Abstract
The European seabass is an active euryhaline teleost that migrates and forages in waters of widely differing salinities. Oxygen uptake (MO2) was measured in seabass (average mass and forklength 510 g and 34 cm, respectively) during exercise at incremental swimming speeds in a tunnel respirometer in seawater (SW) at a salinity of 30‰ and temperature of 14°C, and their maximal sustainable (critical) swimming speed (Ucrit) determined. Cardiac output (Q) was measured via an ultrasound flow probe on their ventral aorta. The fish were then exposed to acute reductions in water salinity, to either SW (control), 10‰, 5‰, or freshwater (FW, 0‰), and their exercise and cardiac performance measured again, 18 h later. Seabass were also acclimated to FW for 3 weeks, and then their exercise performance measured before and at 18 h after acute exposure to SW at 30‰. In SW, seabass exhibited an exponential increase in MO2 and Q with increasing swimming speed, to a maximum MO2 of 339±17 mg kg−1 h−1 and maximum Q of 52.0±1.9 ml min−1 kg−1 (mean±1 SEM; n=19). Both MO2 and Q exhibited signs of a plateau as the fish approached a Ucrit of 2.25±0.08 bodylengths s−1. Increases in Q during exercise were almost exclusively due to increased heart rate rather than ventricular stroke volume. There were no significant effects of the changes in salinity upon MO2 during exercise, Ucrit or cardiac performance. This was linked to an exceptional capacity to maintain plasma osmolality and tissue water content unchanged following all salinity challenges. This extraordinary adaptation would allow the seabass to maintain skeletal and cardiac muscle function while migrating through waters of widely differing salinities.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
European seabass (Family Moronidae) pursue an active pelagic life-history that comprises widescale migrations. Although predominantly marine as adults, seabass are euryhaline and juveniles often enter estuaries where they migrate and forage through waters of different salinities (Pickett and Pawson 1994). Relatively little is known about the cardio-respiratory, exercise and osmoregulatory physiology of seabass (Jensen et al. 1998; Claireaux and Lagardère 1999; Varsamos et al. 2001, 2002; Axelsson et al. 2002) and, therefore, also about the physiological adaptations that they may have evolved for their migratory euryhaline lifestyle.
Previous studies on fish species that migrate through waters of different salinities have shown a direct link between their ability to perform aerobic exercise and their homeostatic regulation of body-fluid osmolality (Brauner et al. 1992, 1994; McKenzie et al. 2001a, 2001b). In coho salmon (Oncorhynchus kisutch) smolts and Adriatic sturgeon (Acipenser naccarii) juveniles, acute increases in water salinity (“seawater challenges”) caused accumulation of plasma ions and large increases in plasma osmolality, which were directly related to a significant reduction in their maximum sustainable (critical) swimming speed (Ucrit) (Brauner et al. 1992, 1994; McKenzie et al. 2001a, 2001b). It has been suggested that this was due to impaired cardiac and skeletal muscle function consequent to ionic imbalances and loss of tissue moisture, plus further strain upon the heart due to haemoconcentration (Brauner et al. 1992, 1994; McKenzie et al. 2001a, 2001b). The effects of acute reductions in salinity have not been studied, but a loss of ions from plasma and tissues, plus increased tissue water content, would also be expected to impair the function of cardiac and skeletal muscle. Kolok and Sharkey (1997) found that gulf killifish (Fundulus grandis) acclimated to freshwater (FW) had a significantly lower Ucrit than fish maintained in brackish water (BW) at a salinity of 10‰, and attributed this to the osmotic stresses the animals suffered in FW.
Studies investigating effects of salinity change upon ion and water balance in seabass have provided contrasting results. Jensen et al. (1998) acclimated seabass to BW at a salinity of 15‰ and found that acute exposure to either FW or concentrated seawater (SW) at a salinity of 50‰ both caused profound iono-osmotic imbalances that lasted for a number of days. However, Varsamos et al. (2001) found that seabass acclimated to BW at 25‰ had an exceptional ability to regulate osmotic homeostasis, with no changes in plasma ion and osmotic status at 24 h following acute exposure to either BW at 5‰ or SW at 39‰. The ability of seabass to maintain ionic and osmotic homeostasis may, clearly, be of critical importance to their ability to negotiate migratory movements through estuaries, where water salinity may vary quite significantly over short temporal and spatial scales.
