher lipid reserves produced larger larvae than females with lower levels of lipid reserves (Fig. 4). Dry mass and energy content of the larvae were also related to the HSI of the females (Fig. 4). The regressions were significant (P<0.002) but the coefficient of determination was low in each case. Temperature treatments were not analyzed separately, as dry mass and energy content of the larvae were not influenced by temperature.
Field–laboratory observations
In October 2001, concentrations of ovigerous females in the St. Lawrence estuary were located at depths between 110 m and 160 m where temperatures were between 1.5°C and 3.6°C. The following April, females were found at depths where the temperature was approximately 2.2°C (Louise Savard, MPO, personal communication).
Mean date of spawning for females in the wild was estimated to be October 15th and our experiment began the 7th of November. We considered the mean temperature for this 22-day period to be 2°C. Thus, 22 days were added to the observed incubation time at 2°C in the experiment to obtain the total incubation time. The ratios between incubation time at 2°C and the other temperatures during the experiment were used to correct incubation time at 5°C (16 days) and 8°C (13 days). With these corrections, total incubation time of 236, 178, and 136 days were estimated at 2°C, 5°C, and 8°C, respectively. The following polynomial regression best fit the relationship between incubation time in days (Y) and temperature (T) (Fig. 5):
Similar values of CF were observed in the laboratory and in wild females in spring. However, significant differences in female condition and biochemical composition were detected between females caught in the St. Lawrence estuary in the spring of 2002 and females from the laboratory at the end of the experiment.
Mean HSI of wild females (6.38±1.19) was significantly higher than in females from all experimental temperature treatments (Z-test P<0.0001). Specific energy content of the hepatopancreas for wild females (17.58±3.22 kJ/g wet mass) was not significantly different than the observed values for females held at 2°C and 8°C (Z-test P>0.43) but was significantly lower than that observed at 5°C (Z-test P<0.0003) (Table 1). Mean specific energy content of the muscle (4.77±0.17 kJ/g wet mass) for wild females was significantly lower than values observed in all temperature treatments (Z-test P<0.01) while mean specific energy of the carcass (4.37±3.22 kJ/g wet mass) was similar to the values observed for females held at 5°C and 8°C (Z-test P>0.04) and higher than in females held at 2°C (Z-test P<0.001) (Table 1). Overall, somatic energy of wild females (51.88±9.88 kJ) was similar to the somatic energy of females in the laboratory (Z-test P>0.52) even though specific somatic energy content of wild females (5.15±0.53 kJ/g wet mass) was higher than in females maintained at 8°C in the laboratory (Z-test P<0.0002).
The fecundity of wild females decreased significantly during the incubation period. Significant differences (ANCOVA P<0.0001) in the relationship between fecundity and carapace length were observed between wild females sampled in the fall of 2001 after spawning and females sampled in the spring of 2002 before the hatching of the larvae (Fig. 6). Egg loss varied from 0% to 69% for females with CL of 23 mm and from 0% to 86% for females with CL of 28 mm (95% confidence interval). Mean mortality rate of eggs during incubation based on these relationships was 14%. In comparison, egg losses during incubation varied between 40% and 75% in the laboratory experiment (Table 2).
The length of the larvae produced by wild females was comparable to the length of the larvae from cold temperature treatments in the experiment. CL of larvae from wild females was not significantly different (Z-test P>0.73) from CL of larvae from females held at 2°C (Tables 5, 6). However, CL of larvae from wild females was significantly larger than CL of larvae from females held at 5°C and 8°C (Z-test P<0.0002). TL of larvae from wild females was similar to the TL of larvae from laboratory females held at 2°C and 5°C (Z-test P>0.07) and significantly larger (Z-test P<0.0001) than TL of larvae from laboratory females held at 8°C (Tables 5, 6).
Water content of larvae from wild females (Table 6) was similar to values observed for larvae hatching at 5°C and 8°C but lower than water content for larvae hatching at 2°C (Table 5). Protein content of the larvae of wild females was significantly higher (Z-test P<0.0001) than the values observed in the laboratory at each temperature (Tables 5, 6) while total lipid content was similar to values observed at 5°C and 8°C (Z-test P>0.13) and higher than the value observed at 2°C (Table 5, 6). TAG content of newly hatched larvae was negligible for most females collected in the field although it was detected in some larvae (eight females) but at a very low concentration (Table 6).
Discussion
Egg survival, embryonic development, and larval characteristics
The incubation temperature of ovigerous females of northern shrimp had marked effects on egg survival, embryonic development and larval characteristics at hatching. The increase in temperature accelerated developmental time but egg survival at the highest temperature (8°C) was significantly reduced. The higher temperature reduced the duration of yolk reserves and the conversion efficiency of yolk into tissue growth. As a result, lower larval size, mass and protein content were observed at the higher temperature.
