Abstract
Whale sharks (Rhincodon typus Smith) aggregate seasonally (March–June) to feed in coastal waters off Ningaloo Reef, Western Australia. Pop-up archival tags were attached to 19 individuals (total lengths 4.5–11.0 m) at this location in early May of 2003 and 2004 to examine their horizontal and vertical movements. The long-term movement patterns of six whale sharks were documented, all of which travelled northeast into the Indian Ocean after departing Ningaloo Reef. They used both inshore and offshore habitats and made extensive vertical movements, occasionally to a depth of at least 980 m. Frequent up-and-down movements, diel vertical migration, and crepuscular descents were evident in the depth records. The sharks experienced ambient temperatures ranging between 4.2 and 28.7°C and encountered gradients of up to 20.8°C on dives.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Understanding the movement patterns of large migratory fishes is important to their conservation and management. To investigate these patterns, satellite-linked radio transmitters have been widely used on species that regularly swim in surface waters. However, this technology is less effective when studying species that may remain submerged for long periods as radio signals are rapidly attenuated in seawater and are also reflected downward at the sea surface. Consequently, the signals either have limited strength or never reach earth-orbiting satellites unless the transmitter’s antenna is above the sea surface. In recent years, new types of tags have been developed that overcome some of these constraints (Block et al. 1998). These “pop-up” archival tags store recorded data until they detach from the fish and float to the surface. They then transmit this information to Argos satellites. This technology has permitted researchers to examine the horizontal and vertical movement patterns of a wide range of fishes, such as tunas (e.g. Wilson et al. 2005), billfishes (e.g. Takahashi et al. 2003), and sharks (e.g. Sims et al. 2003).
Though whale sharks (Rhincodon typus) are the largest fishes (up to at least 12 m) on Earth, they are one of the least known (Stewart and Wilson 2005). Their broad-scale horizontal movements in tropical and warm-temperate seas have been correlated with productivity pulses, ocean circulation, and water temperatures (Compagno 2001; Wilson et al. 2001a). Satellite telemetry studies in the Pacific Ocean have shown that they can migrate over great distances. Indeed, one shark travelled 12,620 km over 37 mo (Eckert and Stewart 2001). Only limited data on their vertical movements have been obtained to date (Gunn et al. 1999; Eckert and Stewart 2001). At some coastal sites (e.g. Ningaloo Reef, Western Australia and along the Belize Barrier Reef), predictable seasonal aggregations of whale sharks have supported the development of lucrative ecotourism industries. At others, fishermen supplying meat and fins to Asian markets target those seasonal aggregations. Because catches have declined in several areas where intensive fisheries occur (CITES 2002), descriptions of whale shark migration routes are urgently needed (Fowler 2000).
Large numbers of whale sharks gather off Ningaloo Reef between March and June each year to feed on schools of euphausiids (Taylor 1994; Wilson and Newbound 2001; Wilson et al. 2001b; Jarman and Wilson 2004) and baitfishes associated with the seasonal development of a closed Leeuwin Current/Ningaloo Current recirculation pattern (Taylor and Pearce 1999; Wilson et al. 2001b, 2003). They are protected when in Australian waters by domestic legislation that prohibits their capture and strictly manages human activities around them (Colman 1997). However, after departing Ningaloo Reef their whereabouts have been unknown, though they might travel to Indonesia and other neighboring nations where they are targeted by artisanal and commercial fisheries (Eckert et al. 2002; Newman et al. 2002). Here, we report the results of our studies using pop-up archival tags to document the horizontal and vertical movements of whale sharks that visit Ningaloo Reef each year.
Materials and methods
We tagged 19 whale sharks (Rhincodon typus Smith) with pop-up archival tags in 2003 (n = 4; model PTT-100, Microwave Telemetry, Inc., Columbia, MD, USA) and 2004 (n = 15; PAT version 4, Wildlife Computers, Inc., Redmond, WA, USA) (Table 1). Both types of tags measured and stored light, depth, and temperature information at regular intervals and then later transmitted those data to Argos satellites once the tag was at the sea surface, either at a programmed date or if it detached prematurely. The Microwave Telemetry tags recorded depth and temperature at 1-h intervals and transmitted this information as raw data. In contrast, these parameters were sampled every minute by Wildlife Computers tags and compressed into depth and temperature histograms (intervals set at 1 or 12-h) for data transmission.