In active migratory fish such as salmonids, the capacity for sustained aerobic exercise is believed to be directly related to the ability of the heart to provide blood flow (Farrell 2002; Claireaux et al. 2005). Relatively little is known about cardiac performance during sustained exercise in other teleosts (reviewed by Farrell and Jones 1992; also Kolok et al. 1993; Kolok and Farrell 1994a, 1994b; Korsmeyer et al. 1997a, 1997b). Cardiac output (Q) during swimming is a product of ventricular stroke volume (VSH) and heart rate (fH) and, in almost all fishes studied to date, increases in VSH make the major contribution to increased Q during exercise, with the tunas and some Antarctic teleosts (Axelsson et al. 1992; Farrell and Jones 1992; Farrell 1996; Thorarensen et al. 1996a; Brill and Bushnell 2001) being considered exceptions to this general rule. This dominant role for inotropic versus chronotropic regulation during exercise has, however, yet to be investigated in the vast majority of teleost fishes.
The current study investigated the performance and energetics of sustained aerobic exercise in seabass, and the associated performance of the heart. It investigated how these were influenced by changes in water salinity, the hypothesis being that maintenance of exercise and cardiac performance following salinity change would be directly dependent upon the regulation of osmotic homeostasis in plasma and tissues.
Materials and methods
Experimental animals
European seabass (Dicentrarchus labrax) with a mean (±SEM) mass of 514±23 g and length of 34.4±0.5 cm were obtained from a commercial supplier on the Île de Ré (Charente Maritime, France), where they had been maintained throughout their lives in SW net pens. They were maintained at CREMA in 1-m2 fibreglass tanks (water volume approximately 400 l) provided with biofiltered SW at a salinity of 30‰ and temperature of 14±0.4°C, for at least 2 weeks prior to any use in experiments. Animals were fed commercial fish feed daily, but were starved for 24 h prior to use. A sub-set of the seabass (n=6) were acclimated for 2 weeks to biofiltered FW (dechlorinated l’Houmeau tapwater, salinity 0‰) at the same temperature. The reduction in salinity was accomplished over 24 h, by flushing the SW in their tank until it had been completely replaced with the FW. The seabass resumed feeding within 48 h of the FW transfer.
Surgical preparation
Bass were anaesthetised with tricaine methane sulphonate (MS-222) at a concentration of 0.1 g l−1, and transferred to an operating table where their gills were irrigated with aerated water containing 0.05 g l−1 MS-222. A 2S-type Transonic ultrasound flow probe (resolution 0.1 ml min−1; absolute accuracy ±15%)was placed around the ventral aorta, as described by Axelsson et al. (2002). For surgery on the seabass acclimated to FW, the MS-222 was buffered with NaHCO3 (0.05 g l−1). The animals were allowed 48 h recovery in opaque PVC chambers provided with a flow of water at the appropriate salinity (either SW or FW).
Exercise and cardiac performance
Swimming respirometry was performed with an automated Brett-type swim-tunnel respirometer designed to exercise fish in a non-turbulent water flow with a uniform velocity profile, as described in detail by McKenzie et al. (2001a). Fish were transferred individually to the respirometer and allowed to recover for at least 12 h (overnight) in a current at a speed of 5 cm s−1. At this low current speed, the bass rested on the bottom and maintained position in the flume by very gentle sculling of their pectoral fins and occasional tailbeats. The following day, the seabass were exposed to progressive increments in swimming speed, of 5, 10 and then each 20 cm s−1 every 30 min, until fatigue. Fish were considered to be fatigued when they were unable to remove themselves from the posterior screen of the swimming chamber despite gentle encouragement by sudden increases in current velocity.