The negative relationship between the duration of the ovigerous period and the temperature is in agreement with previous findings for northern shrimp (Stickney and Perkins 1977; Nunes 1984; Bergström 2000), different species of lobsters (Perkins 1972; Branford 1978; Tong et al. 2000; Smith et al. 2002) and crabs (Shirley et al. 1987). However, absolute values of incubation time at the different temperatures in our study are about 40 days longer than in the study of Stickney and Perkins (1977) if observed incubation times in our study are corrected for the elapsed time between spawning estimated to be October 15th and the beginning of the experiment (November 7th).
During a long period of incubation, some northern shrimp eggs are subject to mortality even though the egg mass is carried by the female. The number of eggs a female can incubate is dependent on female size. The relationship between female size (CL) and number of eggs has been well documented for northern shrimp (Rasmussen 1953; Allen 1959; Haynes and Wigley 1969; Skuladottir et al. 1978; Nunes 1984; Apollonio et al. 1986; Parsons and Tucker 1986; Berenboim and Sheveleva 1989; Burukovsky and Sudnik 1997). At the beginning of the experiment, egg number varied between 867 for females with CL of 21 mm and 2,361 for females with CL of 30 mm (Fig. 6). Egg numbers between 645 and 3,000 are reported for females of the same sizes in other studies (Haynes and Wigley 1969; Shumway et al. 1985; Apollonio et al. 1986). However, a large variability is observed both within and between populations. Some of the variability in the number of eggs can probably be explained by the inclusion of females carrying eggs in different stages of development and therefore exposed to different levels of egg mortality (Bergström 2000). There is evidence that eggs during the incubation period are exposed to different sources of mortality. Laboratory experiments reported mean egg losses during the incubation between 3.4% and 31% for Stickney (1981) and Nunes (1984). Higher egg losses were observed in the present study (Table 3). Individual egg loss varied between 1% and 100%. Mean egg loss at each temperature varied between 51% and 65% with the lowest value being observed at 2°C. Highest egg loss was observed at low temperature (3°C) by Nunes (1984) while no effect of temperature was detected by Stickney (1981). The observation of Nunes (1984) appears surprising as he found that larvae hatched from eggs incubated at 3°C tended to have higher survival rates and higher growth rates than larvae hatched from eggs incubated at higher temperatures (Nunes and Nishiyama 1984). However, temperature effects on egg loss during incubation may be difficult to distinguish from the effect of experimental conditions. Mechanical severance, erosion, abrasion, and volumetric expansion from water intake have been suggested as possible causes of egg loss in laboratory conditions (Balasundaram and Pandian 1982). At higher temperatures, more intense movements of pleopods in ovigerous females to supply the higher oxygen demand of developing embryos could possibly increase egg loss during incubation. Parasitism has also been reported as a possible cause of egg mortality (Stickney and Perkins 1977; Stickney and Perkins 1981). In wild ovigerous females, little or no infestation was observed at temperatures below 5.5°C but rates of infestation between 3% and 48% were observed above that temperature (Apollonio et al. 1986); the highest incidence occurring at temperatures between 7°C and 9°C.
Yolk proteins and/or lipids have been recognized as the major energy sources used during embryogenesis in European lobster Homarus gammarus (Pandian 1970a), American lobster Homarus americanus (Sasaki et al. 1986), spider crab Hyas araneus (Petersen and Anger 1997), giant crab Pseudocarcinus gigas, Lamarck (Gardner 2001), and tropical caridean shrimps, Alpheus saxidomus and Palaemonetes schmitti (Wehrtmann and Graeve 1998). Among lipids, TAG represents the most common fuel used by cells in decapods (Galois 1987). In our experiment, a general decrease in total proteins, lipids, and TAG was observed in the eggs during embryogenesis. A similar decrease was also reported for other crustaceans such as American lobster (Sasaki et al. 1986), Giant crab (Gardner 2001), spider crab (Petersen and Anger 1997) and caridean species, Plesionika martia martia, Palaemon serratus and Palaemon elegans (Morais et al. 2002). Larval size and growth during embryonic development are affected by the rate of yolk absorption and the efficiency with which yolk is converted into tissue (Heming 1982). Yolk contained in eggs has three potential functions (Clarke and Gore 1992): (1) It can be used for the synthesis of new tissue in the developing larvae, (2) to provide energy for the maintenance of tissues once they have been produced, and (3) to provide energy reserves for newly hatched larvae before exogenous feeding.
