Abstract
Partial sequences of the mitochondrial DNA (mtDNA) gene cytochrome oxidase subunit 1 (COI) were analysed from individuals of the coralline demosponge Astrosclera willeyana sensu lato out of ten Indo-Pacific populations from the Red Sea to the central Pacific. This taxon is widely distributed in cryptic coral reef habitats of the Indo-Pacific and is regarded as a modern representative of long-extinct, formerly reef-building stromatoporoid-type sponges. The aims were to clarify phylogeographic and taxonomic relationships in this “living fossil” and to explore mitochondrial DNA sequence variation over a wide geographic range. Very low variability was observed across the Indo-Pacific, as only three COI haplotypes were identified, with a maximum p-distance of 0.418% and low nucleotide diversity (π=0.00049). Very low genetic structure was revealed among populations: Haplotype 1 was found in all specimens from nine Pacific populations (N=45), separated by distances of more than 7,000 km; haplotype 2 was restricted to the Red Sea population (N=4); haplotype 3 was only found in the Tuamoto specimens (N=7). COI data presented here do not support the hypothesis of at least two sibling species belonging to genus Astrosclera in the Pacific. Considering the maximum geographic distance of more than 20,000 km between sampled populations, mtDNA COI sequence variation observed here is among the lowest reported to date for a diploblastic taxon and adds to the growing evidence of a general mtDNA conservation in sponges. It is argued that this low mtDNA variation in A. willeyana s.l. is due to a low rate of mtDNA evolution caused by a combination of long generation time and low metabolic rate.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrosclera willeyana sensu lato Footnote 1 Lister 1900 is the most common coralline sponge (‘sclerosponge’, a sponge with a secondary calcareous basal skeleton) in Indo-Pacific coral reefs today (Reitner et al. 1996). This taxon is found throughout the Indo-Pacific, from the northern Red Sea to Tahiti (Wörheide 1998) and is regarded as a living fossil, a thought-to-be living relative of the long-extinct ‘Stromatoporoidea’ (Wood 1987), an enigmatic fossil group of sponges which were the main reef-building organisms during long periods of the Earth’s history. Taxon Astrosclera first occurred in the upper Triassic reef deposits of Antalya, Turkey (A. cuifi, Wörheide 1998) and today, in shallow water, A. willeyana s.l. is restricted to certain cryptic and light reduced environments such as reef caves.
Phylogeographic and taxonomic relationships of regional populations remain contentious. Vacelet (1981) was the first to observe regional differences in spicule morphology, the main taxonomic character in Porifera, and discussed the possibility that there may be more than one species of A. willeyana throughout its wide Indo-Pacific distribution. This concept was subsequently supported by a detailed morphological study (length and spination of acanthostyle spicules, see Wörheide 1998 for details) that discovered several regional populations, distinguishable by different spicule morphologies.
Based on new data from rDNA internal transcribed spacer (ITS) sequences, which were congruent with the previously reported morphometric differences, Wörheide et al. (2002) proposed that A. willeyana might actually be a complex of at least three sibling species. The Fiji/Vanuatu population allegedly represented A. willeyana sensu stricto, due to the close vicinity to the type locality and similarities in spicule morphology, and the two new sibling species were Astrosclera sp. (1) from the Red Sea and Astrosclera sp. (2) from the Great Barrier and Osprey Reef. In phylogenetic analysis, the Red Sea and GBR/Osprey Reef sequence types formed a closely related sister group to the Fiji/Vanuatu sequence types. However, only 12 samples were sequenced in this study and variation among the sequence types was low (maximum p-distance, 0.0062). This hypothesis is therefore in need of further corroboration by additional loci, such as mitochondrial DNA (mtDNA) genes.
Animal mtDNA is the most frequently used genetic marker to infer phylogeographic relationships in most marine organisms (Avise 2000). Several important characteristics make genes of the animal mitochondrial genome especially suitable for such analyses: substitution rates are mostly high, especially at third codon positions of protein-coding genes, compared to genes of the nuclear genome (e.g. Brown et al. 1979), they are normally maternally inherited and non-recombining (Avise 1994; but see Tsaousis et al. 2005) and the fourfold smaller effective population size compared to nuclear DNA (nDNA) leads to a much faster lineage sorting (Birky et al. 1983) (see also reviews by Moritz et al. 1987; Boore 1999). Among the most frequently used genes for shallow phylogenies is the one coding for cytochrome oxidase subunit 1 (COI), which is easily amplified using PCR methods and conserved primers (e.g. Folmer et al. 1994).