Measurements of O2 uptake (MO2, in mg kg−1 h−1) at each swimming speed were calculated automatically with the custom-designed data-acquisition system described in McKenzie et al. (2001a), from the decline in water O2 saturation in the sealed respirometer, the volume of water, and the mass of the fish. Water O2 saturation was measured with an Orbisphere clarke-type polarographic oxygen electrode and associated meter (Orbisphere Laboratory, Geneva, Switzerland). For each individual fish, a least squares exponential regression was applied to the relationship between swimming speed and MO2. Note that data for swimming speeds close to fatigue were not included in this analysis, to ensure that swimming performance was sustained by aerobic metabolism and that the relationship between MO2 and swimming speed was not, therefore, confounded by contributions to performance from anaerobic metabolic pathways. Extrapolation back to the y-intercept, a notional swimming speed of zero, was then employed to correct for the contribution to MO2 of locomotor-muscle activity (Brett 1964; Fry 1971). The value thus derived was termed inactive metabolic rate (IMR; McKenzie et al. 2003). The maximum metabolic rate of activity (AMR) was identified during swimming (this occurred at speeds approaching Ucrit) and net aerobic scope was then calculated as AMR minus IMR (Fry 1971; McKenzie et al. 2003). Each fish’s Ucrit was calculated in cm s−1, and also bodylengths s−1 (BL s−1), as described by Brett (1964).
At each swimming speed, Q was measured in ml min−1 kg−1, with the signal from the flowprobe displayed on the Transonic amplifier and acquired by a PC with the custom-designed labview software described by Axelsson et al. (2002). The signal was used to calculate fH in beats min−1 and, together with the data for Q, used to calculate VSH, in ml beat−1 kg−1, as described by Axelsson et al. (2002). Cardiac scope during exercise was calculated as maximum Q minus “routine” Q. Maximum Q always occurred at swimming speeds approaching Ucrit. Routine Q was taken as the lowest Q measured when the fish was swimming very gently at a speed of 5 cm s−1, prior to the exercise protocol. Routine fH and VSH were derived from the measures of routine Q.
Salinity challenges
Following the above exercise protocol in the water to which they had been acclimated, the seabass were exposed to an acute change in water salinity, as follows: SW to SW (control); SW to BW at 10‰; SW to BW at 5‰; SW to FW, or FW to SW. The reductions in salinity were accomplished by flushing the respirometer with dechlorinated tapwater, while the FW to SW transfer was accomplished by flushing with seawater. Water salinity was measured with a salinometer (WTW F216, WTW, Weilheim, Germany).
At 18 h after these changes in water salinity, exercise and cardiac performance were measured once again, exactly as described above. Following this second swim test, animals were rapidly removed from the respirometer and killed with a blow to the head. A blood sample was withdrawn from the caudal vein and centrifuged to obtain plasma, which was stored at −20°C for subsequent measurement of osmolality, with a freezing-point osmometer (13/13DR, Herman Roebling MESSTECHNIK, Berlin). Samples of muscle and ventricle were collected, weighed and then dried to constant weight at 50°C to estimate percentage tissue water content.
Statistical analyses
The effects of the first exercise test upon MO2 and cardiac variables were assessed by one-way analysis of variance (ANOVA) for repeated measures. The effects of the salinity challenges upon performance were assessed as their effects upon Ucrit, IMR, AMR, aerobic scope, and maximum Q, using a two-way ANOVA for repeated measures. The effects of the salinity challenges upon plasma osmolality, and skeletal or cardiac water content, were assessed by one-way ANOVA.
Results
Whereas all of the animals acclimated to SW survived anaesthesia and placement of the Transonic cuff (n=19), the seabass acclimated to FW died when this was attempted, so the procedure was only performed upon 3 fish and the remaining three were exercised without the cuff. Data for cardiac performance are not, therefore, available for fish acclimated to FW.
Exercise and cardiac performance in SW
When all data are pooled for the first swim test in SW, the seabass achieved a Ucrit of 80.2±4.3 cm s−1, equivalent to 2.25±0.08 BL s−1 (mean±SEM, n=19). As shown in Fig. 1, there was a significant and profound increase in MO2 during exercise; the increase was exponential up to 60 cm s−1 followed by deviation towards an asymptote as the fish approached Ucrit. Application of an exponential relationship to the combined raw data between 5 cm s−1 and 60 cm s−1 revealed a high correlation coefficient, with R2=0.946. Mean IMR was 56±7 mg O2 kg−1 h−1 and AMR was 339±17 mg O2 kg−1 h−1, such that net aerobic scope was 283±20 mg O2 kg−1 h−1. Thus, the sustained aerobic exercise elicited an approximately fivefold increase in metabolism above IMR.