The present study is the first to examine the effect of temperature on the changes in the biochemical characteristics of P. borealis eggs during embryogenesis. Temperature has previously been shown to have a direct effect on the rate of yolk absorption in Chinook salmon Oncorhynchus tshawytscha (Heming 1982) and American lobster (Sasaki et al. 1986). The size and energy content of newly hatched larvae at 8°C suggest that necessary energy for tissue maintenance during embryonic development is proportionally higher at 8°C than at 2°C and 5°C. Thus, conversion efficiency would decrease at higher temperature and less energy would be available for the synthesis of new tissues in embryos at 8°C. The total energy loss during the embryogenesis estimated from the difference between the energy content of the eggs at the beginning of the experiment and the energy content of the larvae at hatching varied between 37% and 44%. The highest loss (44%) was associated with embryogenesis at 8°C.
The loss in proteins during embryogenesis and hatching accounted for 25% to 34% of the total protein content of the eggs at the beginning of the experiment. Again, the highest loss (34%) was associated with embryogenesis at 8°C. Lipid content in larvae was almost exclusively structural and increased slightly with temperature. The depletion of lipids during embryogenesis was between 56% and 60%. If TAG stored in eggs are inadequate to support embryonic development, then catabolism of phospholipids that are normally reserved for maintenance and structural membrane development may result, which could reduce larval viability by producing smaller underdeveloped larvae that are more susceptible to disease and predation (Palacios et al. 1998). At hatching, TAGs were not detected in northern shrimp larvae hatching from females held at 2°C and 5°C. Similar results have been reported by Ouellet et al. (1992) and Nates and McKenney (2000). This indicates that all TAG reserves had been spent during embryogenesis. However, traces of TAG was detected in larvae from some of the females held at 8°C. The same observation was made for American lobster larvae incubated at an elevated temperature (Sasaki et al. 1986). This higher level of TAG reserves does not necessarily confer an advantage to shrimp larvae. In our experiment, embryos with yolk reserves at hatching frequently showed deficiencies in morphological characteristics (e.g. absence of rostrum, partially developed eyes). We hypothesized from the smaller size at hatching, the shorter incubation time and the higher proportion of abnormal larvae at 8°C that northern shrimp embryo developing at that temperature may have hatched before having completed their full development and/or may be more subject to abnormal development. In Chinook salmon held at elevated temperature (12°C), alevins hatched earlier in development were smaller at hatching (Heming 1982). Disadvantages including decreased foraging ability and increased susceptibility to predation were also expected in relation to precocious emergence.
Northern shrimp larvae need to feed immediately after hatching as they are depleted of TAG reserves at hatching. Larger larvae may be favored as they could possibly show better swimming ability, and could capture prey more easily (Heming 1982). Moreover, newly hatched larvae of larger size could catch larger prey and/or have access to a larger range of prey size (Knutsen and Tilseth 1985; Berejikian et al. 1996). Thus, larger larvae could be less vulnerable to starvation and death (Marsh 1986; Tessier and Consolatti 1989). Smaller larvae of spiny lobster hatching from elevated temperature during their embryonic development had lower survival rates when submitted to different feeding treatments (Smith et al. 2003). Spider crab and American lobster larvae that were larger at hatch maintained their size advantage throughout development (Kunisch and Anger 1984; MacKenzie 1988). Similar differential growth has been described in spiny lobster, with larger larvae growing more at each stage than smaller larvae (Smith et al. 2003). In our experiment, the size of larvae at hatching was inversely related to temperature at which ovigerous females were held. Negative relationships between temperature and size of the larvae at hatching were also observed in lobster (Smith et al. 2002), crab (Shirley et al. 1987) and fish (Heming 1982). This negative relationship between larval size and temperature for northern shrimp was also observed by Nunes (1984) but a greater decrease in the size of the larvae was observed at higher temperatures. The size of the larvae was similar between the two studies at temperatures below 6°C but the mean larval size at spawning observed at 9°C (1.09 mm) by Nunes (1984) was lower than in the present experiment (1.36 mm). The results of these two studies indicate that higher temperatures (8–9°C) during both maturation and incubation of the eggs influence the size of larvae at hatching. In our experiment, the maturation of the females in each temperature treatment occurred in the natural environment at temperatures between 1.5°C and 3.6°C while maturation and incubation in Nunes (1984) study were examined at the same temperatures.