However, while high substitution rates in mtDNA are common in most animals (e.g. mammals, Brown et al. 1979), slow mitochondrial sequence evolution has been reported in some anthozoan cnidarians, making it difficult to reconstruct phylogenies among conspecifics, but factors contributing to this slow mitochondrial evolution still remain largely enigmatic (see review by Shearer et al. 2002). Data on mtDNA variation at lower systematic levels in another diploblast taxon, the sponges (Porifera), are scarce. Wörheide et al. (2000) were the first to report difficulties in resolving phylogenetic and phylogeographic relationships within the calcarean family Leucettidae using the mtDNA gene cytochrome oxidase subunit II (COII), whereas those relationships were clearly resolved using rDNA ITS sequences. Only one study to date explored in more detail the intraspecific variation of COI in sponges, in the demosponge Crambe crambe (Duran et al. 2003), where only two haplotypes and low nucleotide diversity (π=0.0006) were identified in eight populations separated by distances of 20–3,000 km, from the western Mediterranean to the Atlantic. Duran et al. (2003) interpreted their findings in support of the hypothesis of a general high level of mtDNA conservation in diploblast phyla, suggested earlier by Watkins and Beckenbach (1999). The recently published mitochondrial genomes of three demosponges (Lavrov and Lang 2005; Lavrov et al. 2005) provide further insight into gene content and mitochondrial evolution in sponges, and a reduced ratio of evolutionary rates of poriferan mitochondrial small subunit (SSU) rRNA genes compared to nuclear SSU-rDNA genes was observed, compared to rates in mammals (4.3 vs. 10.7 times on an average) (Lavrov et al. 2005). However, no evidence for mechanisms responsible for these rate differences was found.
I have now investigated mtDNA COI sequence variation in individuals of A. willeyana (Agelasida), geographically ranging from the northern Red Sea to the Tuamoto Archipelago in the central Pacific—a distance covering more than 20,000 km—in an attempt to clarify their phylogeographic and taxonomic relationships and to test whether Lavrov et al.’s (2005) statement of “extremely low levels of intraspecific polymorphism in (poriferan) mitochondrial genes” holds true more widely than in the single so-far investigated taxon C. crambe from the Mediterranean (Duran et al. 2003).
Here I will show that very low levels of genetic variation in mtDNA COI partial sequences occur among populations of Astrosclera over their whole geographic range and that putative sibling species in the Pacific cannot be distinguished by the COI sequence comparison. I argue that this low variation is most likely due to a low rate of mtDNA evolution caused by multiple factors.
Material and methods
Partial mtDNA COI sequences were amplified from the samples listed in Table 1. Specimens were collected by SCUBA from subtidal reef caves and were preserved immediately after collection either in silica gel (Wörheide 1998) or in 99% ethanol and stored at −20°C or at room temperature until time of extraction (see also Wörheide et al. 2002). An unidentified species from the genus Agelas from the Great Barrier Reef was used as an outgroup. Voucher samples with registration numbers QMG31xxxx have been deposited at the Queensland Museum and vouchers of the remaining samples are held by the author.
Samples were extracted using the Qiagen DNEasy Tissue extraction kit according to the manufacturer’s instructions or using Chelex (Yue and Orban 2001). Partial COI mtDNA sequences were amplified using the universal primers from Folmer et al. (1994). PCR amplifications were conducted on a MJ Research PTC-200 thermocycler in 25 μl reactions that consisted of 0.25 U BIOTAQ™ Polymerase (Bioline, Luckenwalde, Germany), NH4 Buffer [16 mM (NH4)2SO4, 67 mM Tris–HCl (pH 8.8 at 25°C), 0.01% Tween-20], 2.5 mM MgCl2, 0.4 μM of each primer, 0.4 mM each dNTP, and template of variable concentration. The following temperature profile was used: initial denaturation at 95°C for 5 min, then 35 cycles of 95°C for 60 s, 45°C for 60 s, and 72°C for 60 s, with a final elongation step of 7 min. Amplicons were purified from agarose gels using a silica-based method (Boyle and Lew 1995) and directly sequenced using PCR primers and the ABI BigDye Ready Reaction Kit (version 3) on an ABI 3100.