Metabolic rate and cardiac performance in seabass exercising in seawater (salinity=30‰) at 14°C. Effects of stepwise increase in swimming speed on a oxygen consumption (MO2, squares) and cardiac output (Q, circles) and b heart rate (fH, triangles) and stroke volume (VSH, diamonds). Values are mean±SEM, n = 19.
The mean routine Q of the seabass was 25.0±1.39 ml min−1 kg−1; routine fH was 34.1±2.0 beats min−1 and routine VSH was 0.68±0.04 ml beat−1 kg−1. The changes in Q during exercise showed a similar response profile to that of MO2, with an exponential increase up to 60 cm s−1 followed by an asymptote at higher speeds (Fig. 1). Application of an exponential relationship to the combined raw data between 5 cm s−1 and 60 cm s−1 revealed a high correlation coefficient, R2=0.936. Maximum Q was 52.0±1.9 ml min−1 kg−1 and net cardiac scope was 30.1±1.6 ml min−1 kg−1, so that Q approximately doubled during the exercise protocol. The increase in Q was associated with a significant increase in both fH and VSH (Fig. 1). As can be seen in Fig. 1, however, the increase in fH was much more pronounced than the increase in VSH, and showed a response profile that closely mirrored those of both MO2 and Q. Indeed, fH increased exponentially to 60 cm s−1 whereas VSH showed no further significant increases after 40 cm s−1, and the increase in fH accounted for almost 90% of the measured increase in Q.
Effects of the salinity challenges
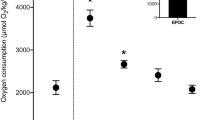
The seabass exhibited an exceptional tolerance of all the salinity challenges, with no mortalities observed. Indeed, as can be seen in Fig. 2, the salinity challenges had no significant effects upon Ucrit, which was maintained at the same level as prior to the challenge. As might be expected from the fact that Ucrit was maintained, the salinity challenges also had no significant effects upon IMR, AMR or aerobic scope (Fig. 3) or upon maximum Q (Fig. 4).
The effects of acute changes in water salinity on mean (±SEM) critical swimming speed (Ucrit). Unfilled columns are means of the first swim test in either seawater (SW) or fresh water (FW). Filled columns are means of the second swim test 18 h after the salinity challenges. These comprised an acute change from seawater to either seawater (SW-SW, n=6); brackish water at a salinity of 10‰ (SW-10‰, n=4); brackish water at a salinity of 5‰ (SW-5‰, n=6), or to fresh water (SW-FW, n=3); or an acute change from fresh water to seawater (FW-SW, n=3).
Effects of acute changes in water salinity on mean (±SEM) immobile metabolic rate (IMR) (a), active metabolic rate (AMR) (b) and resultant aerobic scope (c). Unfilled columns are means of the first swim test in either seawater (SW) or fresh water (FW). Filled columns are means of the second swim test 18 h after the salinity challenges. These comprised an acute change from seawater to either seawater (SW-SW, n=6); brackish water at a salinity of 10‰ (SW-10‰, n=4); brackish water at a salinity of 5‰ (SW-5‰, n=6), or to fresh water (SW-FW, n=3); or an acute change from fresh water to seawater (FW-SW, n=3).
Effects of changes in water salinity on mean (±SEM) maximum cardiac output (Qmax). Unfilled columns are means of the first swim test in seawater (SW). Filled columns are means of the second swim test 18 h after the salinity challenges. These comprised an acute change from seawater to either seawater (SW-SW, n=6); brackish water at a salinity of 10‰ (SW-10‰, n=4); brackish water at a salinity of 5‰ (SW-5‰, n=6), or to fresh water (SW-FW, n=3).
The ability of the seabass to maintain exercise and cardiac performance following the salinity challenges was associated with an extraordinary capacity for homeostatic regulation of plasma osmolality and tissue water balance. That is, the mean plasma osmolality of the seabass when sampled at fatigue from the swim test performed after the various salinity challenges was not significantly different from that of the control seabass fatigued in SW (Table 1). Similarly, the mean water content of cardiac and skeletal muscle from the seabass exposed to the salinity challenges was not significantly different from that of the control seabass in SW (Table 1). Note that water content was not measured in the fish exposed to the SW to FW or FW to SW challenges, but the fact that they regulated plasma osmolality indicates that tissue water balance was also maintained.