Whale sharks were located by aircraft in coastal waters off Ningaloo Reef, Western Australia. Once a shark was spotted, a research vessel was directed to position divers ahead of the approaching shark for tag deployment. A tether, constructed of either monofilament or nylon-coated stainless steel, was used to attach each tag to a titanium dart. The darts were implanted several centimeters into the subdermal tissue of the shark, just below the first dorsal fin, using a Hawaiian-sling polespear. Total lengths of sharks were measured using an open reel tape measure. We identified the sex of each shark by direct visual inspection of the pelvic fins (i.e. for the presence or absence of claspers).
Tagging locations were determined using a Garmin global positioning system (GPS) receiver (accuracy approximately 15 m). Pop-up locations were determined by using the best locations available (location class ≥1 i.e., accuracy ≤1 km) when the tags detached and began communicating with Argos satellites. The routes that the sharks travelled between the tagging and pop-up locations were estimated with a two-step process. First, raw geolocation estimates were calculated from recovered light-level data using astronomical algorithms provided by the tag manufacturers. The accuracy of these estimates was determined by comparing light-based geolocation estimates immediately prior to tag detachment with Argos pop-up locations (cf. Schaefer and Fuller 2002; Stokesbury et al. 2004; Teo et al. 2004). The second step was to apply a state-space Kalman filter model (Sibert and Fournier 2001; Sibert et al. 2003) to estimate movement parameters and provide a ``most probable” trackline for each shark. This processing was conducted using the KFtrack package (Sibert and Nielsen 2004) in the R statistical environment (R Development Core Team 2005).
Results
Seven of the tags never reported (1 of 4 in 2003 and 6 of 15 in 2004) and six others detached within 10 days of deployment. We recovered long-term records (2–9 mo) from six sharks (Table 1).
Horizontal movements
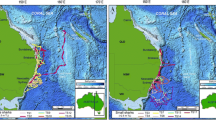
The greatest distance travelled between tagging and pop-up locations was 1,501 km in 57 days (Table 1; minimum speed = 26 km day−1). We calculated root mean square errors of the raw light-based geolocation estimates relative to the known endpoint locations as 1.44° for latitude (approximately 160 km) and 0.68° for longitude (approximately 75 km). We assumed that the errors in raw estimates of latitude and longitude between the tagging and pop-up locations were less than or equal to these calculated errors for periods beyond 14 days of the austral vernal equinox (September 21). Consequently those estimates of error apply to the entire routes travelled by sharks 4, 10, 16, and 17 and virtually all of the routes travelled by sharks 12 and 19 (Fig. 1a, b).
Rhincodon typus. Kalman filtered tracks of sharks. a 10 and 17, b 4, 12, 16, and 19. Geolocation estimates of sharks 12 and 19 occurring within 14 days of the austral vernal equinox are highlighted (white points). The month each tag reported is displayed at the end of each track. The 200-m isobath (solid line) indicates the continental shelf break
All sharks remained near the coast for several weeks after being tagged. They departed Ningaloo Reef between May and June and most travelled northeast along the continental shelf before moving offshore into the northeastern Indian Ocean (Fig. 1a, b). The two longest tracks, with endpoints at Ashmore (shark 17) and Scott Reefs (shark 10), were from tags that reported in July and August, respectively (Fig. 1a). The four other long-term tracks terminated later in the year at locations nearer to Ningaloo Reef, on the North West Shelf (shark 19) and in the open ocean (sharks 4, 12, and 16) (Fig. 1b).
Vertical movements
Whale sharks routinely moved between the sea surface and depth (Figs. 2a–d and 3a, b). They spent >40% (day=36.9%, night=49.7%) of their time in the upper 15 m of the water column and >50% (day=48.3%, night=59.4%) of their time at depths of ≤30 m (Fig. 4). Daytime depths were generally greater than those at night, with some exceptions. For example, when shark 4 was in coastal waters off Ningaloo Reef it spent daylight hours near the surface and nights at depths of 30–80 m (Fig. 2b). That pattern reversed when it moved to the outer North West Shelf where it spent most days swimming just off the seafloor (at depths of approximately 120 m) and nights oscillating between depths of 30–80 m (Fig. 2c). Abrupt changes between day and night swimming patterns and deep dives often occurred at dawn and dusk (Fig. 2b-d).