Sequences were assembled using the software CodonCode aligner (http://codoncode.com/aligner). One sequence (AY561969) from a specimen from Papua New Guinea (PNG) was downloaded from GenBank and all sequences were aligned with a newly generated sequence of an Agelas species, a relative from the same order, Agelasida. Automatic alignments done with ClustalW (Thompson et al. 1994) were manually inspected, optimized, and translated into amino acids using Se-Al v2.0a11 (Rambaut 1996) and MacClade 4.0 (Sinauer Association). The first nucleotide of the sequenced fragment corresponds to nucleotide 11398 in the full Tethya mitochondrial genome (Lavrov et al. 2005). Due to the low variation, the alignment was straightforward. De novo sequences generated in this study were checked against GenBank matches for possible contamination by running BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/), since it is well known that Astrosclera can harbour several commensals or symbionts (Wörheide 1998). An E value of <10−6 was used to define a significant match. Uncorrected nucleotide p-distances were calculated using PAUP*4 (Swofford 1998) and nucleotide diversities in DNAsp v4 (Rozas and Rozas 1999). A neigbour-joining analysis was carried in PAUP*4 using nucleotide p-distances and the default options.
Four new COI haplotypes have been deposited in the EMBL Nucleotide Sequence Database (http://www.ebi.ac.uk/) under accession numbers AJ972396-98 (A. willeyana s.l.) and AJ972399 (Agelas sp.).
Results
Partial COI sequences with an aligned length of 479 bp were obtained de novo from 55 specimens of A. willeyana s.l.. All significant BLAST matches were obtained from other Porifera and Cnidaria. The fact that the sequence of a specimen of A. willeyana s.l. from PNG was identical to the most widespread haplotype here, and the low divergent haplotypes over the geographic range of more than 20,000 km suggests that no contaminants or symbionts were sequenced. Only three haplotypes were detected among all specimens of A. willeyana s.l.. Haplotype 1 is geographically widespread and occurs in 45 specimens in the Pacific. Haplotype 2 occurs only in the four specimens from the Red Sea, and Haplotype 3 was found only in the seven specimens from Tuamoto Archipelago in the central Pacific (see Table 1). Nucleotide diversity was very low (π=0.00049) and maximum p-distance between the three haplotypes across the Indo-Pacific was 0.418%. COI sequences were invariable among the 45 specimens from the western Pacific plus Moorea, spanning a geographic distance of more than 7,000 km. The maximum nucleotide p-distance to Agelas sp., a member of the same order Agelasida, was 10%, with 46 polymorphic sites out of 48 being silent third codon substitutions, and one a silent first codon substitution (see Table 2).
Translated amino acid sequences were invariable among A. willeyana s.l., and showed only one amino acid difference (out of 159 amino acids inferred) to Agelas sp.. This change was a transition in the first codon position of an Isoleucine (ATA) at aa 126 that caused a change into Valine (GTA) (see Table 2)—both non-polar, easily exchangeable amino acids. Among the three A. willeyana s.l. haplotypes, only two polymorphic sites in the nucleotide alignment were observed: in position 89 and 113 (Table 2). Both sites in Agelas sp. examined here are conserved within the A. axifera group (F. Parra, personal communication), to which the specimen here, based on COI sequence comparison, most likely belongs. Implied phylogeographic relationships from neighbour-joining analysis are given in Fig. 1.
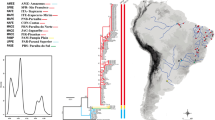
Neighbour-joining tree (using nucleotide p-distances) with estimated phylogenetic relationships of the three haplotypes discovered among specimens from ten populations of Astrosclera willeyana s.l. over a geographic distance of more than 20,000 km across the Indo-Pacific. Note that this phylogeny-estimation is only based on two variable sites (compare Table 2) and is rooted using Agelas sp. (QMG317484). Haplotype frequencies are indicated as pie charts. Character state changes are indicated on branches. Photo insert: A specimen of Astrosclera willeyana s.l. at Osprey Reef (Coral Sea, Australia) with a diameter of about 4 cm. Photo by Dr. Matthias Bergbauer
Discussion and conclusions
This study uncovered very low genetic divergences in partial COI sequences among specimens of the widespread Indo-Pacific coralline demosponge A. willeyana s.l. from the Red Sea to Tuamoto Archipelago in the central Pacific, spanning a geographic distance of more than 20,000 km, with invariable nucleic acid sequences among 45 specimens collected from reef caves up to 7,000 km apart (Guam–Moorea). Considering the geographic coverage of samples, this is among the lowest variation reported to date for COI in metazoan taxa (Shearer et al. 2002), and certainly the lowest variation reported for sponges yet. Although variation in mtDNA genes has been investigated in detail only for COI in two poriferan taxa to date (C. crambe, Mediterranean, Duran et al. 2003; A. willeyana, this study), results presented here add to the growing evidence that low intraspecific variation in mitochondrial gene sequences might be a more general phenomenon in Porifera and the use of this gene fragment for phylogeographic studies in sponges appears to be very limited.