Discussion
The ability of the seabass to maintain exercise performance following changes in salinity differs from the responses observed in coho salmon and Adriatic sturgeon, where seawater challenge caused a significant decline in Ucrit (Brauner et al. 1992, 1994; McKenzie et al. 2001a, 2001b). The impaired responses in these latter species were directly related to significant increases in plasma ion concentrations and osmolality, and significant reductions in tissue water content (Brauner et al. 1992, 1994; McKenzie et al. 2001a, 2001b). Thus, it seems reasonable to assume that the absence of any changes in the seabass’ exercise or cardiac performance following the various salinity challenges was a direct result of their exceptional ability to regulate plasma osmotic homeostasis and tissue water balance.
This extraordinary capacity for homeostatic regulation of osmotic status is consistent with a previous study by Varsamos et al. (2001). Salmonids and sturgeon exhibit significant osmotic imbalances at 24 h following changes in water salinity (Bath and Eddy 1979; Brauner et al. 1992, 1994; Claireaux and Audet 2000; McKenzie et al. 2001a, 2001b), and so do such species as Mozambique tilapia (Oreochromis mossambicus) and its hybrids, which are able to adapt to extreme salinities of up to 95‰ (Hwang et al. 1989; Sardella et al. 2004). The European flounder (Platichthys flesus) is, however, able to tolerate increases in salinity from 10‰ to 30‰ with only a small increase in plasma osmolality at 24 h exposure (Jensen et al. 2002).
In many euryhaline species, changes in water salinity lead to changes in routine MO2 and, although both increases and decreases in MO2 have been reported, these have all been attributed to the existence of osmotic stress (e.g. Morgan and Iwama 1991, 1998; Swanson 1998; McKenzie et al. 2001a, 2001b; Sardella et al. 2004). Thus, the absence of any changes whatsoever in IMR of the seabass, whether acclimated to SW or FW, and following the various salinity challenges, may indicate that the maintenance of osmotic homeostasis did not cause any significant stress or metabolic costs. There was one indication that acclimation to FW was, however, significantly stressful to the seabass, which was their inability to tolerate anaesthesia and placement of the ventral aortic flow cuff. It is not clear whether seabass spend extended periods in FW in nature (Pickett and Pawson 1994), but there are anecdotal reports that they are particularly sensitive to husbandry stresses when reared in FW.
Varsamos et al. (2002) suggested that the exceptional capacity for osmotic regulation may reflect extremely plastic morphofunctional adaptations of mitochondria-rich (“chloride”) cell populations in the gills, and the osmoregulatory physiology of the seabass clearly represents a fascinating area for future research. Whatever the mechanism by which the seabass can maintain a constant internal milieu when faced by variations in environmental salinity, this ability would allow them to migrate freely throughout estuaries, and between marine, BW and FW habitats (Pickett and Pawson 1994). It is interesting that the European flounder, which is also reported to perform such facultative migrations, also possesses a similar ability to regulate the osmotic status of their internal milieu when exposed to acute changes in water salinity (Jensen et al. 2002).
The performance and energetics of sustained exercise in the seabass at 14°C were comparable to those of farmed and instrumented salmonids of a similar size at temperatures between 10°C and 14°C (e.g. Gallaugher et al. 2001; Shingles et al. 2001; Beaumont et al. 2003). For example, Shingles et al. (2001) exercised cannulated rainbow trout (Oncorhynchus mykiss) of a similar size at 14°C in the same respirometer as used in the current study, and reported a Ucrit of 2.23 BL s−1, compared with 2.25 BL s−1 measured in the current study. This required a similar, over fivefold, increase in metabolic rate above IMR (Shingles et al. 2001). A temperature of 14°C is at the lower end of the European seabass’s thermal range; they perform better at warmer temperatures around their thermal optimum. Aerobic scope at 26°C is up to double that observed at 14°C (Claireaux and Lagardère 1999) and Ucrit some 1.5 times higher (G. Claireaux, unpublished observations).
Seabass cardiac scope during exercise, and the maximum Q achieved, were similar to those measured directly by ventral aortic flowprobes in salmonids (Thorarensen et al. 1996b; Gallaugher et al. 2001; Beaumont et al. 2003) and other active (i.e. non-sedentary) teleosts such as the northern squawfish Ptychocheilus oregonesis (Kolok and Farrell 1994a, 1994b) of a similar size and at similar temperatures (10–15°C). The fact, however, that seabass exhibit much greater aerobic scope and Ucrit at temperatures above 20°C (Claireaux and Lagardère 1999; G. Claireaux, unpublished observations) indicates that they may also have much greater cardiac scope and maximum Q at such temperatures. There is preliminary evidence to indicate that this is indeed the case at 20°C (A. Chatelier, unpublished observations).