Deeper dives were recorded in the open ocean, in contrast with the bathymetrically constrained swimming depths observed on the continental shelf (Figs. 2a and 3a, b). Overall, the sharks spent approximately 1% of the time at depths >300 m. The deepest recorded depth was 980 m, the measurement limit of tags used in 2004. Most deep dives (>300 m) were during the day (Fig. 4). The tag attached to shark 12 recorded a constant depth of 980 m and temperature of 2.2°C for at least 12 h prior to reporting.
Temperature records indicated that the upper 50–80 m of the water column was isothermal regardless of location (Fig. 5a, b). The sharks spent approximately 75% of their time in that mixed surface layer. Beneath the mixed layer, the thermocline extended to a depth of approximately 500 m (Fig. 5b). They spent >90% of their time in water of 23–28°C (Fig. 6). They inhabited sea surface temperatures (SSTs) ranging from 23.3–28.4°C and experienced ambient water temperatures ranging from 4.2 to 28.7°C (Table 1). Only small fluctuations in ambient temperature were experienced by whale sharks in coastal waters (Figs. 2a and 3a, b). As they moved beyond the shelf break, deep diving activity increased and a broader range of ambient temperatures was encountered. Differentials of up to 20.8°C were recorded on some dives.
Discussion and conclusions
Frequent deep diving by whale sharks may account for the large number of non-reporting tags in the second year of this study. Unlike the Microwave Telemetry tags used in 2003, the Wildlife Computers tags used in 2004 could not be programmed to jettison when they approached their crush depth. Rather, the manufacturer provided a mechanical guillotine that severed monofilament tethers when the tag approached that depth. Because we used nylon-coated stainless steel tethers in 2004, these devices were omitted. High tag failure rates have been reported in species that make frequent and extensive vertical movements (e.g. 63% in swordfish, 63% in bigeye thresher sharks, 50% in blue sharks; M. K. Musyl et al., in preparation), perhaps indicating that repeated contraction and expansion of pressure housings might compromise structural integrity. Rapid biofouling of tags in tropical waters might also account for some reporting failures. Tags deployed in 2004 were not coated with anti-fouling paint and photographs taken by whale shark tour operators 4–6 weeks after tagging revealed heavy growth that may have interfered with tag buoyancy and data transmission.
After departing Ningaloo Reef, all whale sharks moved offshore into the northeastern Indian Ocean, a known “hotspot” of predator diversity (Worm et al. 2003) and the only known spawning area of southern bluefin tuna (Thunnus maccoyii) (Matsuura et al. 1997). Two of three whale sharks tracked in an earlier study took similar northeasterly routes after departing Ningaloo Reef (J.D. Stevens, CSIRO Marine Research, personal communication). The two tags (sharks 10 and 17) that reported during the austral winter (July and August) were farthest from Ningaloo Reef whereas the four (sharks 4, 12, 16, and 17) that reported in spring (September, November, December) were closer to the tagging site. Although none of the tagged sharks have returned to Ningaloo Reef, some individuals have been re-sighted at this location over successive years (Taylor 1994; M.S. Press et al., in preparation). Distinct inshore and offshore residency patterns have been documented in several other shark species, including tiger sharks (Galeocerdo cuvier) (Holland et al. 1999), school sharks (Galeorhinus galeus) (West and Stevens 2001), and white sharks (Carcharodon carcharias) (Boustany et al. 2002). Our observations of whale shark movements are consistent with those general patterns.
Our representations of the movements of whale sharks between the tagging and the pop-up locations are conditioned on error associated with light-based latitude and longitude estimates. Pop-up archival tags use recovered light-level data to calculate latitude (from day length) and longitude (from time of local noon). Their accuracies are influenced by equinoxes, light attenuation, water clarity, weather conditions, light sensor resolution, clock error, and diving behavior (Musyl et al. 2001). Latitude estimates are considerably less accurate than longitude estimates, especially near the equinoxes when day length is similar at all latitudes. Of the six long-term tracks presented here, three (sharks 10, 16, and 17) did not extend beyond the austral vernal equinox (September 21). Only two geolocation estimates from the remaining three tracks occurred within 14 days of this date (Fig. 2b).