Physiological condition of ovigerous females and larval characteristics
Female condition was significantly influenced by temperature. A significant increase in female somatic energy was observed during the incubation period but at the time of hatching, ovigerous females held at 8°C had a lower energetic condition than females held at 2°C and 5°C (Table 1). Lower HSI and lower specific energy content in the hepatopancreas also indicated that ovigerous females at 8°C accumulated less lipid reserves than ovigerous females at lower temperatures. In crustaceans, the hepatopancreas is considered the most important organ for lipid metabolism (Galois 1987). Lower lipid reserves could indicate that higher metabolic costs are associated with respiration and parental care in ovigerous females held at high temperature. These observations are consistant with Apollonio et al.’s (1986) hypothesis that later in its life cycle (i.e. female size), northern shrimp is less tolerant of warmer temperatures.
Food rations used in the experiment did not result in any difference in the mean condition of the females. However, within each food ration a large range in the physiological condition of the females was observed. The significant relationships between HSI (i.e. lipid reserves) of the females and larval size, dry mass and energy content could indicate a possible effect of the physiological condition of ovigerous females on the characteristics of the embryos. However, physiological condition should not directly influence larval characteristics as the embryos rely on energy reserves accumulated during vitellogenesis for their development. On the other hand, parental care provided through the oxygenation of the egg mass by the beating of the pleopods may potentially be reduced in ovigerous females with a lower physiological condition. It has been demonstrated that parental care is important for the development of embryos, especially for embryos arranged in large and compact masses. Oxygen limitation caused by the position of the embryos in the mass can lead to longer development times or smaller size at hatching (Chaffee and Strathmann 1984; Strathmann and Strathmann 1995). Marine invertebrates such as crabs show an active brooding behavior that seems to provide oxygen to the embryo mass. Abdomen flapping and beating of the pleopods are behaviors associated with brooding in Cancer pagurus and Maja squinado (Fernandez et al. 2000). Stickney and Perkins (1977) reported that females of northern shrimp use their pereiopod periodically to clean the eggs from debris and epizoans and females of M. squinado also frequently introduced the chelae into the embryo mass probably for the same purpose. In this experiment, the behavior of ovigerous females was not quantified but beating of pleopods and pereiopod movements in the eggs mass were frequently observed. Considerable cost has been associated with parental care in brooding female crabs (Fernandez et al. 2000; Baeza and Fernandez 2002). Ovigerous northern shrimp females in good physiological condition (i.e. high HSI and lipid reserves) could provide better parental care to their embryos and produce larger larvae with a higher energy content. However, this hypothesis is speculative as higher costs associated with parental care do not necessarily indicate a decrease in parental care. Other studies, with more appropriate experimental designs are needed to test these possible relationships.
Reproductive characteristics of wild northern shrimp females
Comparisons of relationships between CL of ovigerous females and number of eggs in October 2001 and April 2002 indicate that mean egg loss during incubation was 14%. Based on a 95% confidence interval for the relationship observed in April, egg loss during incubation varied between 0% and 86%. In Icelandic waters, egg loss between 30% and 54% has been reported for ovigerous females between September and March (Skuladottir et al. 1978).
Larval extrusion began at the beginning of May and 50% of the females had released their larvae by May 12th. No ovigerous females were found after May 20th. Based upon incubation time at different temperatures (Fig. 5), ovigerous females in the St. Lawrence estuary should have been submitted to a mean temperature between 3°C and 3.5°C during the incubation of eggs. Hatching date of larvae influences survival probability, as larvae need to feed rapidly after hatching. In the laboratory, it has been observed that larvae must ingest some food within 30 h after hatching or death is insured even when food is obtained thereafter (Stickney and Perkins 1977). Wienberg (1982) mentioned that northern shrimp larvae could survive 6 days without food. This implies that the timing of larvae appearance is crucial in determining their food supply and survival. Although limited, data on the nutrition of larvae during initial stages of development indicate that they feed on both phytoplankton and zooplankton within the size range 50–1,000 µm (Stickney and Perkins 1981). A larger larval size at hatching could be advantageous given the size spectrum of the prey. The mean size of larvae from wild females caught in the St. Lawrence estuary was similar to the larger size of larvae observed at 2°C in the laboratory experiment (Tables 5, 6). The onset of spring phytoplankton bloom in the St. Lawrence estuary occurred in early May between 1998 and 2001 and the annual maximum in zooplankton biomass in 2002 was observed in April while in 2001, the total biomass was at its highest level during April and May (Starr et al. 2002; Harvey et al. 2003). Thus, the migration pattern and distribution of ovigerous females during the incubation period (Shumway et al. 1985) is important in determining the match or mismatch between larval extrusion and phytoplankton and zooplankton abundance, and the size of the larvae at hatching.