In many of the active teleost species studied to date, both MO2 and Q plateau as the fish approaches Ucrit (Kiceniuk and Jones 1977; Kolok and Farrell 1994a; Thorarensen et al. 1996b; Gallaugher et al. 2001). This has been proposed as circumstantial evidence that maximum cardiac output is linked to, and may limit, maximum O2 uptake and aerobic exercise performance in these species (Farrell 2002). This plateau effect occurred in the seabass, and visual observation of the ventral aortic probe trace revealed that both fH and Q became extremely irregular as the fish approached Ucrit and started the intermittent “burst and coast” swimming pattern that indicates recruitment of anaerobic white muscle (Day and Butler 1996) and which precedes fatigue. Thus, cardiac performance may limit AMR and Ucrit in the seabass.
In most teleost species studied to date, increases in VSH are responsible for at least 50% of the increase in Q observed during exercise (reviewed by Farrell and Jones 1992; also Kolok and Farrell 1994a, 1994b; Thorarensen et al. 1996a, 1996b; Gallaugher et al. 2001) whereas, in the seabass, 90% of the increase in Q was attributable to increased fH. The tunas have often been cited as one of the few teleost groups that accomplish exercise-related increases in Q almost exclusively through increased fH (Farrell 1996; Brill and Bushnell 2001). It has been suggested that this is because they have extremely high VSH under routine conditions and so have little scope to increase it during exercise (Farrell 1996; Brill and Bushnell 2001). The routine VSH of the seabass was not, however, exceptionally high, being only some 20% higher than the routine values reported for salmonids at similar temperatures (e.g. Taylor et al. 1996; Thorarensen et al. 1996b; Gallaugher et al. 2001). However, routine fH of the seabass was some 35% lower than resting values for salmonids reported in these previous studies (e.g. Taylor et al. 1996; Thorarensen et al. 1996b; Gallaugher et al. 2001). In tunas, regulation of fH during exercise appears to occur exclusively as a result of relaxation of inhibitory vagal cholinergic tone, whereas in other teleosts it occurs both by this mechanism and by increased adrenergic stimulation (Farrell and Jones 1992; Brill and Bushnell 2001). Investigating the pharmacology of cardiac control during exercise would be an interesting area for future research in the seabass.
Conclusions
The seabass possesses an exceptional capacity for homeostatic regulation of plasma osmolality and tissue water balance following changes in water salinity. This adaptation allows them to maintain the performance of their skeletal and cardiac muscle, and so would allow the animals to negotiate facultative migrations through waters of widely differing salinities. At 14°C, seabass exercise performance, and underlying cardiac performance, are similar to those reported for other teleosts of a similar size and at similar temperatures. The seabass increased Q during exercise almost exclusively through increases in fH, with only a minor change in VSH. This cardiac response to exercise is similar to that of the tunas but different from that of most other fishes, in which the predominant modulation is of VSH.