To validate light-based geolocation estimates, researchers have attached tags to fixed moorings (Welch and Eveson 1999; Musyl et al. 2001), moving vessels (Welch and Eveson 1999), and captive fish being towed in a sea cage (Gunn et al. 1994). Only one study to date has compared geolocation estimates from pop-up archival tags with Argos positions from satellite-linked radio transmitters in free-swimming animals (Teo et al. 2004). These experiments found errors in raw latitude and longitude estimates of approximately 0–2°. Our analysis of the accuracy of track endpoint data identified errors of similar magnitude. Raw geolocation estimates can be improved by (1) removing clearly anomalous locations (e.g. Block et al. 2001; Schaefer and Fuller 2002; Sims et al. 2003); (2) using smoothing procedures such as moving averages (e.g. Matsumoto et al. 2005); (3) applying state-space movement models such as the Kalman filter (Sibert and Fournier 2001; Sibert et al. 2003) or the particle filter (Royer et al. 2005); or (4) matching tag sea surface temperature (SST) records with remotely sensed SSTs (e.g. Delong et al. 1992; Block et al. 2001; Teo et al. 2004; Domeier et al. 2005). In 2005, we conducted double-tagging experiments on whale sharks at Ningaloo Reef using pop-up archival tags and satellite-linked radio transmitters. Preliminary data obtained for this study suggest that the light-based movement patterns that we reconstructed for whale sharks are reasonable proxies for actual movements. A subsequent paper will examine the accuracy of the raw, processed, and SST-refined geolocation estimates derived from the pop-up archival tag data (S. G. Wilson et al., in preparation).
Using photo-identification data, a recent study found that some of the whale sharks aggregating at Ningaloo Reef return to this location in successive years (M.S. Press et al., in preparation). The current study lends limited support to the hypothesis that whale sharks aggregating at Ningaloo Reef represent a local population that undertakes short distance seasonal migrations. The movements of whale sharks appeared to correlate with the retreat of warm SST isotherms towards the equator in late winter. Circulation in this region is dominated by the exchange of tropical water from the Pacific to the Indian Ocean (Cresswell et al. 1993; Matsuura et al. 1997). Surface flow is to the southwest between March and August, when the sea level gradient between the two ocean basins is greatest (Cresswell et al. 1993). The direction of flow shifts to the northeast between September and January, associated with the transition of monsoons and a weakening sea level gradient. Thus, the gross horizontal movement patterns that we documented do not appear to reverse in phase with the prevailing surface currents at different times of the year.
The in situ oceanographic data (depth and temperature) that we collected reveal much about the habits and habitats of whale sharks. Depth records showed that these filter-feeding sharks have a broader ecological niche than previously suspected. In addition to exploiting coastal and epipelagic (0–200 m) zones, their vertical behavior suggests that they also target mesopelagic (200–1000 m) prey. We propose that whale sharks might exploit organisms of the deep scattering layer (e.g., euphausiids, myctophids, squid, jellyfish, etc.) when seaward of the continental shelf break. Young whale sharks may do so exclusively, as specimens between 1 and 3 m are rarely observed.
Maximum depths attained by whale sharks approach or exceed those reported for other large pelagic fishes, such as blue sharks (Prionace glauca) (600 m; Carey and Scharold 1990), bigeye thresher sharks (Alopias superciliosus) (723 m; Nakano et al. 2003), white sharks (1280 m; Compagno 2001), bluefin tuna (Thunnus thynnus) (>1,000 m; Block et al. 2001), bigeye tuna (Thunnus obesus) (>1,000 m; Gunn and Block 2001), swordfish (Xiphias gladius) (900 m; Takahashi et al. 2003), and sharptail mola (Masturus lanceolatus) (>700 m; Seitz et al. 2002). The two other species of large, filter-feeding sharks are also known to enter deepwater habitats. Pop-up archival tag data have recently shown that basking sharks (Cetorhinus maximus) dive to >750 m (Sims et al. 2003). Similarly, the rare megamouth shark (Megachasma pelagios) is thought to dive to considerable depths in its open ocean habitat. Tags used in the second year of this study had a depth limit of 980 m and any deeper dives were recorded as that limit. Therefore, it seems likely that some dives exceeded 980 m in 2004.
Many of the diving patterns observed in whale sharks have been reported in other large pelagic fishes. Frequent up-and-down movements in the water column are thought to function (1) to detect concentrations of prey (Josse et al. 1998) or olfactory trails leading to them (Westerberg 1984; Carey and Scharold 1990; Gunn et al. 1999); (2) to conserve energy, i.e., swim up and glide down (Weihs 1973); or (3) as a form of behavioral thermoregulation (Carey and Scharold 1990).