Populations of northern shrimp, which are largely distributed in the Northwest Atlantic from the Davis Strait to the Gulf of Maine, are subject to a large range of temperatures according to their geographic position. For example, the Scotian Shelf may represent a transition area in oceanographic conditions for northern shrimp (Koeller 2000). While western Scotian Shelf and Gulf of Maine populations evolve in similar conditions of temperature near the upper physiological and/or ecological limit, eastern Scotian Shelf populations are found in conditions similar to those on the southern Newfoundland Shelf, which are believed to represent the preferred temperature range for northern shrimp. However, the optimum temperature for physiological conditioning of females during maturation and embryogenesis, and consequently for larval characteristics at hatching, is not necessarily the same for the other developmental stages of the life cycle. While lower temperatures appear optimal for maturation and reproduction (Stickney and Perkins 1977; Nunes 1984; Bergström 1991; this study), larval survival and growth, as well as juvenile growth seem to be favored at higher temperatures or in environments with continuously increasing temperature (Nunes and Nishiyama 1984; Shumway et al. 1985; Rasmussen 1992; Tande et al. 1994; Rasmussen and Tande 1995). Further research on ontogenetic changes in the response of shrimp to temperature is needed to understand how abiotic and biotic factors affect shrimp population dynamics.
Conclusion
The results of this study suggest that the recruitment success of northern shrimp is likely influenced by temperature. Data suggest that egg loss could be higher when temperature encountered by ovigerous females increases. Duration of the incubation period is inversely related to temperature and the conversion efficiency of yolk reserves in developing embryos is significantly decreased at elevated temperatures. Finally, hatching larvae from eggs incubated at 2°C and 5°C are larger, heavier and possess more energy than hatching larvae at 8°C. Larger accumulation of energy reserves in ovigerous females kept at 2°C and 5°C indicates that low temperatures are more favorable to their physiological condition than high temperatures (i.e. 8°C). The experimental design did not result in any effect of food ration on the energetic condition of females and on egg and larval characteristics and biochemical composition. Within treatments, however, the variability in the energy reserves of ovigerous females indicated some relationships between lipid reserves (i.e. HSI) of the females and characteristics of the larvae (Fig. 4). Further studies are needed to ascertain relationships between physiological condition, parental care, and larval characteristics.
References
Allen JA (1959) On the biology of the Pandalus borealis Kroyer, with reference to a population off the Northumberland coast. J Mar Biol Assoc UK 38:189–220
Anderson PJ (1999) Pandalid shrimp as indicator of ocean climate regime shift. NAFO SCR Doc. 99/80
Anderson PJ, Piatt JF (1999) Community reorganization in the Gulf of Alaska following ocean climate regime shift. Mar Ecol Prog Ser 189:117–123
Apollonio S, Stevenson DK, Dunton EEJ (1986) Effects of temperature on the biology of the Northern Shrimp, Pandalus borealis, in the Gulf of Maine. NOAA Tech Rep NMFS 42
Attard J, Hudon C (1987) Embryonic development and energetic investment in egg production in relation to size of female lobster (Homarus americanus ). Can J Fish Aquat Sci 44:1157–1164
Baeza JA, Fernandez M (2002) Active brood care in Cancer setosus (Crustacea: Decapoda): the relationship between female behaviour, embryo oxygen consumption and the cost of brooding. Funct Ecol 16:241–251
Balasundaram C, Pandian TJ (1982) Egg loss during incubation in Macrobrachium nobilii (Henderson and Mathai). J Exp Mar Biol Ecol 59:289–299
Berejikian BA, Mathews SB, Quinn TP (1996) Effects of hatchery and wild ancestry and rearing environments on the development of agonistic behaviour in steelhead trout (Oncorhynchus mykiss) fry. Can J Fish Aquat Sci 53:2004–2014
Berenboim BI, Sheveleva GK (1989) Data on the deepwater shrimp (Pandalus borealis Koeyer) fecundity in the Barents Sea. ICES C.M. 1989/K:16
Bergström BI (1991) Reproductive cycle and the effect of temperature on oogenesis of Pandalus borealis Kroyer, 1838. J Shellfish Res 10:327–331
Bergström BI (2000) The biology of Pandalus. Adv Mar Biol 38:55–245