References
Axelsson M, Davison W, Forster ME, Farrell AP (1992) Cardiovascular-responses of the red-blooded Antarctic fishes Pagothenia bernacchii and P borchgrevinki. J Exp Biol 167:179–201
Axelsson M, Altimiras J, Claireaux G (2002) Post-prandial blood flow to the gastrointestinal tract is not compromised during hypoxia in the seabass Dicentrarchus labrax. J Exp Biol 205:2891–2896
Bath RN, Eddy FB (1979) Salt and water balance in rainbow trout Salmo gairdneri rapidly transferred from freshwater to seawater. J Exp Biol 83:193–202
Beaumont MW, Butler PJ, Taylor EW (2003) Exposure of brown trout, Salmo trutta, to a sub-lethal concentration of copper in soft acidic water: effects upon gas exchange and ammonia accumulation. J Exp Biol 206:153–162
Brauner CJ, Shrimpton JM, Randall DJ (1992) The effect of short-duration seawater exposure on plasma ion concentrations and swimming performance in coho salmon (Oncorhynchus kisutch). Can J Fish Aquat Sci 49:2399–2405
Brauner CJ, Iwama GK, Randall DJ (1994) The effect of short-duration seawater exposure on the swimming performance of wild and hatchery-reared juvenile coho salmon (Oncorhynchus kisutch) during smoltification. Can J Fish Aquat Sci 51:2188–2194
Brett JR (1964) The respiratory metabolism and swimming performance of young sockeye salmon. J Fish Res Board Can 21:1183–1226
Brill RW, Bushnell PG (2001) The cardiovascular system of tunas. In: Block BA, Stevens ED (eds) Tuna: physiology, ecology and evolution. Academic, San Diego, pp 79–120
Claireaux G, Audet C (2000) Seasonal changes in hyperosmoregulatory ability of brook char: the role of environmental factors. J Fish Biol 56:347–373
Claireaux G, Lagardère JP (1999) Influence of temperature, oxygen and salinity on the metabolism of the European seabass. J Sea Res 42:157–168
Claireaux G, McKenzie DJ, Genge G, Chatelier A, Aubin J, Farrell AP (2005) Linking swimming performance, cardiac performance and cardiac morphology in rainbow trout. J Exp Biol (in press)
Day N, Butler PJ (1996) Environmental acidity and white muscle recruitment during swimming in the brown trout (Salmo trutta). J Exp Biol 199:1947–1959
Farrell AP (1996) Features heightening cardiovascular performance in fishes, with special reference to tunas. Comp Biochem Physiol 113A:61–67
Farrell AP (2002) Cardiorespiratory performance in salmonids during exercise at high temperature: insights into cardiovascular design limitations in fishes. Comp Biochem Physiol 132A:797–810
Farrell AP, Jones DR (1992) The heart. In: Hoar WS, Randall DJ, Farrell AP (eds) Fish physiology, vol 12A. Academic, New York, pp 1–88
Fry FEJ (1971) The effect of environmental factors on the physiology of fish. In: Hoar WS, Randall DJ (eds) Fish physiology, vol 6. Academic, New York, pp 1–99
Gallaugher PE, Thorarensen H, Kiessling A, Farrell AP (2001) Effects of high intensity exercise training on cardiovascular function, oxygen uptake, internal oxygen transfer and osmotic balance in chinook salmon (Oncorhynchus tshawytscha) during critical speed swimming. J Exp Biol 204:2861–2872
Hwang PP, Sun CM, Wu SM (1989) Changes in plasma osmolality, chloride concentration and gill Na-K-ATPase activity in tilapia Oreochromis mossambicus during seawater adaptation. Mar Biol 100:295–300
Jensen FB, Lecklin T, Busk M, Bury NR, Wilson RW, Wood CM, Grosell M (2002) Physiological impact of salinity increase at organism and red blood cell levels in the European flounder (Platichthys flesus). J Exp Mar Biol Ecol 274:159–174
Jensen MK, Madsen SS, Kristiansen K (1998) Osmoregulation and salinity effects on the expression and activity of Na+,K+-ATPase in the gills of European seabass, Dicentrarchus labrax (L.). J Exp Zool 282:290–300
Kiceniuk JW, Jones DR (1977) The oxygen transport system in trout (Salmo gairdneri) during sustained exercise. J Exp Biol 69:247–260
Kolok AS, Farrell AP (1994a) Individual variation in the swimming performance and cardiac performance of northern squawfish, Ptychocheilus oregonensis. Physiol Zool 67:706–722
Kolok AS, Farrell AP (1994b) The relationship between maximum cardiac output and swimming performance in northern squawfish, Ptychocheilus oregonensis: the effect of coronary artery ligation. Can J Zool 72:1687–1690