However, mechanisms responsible for such low variation/low mutation rates still remain enigmatic. While bacterial-like rRNAs and tRNAs were identified in the three demosponge mitogenomes published to date (Lavrov and Lang 2005; Lavrov et al. 2005), no evidence for the presence of a homologue of a bacterial MutSLH mismatch repair system was found. Such a mismatch repair system is present in some octocorals (Pont-Kingdon et al. 1995), but its contribution to the low mtDNA variation observed in some anthozoans remains contentious (Shearer et al. 2002). Other mechanisms that could maintain genetic similarity/low variation over such a vast distance include active high (larval) dispersal and panmixia, a recent origin of the species, a population bottleneck or a slowdown/low rate of mitochondrial evolution.
Active high (larval) dispersal resulting in panmixia appears unlikely, since A. willeyana s.l. broods low numbers of lecithothrophic larvae (Wörheide 1998) and sponges are generally thought to have low dispersal capabilities (e.g. Uriz et al. 1998). A recent origin of the species or a population bottleneck also appears to be a dubious explanation because A. willeyana s.l. is widespread and common in certain habitats throughout the Indo-Pacific, with sampling in this study covering all of its W–E geographic range. Further, geographic populations of A. willeyana s.l. can be differentiated based on morphometric differences of their spicule morphology (for details see Wörheide 1998), the main taxonomic character in Porifera, indicating that sufficient genetic diversity and evolutionary depth exist to generate such morphometric differences. Further, based on rDNA ITS sequence analysis, Wörheide et al. (2002) proposed that A. willeyana s.l. might consist of at least three sibling species, although variation among sequence types was also very low (0.6% p-distance). COI data from the present study does not support distinction of the alleged A. willeyana s.s. and Wörheide et al. (2002) species (2) because sequences were invariable. The present data only supports a distinct Red Sea population due to the possession of a private COI haplotype, in addition to the private ITS sequence type found by Wörheide et al. (2002). Clearly, larger sample sizes, especially from the Indian Ocean and Central Pacific, are needed for further inferences.
The most appealing explanation for the observed very low cross-Indo-Pacific COI haplotype and nucleotide diversity appears to be a slowdown or generally low rate of COI evolution in A. willeyana s.l. Lavrov et al. (2005) postulated that a relaxed selection pressure is responsible for the lower rates of mitochondrial versus nuclear evolution in Porifera. Why selection on sponges should be relaxed despite immense competition for space in coral reefs (Aerts 2000; Diaz and Rutzler 2001) remains a relevant question. In contrast, other authors report that relaxed selection pressure increases mtDNA variation (e.g. Quesada et al. 1998).
Several other factors have been proposed to promote low mutational rates in the mitochondrial genome, among them long generation time (Bailey et al. 1991) and low metabolic rate (Martin and Palumbi 1993). A combination of both might be at play here. First, A. willeyana s.l. is a very slow growing organism (~1 mm vertical growth per year, Wörheide 1998), suggesting that its metabolic rate is indeed low. Second, A. willeyana s.l. is a K-strategist, with a long life span where individuals can reach ages of more than 500 years, small offspring number of viviparous larvae, and stable and predictable habitat preferences (Wörheide 1998). So a combination of a long generation time with low metabolic rate might provide a hypothesis on what causes the low mtDNA (and ITS) variation in Astrosclera—whether this holds true for other sponge taxa remains to be tested.
Notes
The concept of an aggregate of sibling species within Recent genus Astrosclera is followed here by adding sensu lato (= in the broad sense) to the specific name and sensu stricto (= in the strict sense) when referring to individuals from populations in the vicinity of the type locality Lifou (New Caledonia) (Lister 1900).