Biesiot PM, Perry HM (1995) Biochemical composition of the deep-sea red crab Chaceon quinquedens (Geryonidae): organic reserves of developing embryos and adults. Mar Biol 124:407–416
Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37:911–917
Bolger T, Connolly PL (1989) The selection of suitable indices for the measurement and analysis of fish condition. J Fish Biol 34:171–182
Branford JR (1978) Incubation period for the lobster Homarus gammarus at various temperatures. Mar Biol 47:363–368
Braum E (1967) The survival of fish larvae with reference to their feeding behaviour and food supply. In: Gerking SD (ed) The biological basis of freshwater fish production. Blackwell, Oxford, pp, 113–131
Burukovsky RN, Sudnik SA (1997) On realized fecundity of northern shrimp (Pandalus borealis) at Flemish Cap during spring–summer 1996. NAFO SCR Doc 97/94
Carr RS, Neff J (1984) Quantitative semi-automated enzymatic assay for tissue glycogen. Comp Biochem Physiol B 77:447–449
Chaffee C, Strathmann RR (1984) Constraints on egg masses. I. Retarded development within thick egg masses. J Exp Mar Biol Ecol 84:73–83
Clarke A (1982) Lipid synthesis and reproduction in the Polar Shrimp Chorismus antarcticus. Mar Ecol Prog Ser 9:81–90
Clarke A (1993) Egg size and egg composition in polar shrimps (Caridea; Decapoda). J Exp Mar Biol Ecol 168:189–203
Clarke A, Gore DJ (1992) Egg size and composition in Ceratoserolis (Crustacea: Isopoda) from the Weddell Sea. Polar Biol 12:129–134
Fernandez M, Bock C, Poertner H (2000) The cost of being a caring mother: the ignored factor in the reproduction of marine invertebrates. Ecol Lett 3:487–494
Galois R (1987) Les lipides neutres chez les crustacés décapodes: métabolismes et besoins. Oceanis 13:197–215
Gardner C (2001) Composition of eggs in relation to embryonic development and female size in giant crabs [Pseudocarcinus gigas (Lamarck)]. Mar Freshw Res 52:333–338
Gimenez L, Anger K (2001) Relationships among salinity, egg size, embryonic development, and larval biomass in the estuarine crab Chasmagnathus granulata Dana, 1851. J Exp Mar Biol Ecol 260:241–257
Grant SM, Brown JA (1999) Variation in condition of coastal Newfoundland 0-group Atlantic cod (Gadus morhua): field and laboratory studies using simple condition indices. Mar Biol 133:611–620
Hartman KJ, Brandt SB (1995) Estimating energy density of fish. Trans Am Fish Soc 124:347–355
Harvey M, St-Pierre JF, Gagne A, Beaulieu MF, Gagnon Y (2003) Conditions océanographiques dans l’estuaire et le golf du Saint-Laurent en 2002: zooplancton. Sécretariat canadien de recherche scientifique. Document de recherche 2003/077
Hawkes CR, Meyers TR, Shirley TC (1986) Length-weight relationships of blue, Paralithodes platypus, and golden, Lithodes aequispina, king crabs parasitized by the rhizocephalan, Briarosaccus callosus Boschma. Fish Bull 84:327–332
Haynes EB, Wigley RL (1969) Biology of the Northern Shrimp, Pandalus borealis, in the Gulf of Maine. Trans Am Fish Soc 98:60–76
Heming TA (1982) Effects of temperature on utilization of yolk by Chinook Salmon (Oncorhynchus tshawytscha ) eggs and alevins. Can J Fish Aquat Sci 39:184–190
Holdway DA, Beamish FWH (1984) Specific growth rate and proximate body composition of Atlantic cod (Gadus morhua L.). J Exp Mar Biol Ecol 81:147–170
Holland DL, Gabbott PA (1971) A micro-analytical scheme for the determination of protein, carbohydrate, lipid and RNA levels in marine invertebrate larvae. J Mar Biol Assoc UK 5:659–668
Jeckel WH, Aizpun de Moreno JE, and Moreno VJ (1990) Changes in biochemical composition and lipids of the digestive gland in females of the shrimp Pleoticus muelleri (Bate) during the molting cycle. Comp Biochem Physiol B 96:521–525
Knutsen GM, Tilseth S (1985) Growth, development, and feeding success of Atlantic cod larvae Gadus morhua related to egg size. Trans Am Fish Soc 114:507–511
Koeller PA (2000) Relative importance of abiotic and biotic factors to the management of the northern shrimp (Pandalus borealis) fishery on the Scotian Shelf. J North Atl Fish Sci 27:21–33
Kunisch M, Anger K (1984) Variation in development and growth rates of larval and juvenile spider crabs Hyas areneus reared in the laboratory. Mar Ecol Prog Ser 15:293–301
Lambert Y, Dutil JD (1997 a) Can simple condition indices be used to monitor and quantify seasonal changes in the energy reserves of Atlantic cod (Gadus morhua)? Can J Fish Aquat Sci 54:104–112