Kolok AS, Sharkey D (1997) Effect of freshwater acclimation on the swimming performance and plasma osmolarity of the euryhaline gulf killifish. Trans Am Fish Soc 126:866–870
Kolok AS, Spooner RM, Farrell AP (1993) The effect of exercise on the cardiac output and blood flow distribution of the largescale sucker Catostomus macrocheilus. J. Exp Biol 183:301–321
Korsmeyer KE, Lai NC, Shadwick RE, Graham JB (1997a) Heart rate and stroke volume contributions to cardiac output in swimming yellowfin tuna: response to exercise and temperature. J Exp Biol 200:1975–1986
Korsmeyer KE, Lai NC, Shadwick RE, Graham JB (1997b) Oxygen transport and cardiovascular responses to exercise in yellowfin tuna Thunnus albacores. J Exp Biol 200:1987–1997
McKenzie DJ, Cataldi E, Owen S, Taylor EW, Bronzi P (2001a) Effects of acclimation to brackish water on the growth, respiratory metabolism and exercise performance of Adriatic sturgeon (Acipenser naccarii). Can J Fish Aquat Sci 58:1104–1112
McKenzie DJ, Cataldi E, Taylor EW, Cataudella S, Bronzi P (2001b) Effects of acclimation to brackish water on tolerance of salinity challenge by Adriatic sturgeon (Acipenser naccarii). Can J Fish Aquat Sci 58:1113–1120
McKenzie DJ, Martinez R, Morales A, Acosta J, Morales R, Taylor EW, Steffensen JF, Estrada MP (2003) Effects of growth hormone transgenesis on metabolic rate, exercise performance and hypoxia tolerance in tilapia hybrids. J Fish Biol 63:398–409
Morgan JD, Iwama GK (1991) Effects of salinity on growth, metabolism and ion regulation in juvenile rainbow and steelhead trout (Oncorhynchus mykiss) and fall chinook salmon (Oncorhynchus tshawytscha). Can J Fish Aquat Sci 48:2083–2094
Morgan JD, Iwama GK (1998) Salinity effects on oxygen consumption, gill Na+,K+-ATPase activity and ion regulation in juvenile coho salmon. J Fish Biol 53:1110–1119
Pickett GD. Pawson MG (1994) Seabass. Chapman and Hall, London
Sardella BA, Matey V, Cooper J, Gonzalez RJ, Brauner CJ (2004) Physiological, biochemical and morphological indicators of osmoregulatory stress in ‘California’ Mozambique tilapia (Oreochromis mossambicus x O. urolepis hornorum) exposed to hypersaline water. J Exp Biol 207:1399–1413
Shingles A, McKenzie DJ, Taylor EW, Moretti A, ButlerPJ, Ceradini S (2001) Effects of sublethal ammonia exposure on swimming performance in rainbow trout (Oncorhynchus mykiss). J Exp Biol 204:2691–2698
Swanson C (1998) Interactive effects of salinity on metabolic rate, activity, growth and osmoregulation in the euryhaline milkfish (Chanos chanos). J Exp Biol 201:3355–3366
Taylor SE, Egginton S, Taylor EW (1996) Seasonal temperature acclimatisation of rainbow trout: cardiovascular and morphometric influences on maximum sustainable exercise level. J Exp Biol 199:835–845
Thorarensen H, Gallaugher PE, Farrell AP (1996a) The limitations of heart rate as a predictor of metabolic rate in fish. J Fish Biol 49:226–236
Thorarensen H, Gallaugher PE, Farrell AP (1996b) Cardiac output in swimming rainbow trout, Oncorhynchus mykiss, acclimated to seawater. Physiol Zool 69:139–153
Varsamos S, Connes R, Diaz JP, Barnabé G, Charmantier G (2001) Ontogeny of osmoregulation in the European seabass Dicentrarchus labrax L. Mar Biol 138:909–915
Varsamos S, Diaz JP, Charmantier G, Flik G, Blasco C, Connes R (2002) Branchial chloride cells in seabass (Dicentrarchus labrax) adapted to freshwater, seawater and doubly concentrated seawater. J Exp Zool 293:12–26
Acknowledgements
The authors are grateful to G. Guillou and M. Prineau for their assistance during the study. A.C. was supported by a doctoral bursary provided jointly by the Conseil Régionale Charente Maritime and Ifremer. D.J.M. was employed on a research project funded by the European Commission (Ethofish QLRT-2001-00799). These experiments complied with the current laws in France.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S.A. Poulet, Roscoff
Rights and permissions
About this article
Cite this article
Chatelier, A., McKenzie, D.J. & Claireaux, G. Effects of changes in water salinity upon exercise and cardiac performance in the European seabass (Dicentrarchus labrax). Marine Biology 147, 855–862 (2005). https://doi.org/10.1007/s00227-005-1624-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-1624-7