Diel changes in the vertical behavior of whale sharks may be in response to diel vertical migrations of their prey. Several other sharks are reported to be deeper during the day and shallower at night, including megamouth sharks (Nelson et al. 1997), school sharks (West and Stevens 2001), bigeye thresher sharks (Weng and Block 2004), and white sharks (Dewar et al. 2004). This pattern was not always evident in the depth records of whale sharks, however, and might be related to variation in prey behavior. Off Ningaloo Reef, whale sharks often target daytime surface schools of Pseudeuphausia latifrons (Taylor 1994; Wilson and Newbound 2001; Wilson et al. 2001b; Jarman and Wilson 2004) and, perhaps as a consequence, their daytime depths were often shallower at this location. When on the outer North West Shelf, one shark swam just off the seafloor during the day where large numbers of P. latifrons have previously been captured (Wilson et al. 2003).
Crepuscular diving has been reported in many large pelagic fishes (e.g. Nelson et al. 1997; Wilson et al. 2005). A number of hypotheses have been proposed to explain deep bounce dives at times of light transition (i.e. dawn and dusk): (1) the dives are made to visually locate the deep scattering layer and associated prey at the beginning and end of each day (Davis and Stanley 2002); (2) fish are avoiding twilight periods in which predators would have a visual advantage (Itoh et al. 2003); and (3) the dives represent a switch between two modes of behavior (Newlands et al. 2004). Alternatively, these descents may allow visual predators to exploit vertically migrating prey during a brief window of vulnerability. Whale sharks made most of their deep dives during the day, as found in mako sharks (Sepulveda et al. 2004) and bluefin tuna (Wilson et al. 2005). These may represent foraging dives that specifically target prey seeking refuge at depth during the day.
The warm SSTs inhabited by whale sharks were within previously reported ranges (Iwasaki 1969; Arnbom and Papastavrou 1988; Eckert and Stewart 2001). Our data indicate that whale sharks can tolerate a wide range of environmental temperatures (i.e. are eurythermal). Anatomical, physiological, and behavioral adaptations have evolved in a number of tunas (e.g. Holland et al. 1992), billfishes (e.g. Carey 1982), and sharks (e.g. Carey and Teal 1969) that allow them to conserve heat while exploiting cold, deep ocean habitats. It is not known if whale sharks have similar characteristics, but their large body mass may provide sufficient thermal inertia to avoid excessive heat loss during deep dives (Neill and Stevens 1974; Neill et al. 1976; Sims 2003).
Whale sharks that aggregate annually at Ningaloo Reef evidently range up to 1,500 km to the northeast after departing this area. Our data are not inconsistent with the hypothesis that they undertake short-distance migrations and return to Ningaloo Reef at 1-year intervals. Whale sharks may be particularly susceptible to overexploitation due to their migratory nature, apparent k-selected life history traits, behavioral vulnerability, and small population size (Stewart and Wilson 2005). Their horizontal movements suggest that some sharks might enter Indonesian waters where they are hunted. While the impact of such fisheries to regional whale shark abundance is uncertain, it may be substantial if limited mixing is occurring between populations. This highlights the need for conservation and management efforts to be coordinated at a regional or international level. The vertical movements of whale sharks suggest they exploit prey in both epipelagic and mesopelagic habitats and that they have a complex pattern of vertical migration that differs between coastal and offshore locations.