Lambert Y, Dutil JD (1997 b) Condition and energy reserves of Atlantic cod (Gadus morhua) during the collapse of the northern Gulf of St. Lawrence stock. Can J Fish Aquat Sci 54:2388–2400
Lysy A, Dvinina EA (1991) On relation of the deep sea shrimp stock size with oceanographic conditions in the Barents Sea. ICES C.M. 1991/K:52
MacKenzie BR (1988) Assessment of temperature effects on interrelationships between stage durations, mortality, and growth in laboratory-reared Homarus americanus Milne Edwards larvae. J Exp Mar Biol Ecol 116:87–98
Marsh E (1986) Effects of egg size on offspring fitness and maternal fecundity in the orangethroat darter, Etheostoma spectabile (Pisces: Percidae). Copeia 1:18–30
Meyer E, Walther A (1988) Methods for the estimation of protein, lipid, carbohydrate and chitin levels in fresh water invertebrates. Arch Hydrobiol 113:161–177
Morais S, Narciso L, Calado R, Nunes ML, Rosa R (2002) Lipid dynamics during the embryonic development of Plesionika martia martia (Decapoda;Pandalidae), Palaemon serratus and Palaemon elegans (Decapoda;Palaemonidae): relation to metabolic consumption. Mar Ecol Prog Ser 242:195–204
MPO (2001) Les conditions océanographiques dans le golfe du Saint-Laurent en 2001. MPO—Sciences, Rapport sur l’état des stocks G4–01
Narciso L, Morais S (2001) Fatty acid profile of Palaemon serratus (Palaemonidae) eggs and larvae during embryonic and larval development using different live diets. J Crustac Biol 21:566–574
Nates SF, McKenney CL Jr (2000) Ontogenetic changes in biochemical composition during larval and early postlarval development of Lepidophthalmus louisianensis, a ghost shrimp with abbreviated development. Comp Biochem Physiol B 127:459–468
Nunes P (1984) Reproductive and larval biology of northern shrimp Pandalus borealis (Krøyer) in relation to temperature. PhD thesis, University of Alaska, Fairbanks, Alaska
Nunes P, Nishiyama T (1984) Effects of temperature and food availability on the survival and growth of larvae of the northern pink shrimp Pandalus borealis Kroyer. J Shellfish Res 1:96–97
Ouellet P, Taggart CT, Frank KT (1992) Lipid condition and survival in shrimp (Pandalus borealis ) larvae. Can J Fish Aquat Sci 49:368–378
Palacios E, Ibarra AM, Ramirez JL, Portillo G, Racotta IS (1998) Biochemical composition of eggs and nauplii in white Pacific shrimp, Penaeus vannamei (Boone), in relation to the physiological condition of spawners in a commercial hatchery. Aquacult Res 29:183–189
Pandian TJ (1970a) Ecophysiological studies on the developing eggs and embryos of the European lobster Homarus gammarus. Mar Biol 5:154–167
Pandian TJ (1970b) Yolk utilization and hatching time in the Canadian lobster, Homarus americanus. Mar Biol 7:249–254
Parsons DG, Tucker GE (1986) Fecundity of northern shrimp, Pandalus borealis, (Crustacea, Decapoda) in areas of the Northwest Atlantic. Fish Bull 84:549–558
Perkins HC (1972) Developmental rates at various temperatures of embryos of the northern lobster (Homarus americanus Milne-Edwards). Fish Bull 70:95–99
Petersen S, Anger K (1997) Chemical and physiological changes during the embryonic development of the spider crab, Hyas araneus L. (Decapoda: Majidae). Comp Biochem Physiol B 117:299–306
Rasmussen B (1953) On the geographical variation in growth and sexual development of deep sea prawn (Pandalus borealis Kr.). Fiskeridir Skr (Havunders.) Rep Norw Fish Invest 10:1–160
Rasmussen T (1992) Temperature dependent growth in larvae of the deep water prawn, Pandalus borealis Kroyer, reared in the laboratory. ICES C.M. 1992/K:17
Rasmussen T, Tande K (1995) Temperature-dependent development, growth and mortality in larvae of the deep-water prawn Pandalus borealis reared in the laboratory. Mar Ecol Prog Ser 118:149–157
Sasaki GC, Capuzzo JM, Biesiot P (1986) Nutritional and bioenergetic considerations in the development of the American lobster Homarus americanus. Can J Fish Aquat Sci 43:2311–2319
Shirley SM, Shirley TC, Rice SD (1987) Latitudinal variation in the Dungeness crab, Cancer magister, zoeal morphology explained by incubation temperature. Mar Biol 95:371–376
Shumway SE, Perkins HC, Schick DF, Stickney AP (1985) Synopsis of biological data on the pink shrimp, Pandalus borealis Krøyer, 1838. FAO Fisheries Synopsis No. 144
Sibert V, Ouellet P, Brêthes JC (2004) Changes in yolk total proteins and lipid components and embryonic growth rates during lobster (Homarus americanus) egg development under a simulated seasonal temperature cycle. Mar Biol 144:1075–1086