References
Arnbom T, Papastavrou V (1988) Fish in association with whale sharks Rhiniodon typus near the Galápagos Islands. Not Galápagos 46:13–15
Block BA, Dewar H, Farwell C, Prince ED (1998) A new satellite technology for tracking the movements of Atlantic bluefin tuna. Proc Natl Acad Sci USA 95:9384–9389
Block BA, Dewar H, Blackwell SB, Williams TD, Prince ED, Farwell CJ, Boustany A, Teo SL, Seitz A, Walli A, Fudge D (2001) Migratory movements, depth preferences, and thermal biology of Atlantic bluefin tuna. Science 293:1310–1314
Boustany AM, Davis SF, Pyle P, Anderson SD, Le Boeuf BJ, Block BA (2002) Expanded niche for white sharks. Nature 415:35–36
Carey FG (1982) A brain heater in the swordfish Xiphias gladius. Science 216:1327–1329
Carey FG, Scharold JV (1990) Movements of blue sharks (Prionace glauca) in depth and course. Mar Biol 106:329–342
Carey FG, Teal JM (1969) Mako and porbeagle: warm-bodied sharks. Comp Biochem Physiol 28:199–204
CITES (2002) Inclusion of the whale shark (Rhincodon typus) on Appendix II of CITES. Proposal 12.35 presented at the 12th Meeting of the Conference of the Parties, Santiago, Chile. Available at: http://www.cites.org/eng/cop/12/prop/E12-P35.pdf
Colman JG (1997) A review of the biology and ecology of the whale shark. J Fish Biol 51:1219–1234
Compagno LJV (2001) Sharks of the world: an annotated and illustrated catalogue of shark species known to date. vol. 2. Bullhead, mackerel and carpet sharks (Heterodontiformes, Lamniformes and Orectolobiformes). FAO, Rome
Cresswell G, Frische A, Peterson J, Quadfasel D (1993) Circulation in the Timor Sea. J Geophys Res 98:14379–14389
Davis TLO, Stanley CA (2002) Vertical and horizontal movements of southern bluefin tuna (Thunnus maccoyii) in the Great Australian Bight observed with ultrasonic telemetry. Fish Bull 100:448–465
Delong RL, Stewart BS, Hill RD (1992) Documenting migrations of Northern elephant seals using day length. Mar Mamm Sci 8:155–159
Dewar H, Domeier M, Nasby-Lucas N (2004) Insights into young of the year white shark, Carcharodon carcharias, behavior in the Southern California Bight. Environ Biol Fish 70:133–143
Domeier ML, Kiefer D, Nasby-Lucas N, Wagschal A, O’Brien F (2005) Tracking Pacific bluefin tuna (Thunnus thynnus orientalis) in the northeastern Pacific with an automated algorithm that estimates latitude by matching sea-surface-temperature data from satellites with temperature data from tags on fish. Fish Bull 103:292–306
Eckert SA, Stewart BS (2001) Telemetry and satellite tracking of whale sharks, Rhincodon typus, in the Sea of Cortez, Mexico, and the north Pacific Ocean. Environ Biol Fish 60:299–308
Eckert SA, Dola LA, Kooyman GL, Perrin W, Rahman RA (2002) Movements of whale sharks (Rhincodon typus) in south-east Asian waters as determined by satellite telemetry. J Zool 257:111–115
Fowler S (2000) Whale shark Rhincodon typus: policy and research scoping study. Nature Conservation Bureau, Newbury, UK
Gunn J, Block B (2001) Advances in acoustic, archival and satellite tagging of tunas. In: Block BA, Stevens ED (eds) Tuna: physiology, ecology and evolution. Academic Press, San Diego, pp 167–224
Gunn J, Polacheck T, Davis T, Sherlock M, Betlehem A (1994) The development and use of archival tags for studying the migration, behaviour and physiology of southern bluefin tuna, with an assessment of the potential for transfer of the technology to groundfish research. ICES CM 1994/Mini 21:1–23
Gunn JS, Stevens JD, Davis TLO, Norman BM (1999) Observations on the short-term movements and behaviour of whale sharks (Rhincodon typus) at Ningaloo Reef, Western Australia. Mar Biol 135:553–559
Holland KN, Brill RW, Sibert JR, Fournier DA (1992) Physiological and behavioral thermoregulation in bigeye tuna Thunnus obesus. Nature 358:410–412
Holland KN, Wetherbee BM, Lowe CG, Meyer CG (1999) Movements of tiger sharks (Galeocerdo cuvier) in coastal Hawaiian waters. Mar Biol 134:665–673
Itoh T, Tsuji S, Nitta A (2003) Swimming depth, ambient water temperature preference, and feeding frequency of young Pacific bluefin tuna (Thunnus orientalis) determined with archival tags. Fish Bull 101:535–544
Iwasaki Y (1969) On the distribution and environment of the whale shark, Rhincodon typus, in skipjack fishing grounds in the western Pacific Ocean. J Coll Mar Sci Tech Tokai Univ 4:37–51
Jarman SN, Wilson SG (2004) DNA-based species identification of krill consumed by whale sharks. J Fish Biol 65:586–591
Josse E, Bach P, Dagorn L (1998) Simultaneous observations of tuna movements and their prey by sonic tracking and acoustic surveys. Hydrobiologia 372:61–69
Matsumoto T, Saito H, Miyabe N (2005) Swimming behavior of adult bigeye tuna using pop-up tags in the central Atlantic Ocean. ICCAT Coll Vol Sci Pap 57:151–170
Matsuura H, Sugimoto T, Nakai M, Tsuji S (1997) Oceanographic conditions near the spawning ground of southern bluefin tuna; northeastern Indian Ocean. J Oceanogr 53:421–433
Musyl MK, Brill RW, Curran DS, Gunn JS, Hartog JR, Hill RD, Welch DW, Eveson JP, Boggs CH, Brainard RE (2001) Ability of archival tags to provide estimates of geographical position based on light intensity. In: Sibert JR, Nielsen JL (eds) Electronic tagging and tracking in marine fisheries. Kluwer, Dordrecht, pp 343–367
Nakano H, Matsunaga H, Okamoto H, Okazaki M (2003) Acoustic tracking of bigeye thresher shark Alopias superciliosus in the eastern Pacific Ocean. Mar Ecol Prog Ser 265:255–261
Neill WH, Stevens ED (1974) Thermal inertia versus thermoregulation in ‘warm’ turtles and tunas. Science 184:1008–1010
Neill WH, Chang RKC, Dizon AE (1976) Magnitude and ecological implications of thermal inertia in skipjack tuna, Katsuwonus pelamis (Linnaeus). Environ Biol Fish 1:61–80
Nelson DR, McKibben JN, Strong Jr WR, Lowe CG, Sisneros JA, Schroeder DM, Lavenberg RJ (1997) An acoustic tracking of a megamouth shark, Megachasma pelagios—a crepuscular vertical migrator. Environ Biol Fish 49:389–399
Newlands NK, Lutcavage ME, Pitcher TJ (2004) Analysis of foraging movements of Atlantic bluefin tuna (Thunnus thynnus): individuals switch between two modes of search behaviour. Popul Ecol 46:39–53
Newman HE, Medcraft AJ, Colman JG (2002) Whale shark tagging and ecotourism. In: Fowler SL, Reed TM, Dipper FA (eds) Elasmobranch biodiversity, conservation and management. IUCN, Gland, pp 230–235
R Development Core Team (2005) R: a language and environment for statistical computing. Available at: http://www.R-project.org
Royer F, Fromentin J-M, Gaspar P (2005) A state-space model to derive bluefin tuna movement and habitat from archival tags. Oikos 109:473–484
Schaefer KM, Fuller DW (2002) Movements, behavior, and habitat selection of bigeye tuna (Thunnus obesus) in the eastern equatorial Pacific, ascertained through archival tags. Fish Bull 100:765–788
Seitz AC, WengKC, Boustany AM, Block BA (2002) Behavior of a sharptail mola in the Gulf of Mexico. J Fish Biol 60:1597–1602
Sepulveda CA, Kohin S, Chan C, Vetter R, Graham JB (2004) Movement patterns, depth preferences, and stomach temperatures of free-swimming juvenile mako sharks, Isurus oxyrinchus, in the Southern California Bight. Mar Biol 145:191–199
Sibert J, Fournier D (2001) Possible models for combining tracking data with conventional tracking data. In: Sibert JR, Nielsen JL (eds) Electronic tagging and tracking in marine fisheries. Kluwer, Dordrecht, pp 443–456
Sibert JR, Nielsen A (2004) The KFtrack package. Available at: http://www.dina.kvl.dk/~anielsen/kftrack
Sibert J, Musyl MK, Brill RW (2003) Horizontal movements of bigeye tuna (Thunnus obesus) near Hawaii determined by Kalman filter analysis of archival tagging data. Fish Oceanogr 12:141–152
Sims DW (2003) Tractable models for testing theories about natural strategies: foraging behaviour and habitat selection of free-ranging sharks. J Fish Biol 63(Suppl A):53–73
Sims DW, Southall EJ, Richardson AJ, Reid PC, Metcalfe JD (2003) Seasonal movements and behaviour of basking sharks from archival tagging: no evidence of winter hibernation. Mar Ecol Prog Ser 248:187–196