Simard Y, Savard L (1990) Variability, spatial patterns and scales of similarity in size-frequency distributions of the northern shrimp (Pandalus borealis ) and its migrations in the Gulf of St. Lawrence. Can J Fish Aquat Sci 47:794–804
Skuladottir U, Jonsson E, Hallgrimsson I (1978) Testing for heterogeneity of Pandalus borealis populations at Iceland. ICES C.M. 1978/K:27
Smith GG, Ritar AJ, Dunstan GA (2003) An activity test to evaluate larval competency in spiny lobsters (Jasus edwardsii) from wild and captive ovigerous broodstock held under different environmental conditions. Aquaculture 218:1–4
Smith GG, Ritar AJ, Thompson PA, Dunstan GA, Brown MR (2002) The effect of embryo incubation temperature on indicators of larval viability in Stage I phyllosoma of the spiny lobster, Jasus edwardsii. Aquaculture 209:1–4
Starr M, St-Amand L, Berard-Therriault L (2002) Etat de phytoplancton dans l’estuaire et le golfe du Saint-Laurent en 2001. Sécretariat canadien de recherche scientifique. Document de recherche 2002/067
Stickney AP (1981) Laboratory studies on the development and survival of Pandalus borealis eggs in the Gulf of Maine. In: Frady T (ed) Proceedings of the International Pandalid Shrimp Symposium. February 13–15, 1979. Univ Alaska Sea Grant Rep 81–3, Kodiak, Alaska, pp, 395–405
Stickney AP, Perkins HC (1977) Environmental physiology of commercial shrimp, Pandalus borealis. Project 3-202-R Completion Report, February 1, 1974 to January 31, 1977. Dept Mar Resour, W Boothbay Harbor, Maine
Stickney AP, Perkins HC (1981) Observations on the food of the larvae of the Northern Shrimp, Pandalus borealis Kroeyer (Decapoda, Caridea). Crustaceana 40:36–49
Strathmann RR, Strathmann MF (1995) Oxygen supply and limits on aggregation of embryos. J Mar Biol Assoc UK 75:413–428
Tande KS, Rasmussen T, Pedersen G (1994) Thermal increase enhancement: a possible link between recruitment and climate in high latitude environments. ICES Mar Sci Symp 198:502–509
Tessier AJ, Consolatti NL (1989) Variation in offspring size in Daphnia and consequences for individual fitness. Oikos 56:269–276
Tong LJ, Moss GA, Pickering TD, Paewai MP (2000) Temperature effects on embryo and early larval development of the spiny lobster Jasus edwardsii, and description of a method to predict larval hatch times. Mar Freshw Res 51:243–248
Wehrtmann IS, Graeve M (1998) Lipid composition and utilization in developing eggs of two tropical marine caridean shrimps (Decapoda: Caridea: Alpheidae, Palaemonidae). Comp Biochem Physiol B 121:457–463
Wehrtmann IS, Kattner G (1998) Changes in volume, biomass, and fatty acids of developing eggs in Nauticaris magellanica (Decapoda: Caridea): a latitudinal comparison. J Crustac Biol 18:413–422
Wienberg R (1982) Studies on the influence of temperature, salinity, light, and feeding rate on laboratory reared larvae of deep sea shrimp, Pandalus borealis Kroyer 1838. Meeresforsch Rep Mar Res 29:136–153
Acknowledgements
Special thanks to Mario Peloquin for the construction and maintenance of the experimental set-up. We also thank Louise Savard for helpful discussions on the biology of northern shrimp and for the collection of wild shrimp samples and live shrimp specimens for the experiments. We are grateful to Brigitte Desrosiers for her assistance with the biochemical analysis and to Gaétan Daigle for his assistance with the statistics. Gilles Gauthier and two anonymous reviewers provided helpful comments on earlier versions of the manuscript. This study was made possible through funds provided by the Department of Fisheries and Oceans under the Science Strategic Fund program on Growth and Recruitment of Northern Shrimp and by the shrimp fisherman associations of Québec and New-Brunswick under the DFO—Group B Fishermen comanagement agreement. Sophie Brillon was supported by a FCAR postgraduate scholarship (fond québécois pour la Formation de Chercheurs et l’Aide à la Recherche). All experiments were conducted in compliance with the current laws of Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R.J. Thompson, St. John’s
Rights and permissions
About this article
Cite this article
Brillon, S., Lambert, Y. & Dodson, J. Egg survival, embryonic development, and larval characteristics of northern shrimp (Pandalus borealis) females subject to different temperature and feeding conditions. Marine Biology 147, 895–911 (2005). https://doi.org/10.1007/s00227-005-1633-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1633-6