Stewart BS, Wilson SG (2005) Threatened fishes of the world: Rhincodon typus (Smith 1828) (Rhincodontidae). Environ Biol Fish (in press)
Stokesbury MJW, Teo SLH, Seitz A, O’Dor RK, Block BA (2004) Movement of Atlantic bluefin tuna (Thunnus thynnus) as determined by satellite tagging experiments initiated off New England. Can J Fish Aquat Sci 61:1976–1987
Takahashi M, Okamura H, Yokawa K, Okazaki M (2003) Swimming behaviour and migration of a swordfish recorded by an archival tag. Mar Freshw Res 54:527–534
Taylor G (1994) Whale sharks: the giants of Ningaloo Reef. Angus and Robertson, Sydney
Taylor JG, Pearce AF (1999) Ningaloo Reef currents: implications for coral spawn dispersal, zooplankton and whale shark abundance. J R Soc West Aust 82:57–65
Teo SLH, Boustany A, Blackwell S, Walli A, Weng KC, Block BA (2004) Validation of geolocation estimates based on light level and sea surface temperature from electronic tags. Mar Ecol Prog Ser 283:81–98
Weihs D (1973) Mechanically efficient swimming techniques for fish with negative buoyancy. J Mar Res 31:194–209
Welch DW, Eveson JP (1999) An assessment of light-based geoposition estimates from archival tags. Can J Fish Aquat Sci 56:1317–1327
Weng KC, Block BA (2004) Diel vertical migration of the bigeye thresher (Alopias superciliosus), a species possessing orbital retia mirabilia. Fish Bull 102:221–229
West GJ, Stevens JD (2001) Archival tagging of school shark, Galeorhinus galeus, in Australia: initial results. Environ Biol Fish 60:283–298
Westerberg H (1984) The orientation of fish and the vertical stratification at fine- and micro-structure scales. In: McCleave JD, Arnold GP Dodson JJ, Neill WH (eds) Mechanisms of migration in fishes. Plenum Press, New York, pp 179–203
Wilson SG, Newbound DR (2001) Two whale shark faecal samples from Ningaloo Reef, Western Australia. Bull Mar Sci 68:361–362
Wilson SG, Taylor JG, Pearce AF (2001a) The seasonal aggregation of whale sharks at Ningaloo Reef, Western Australia: currents, migrations and the El Niño/ Southern Oscillation. Environ Biol Fish 61:1–11
Wilson SG, Pauly T, Meekan MG (2001b) Daytime surface swarming by Pseudeuphausia latifrons (Crustacea, Euphausiacea) off Ningaloo Reef, Western Australia. Bull Mar Sci 68:157–162
Wilson SG, Meekan MG, Carleton JH, Stewart TC, Knott B (2003) Distribution, abundance and reproductive biology of Pseudeuphausia latifrons and other euphausiids on the southern North West Shelf, Western Australia. Mar Biol 142:369–379
Wilson SG, Lutcavage ME, Brill RW, Genovese MP, Cooper AB, Everly AW (2005) Movements of bluefin tuna (Thunnus thynnus) in the northwestern Atlantic Ocean recorded by pop-up archival tags. Mar Biol 146:409–423
Worm B, Lotze HK, Myers RA (2003) Predator diversity hotspots in the blue ocean. Proc Natl Acad Sci USA 100:9884–9888
Acknowledgements
This project received funding and support from the NOAA Ocean Exploration Program, Woodside Energy, BHP Billiton Petroleum, Chevron, the whale shark research fund administered by the Western Australian Department of Conservation and Land Management (CALM), the Australian Institute of Marine Science, the U.S. National Marine Fisheries Service, Hubbs-SeaWorld Research Institute, the University of New Hampshire, U.S. Scuba and PADI Australia. We thank the staff of CALM in Exmouth, particularly Roland Mau, Jennie Cary and Ben Fitzpatrick, for helping with logistics. This research was reviewed and approved by the animal ethics committees of Hubbs-SeaWorld Research Institute and CALM.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J.P.Grassle, New Brunswick
Rights and permissions
About this article
Cite this article
Wilson, S., Polovina, J., Stewart, B. et al. Movements of whale sharks (Rhincodon typus) tagged at Ningaloo Reef, Western Australia. Marine Biology 148, 1157–1166 (2006). https://doi.org/10.1007/s00227-005-0153-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0153-8