References
Aerts LAM (2000) Dynamics behind standoff interactions in three reef sponge species and the coral Montastraea cavernosa. Mar Ecol 21:191–204
Avise J (2000) Phylogeography: the history and formation of species. Harvard University Press, Cambridge
Avise JC (1994) Molecular markers, natural history and evolution. Chapman & Hall, New York
Bailey WJ, Fitch DH, Tagle DA, Czelusniak J, Slightom JL, Goodman M (1991) Molecular evolution of the psi eta-globin gene locus: gibbon phylogeny and the hominoid slowdown. Mol Biol Evol 8:155–184
Birky CWJ, Maruyama T, Fuerst P (1983) An approach to population and evolutionary genetic theory for genes in mitochondria and chloroplasts, and some results. Genetics 103:513–527
Boore JL (1999) Animal mitochondrial genomes. Nucleic Acids Res 27:1767–1780
Boyle JS, Lew AM (1995) An inexpensive alternative to glassmilk for DNA purification. Trends Genet 11:8
Brown WM, George MJ, Wilson AC (1979) Rapid evolution of animal mitochondrial DNA. Proc Natl Acad Sci USA 76:1967–1971
Diaz MC, Rutzler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69:535–546
Duran S, Pascual M, Turon X (2003) Low levels of genetic variation in mtDNA sequences over the western Mediterranean and Atlantic range of the sponge Crambe crambe (Poecilosclerida). Mar Biol 144:31–35
Folmer O, Black M, Hoeh W, Lutz R, Vrijenhoek R (1994) DNA primers for amplification of mitochondrial cytochrome C oxidase subunit I from diverse metazoan invertebrates. Mol Mar Biol Biotechnol 3:294–299
Lavrov DV, Forget L, Kelly M, Lang BF (2005) Mitochondrial genomes of two demosponges provide insights into an early stage of animal evolution. Mol Biol Evol 22:1231–1239
Lavrov DV, Lang BF (2005) Transfer RNA gene recruitment in mitochondrial DNA. Trends Genet 21:129–133
Lister JJ (1900) Astrosclera willeyana, the type of a new family of sponges. Zool Results 4:461–482
Martin AP, Palumbi SR (1993) Body size, metabolic rate, generation time, and the molecular clock. Proc Natl Acad Sci USA 90:4087–4091
Moritz C, Dowling TE, Brown WM (1987) Evolution of the animal mitochondrial DNA: relevance for population biology and systematics. Annu Rev Ecol Syst 18:269–292
Pont-Kingdon GA, Okada NA, Macfarlane JL, Beagley CT, Wolstenholme DR, Cavalier Smith T, Clark Walker GD (1995) A coral mitochondrial mutS gene. Nature 375:109–111
Quesada H, Warren M, Skibinski DOF (1998) Nonneutral evolution and differential mutation rate of gender-associated mitochondrial DNA lineages in the marine mussel Mytilus. Genetics 149:1511–1526
Rambaut A (1996) Se-Al: Sequence Alignment Editor. available at: http://evolve.zoo.ox.ac.uk/
Reitner J, Wörheide G, Thiel V, Gautret P (1996) Reef caves and cryptic habitats of Indo-Pacific reefs; distribution patterns of coralline sponges and microbialites. Global and regional controls on biogenic sedimentation; 1: reef evolution, research reports. Göttinger Arbeiten zur Geologie und Palaeontologie Sb2:91–100
Rozas J, Rozas R (1999) DnaSP version 3: an integrated program for molecular population genetics and molecular evolution analysis. Bioinformatics 15:174–175
Shearer T, van Oppen MJH, Romano SL, Wörheide G (2002) Slow mitochondrial DNA sequence evolution in the Anthozoa (Cnidaria). Mol Ecol 11:2475–2487
Swofford DL (1998) PAUP*. Phylogenetic analysis using parsimony (*and other methods), version 4. Sinauer Associates, Sunderland
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, positions-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Tsaousis AD, Martin DP Ladoukakis ED, Posada D, Zouros E (2005) Widespread recombination in published animal mtDNA sequences. Mol Biol Evol 22:925–933
Uriz MJ, Maldonado M, Turon X, Marti R (1998) How do reproductive output, larval behaviour, and recruitment contribute to adult spatial patterns in Mediterranean encrusting sponges? Mar Ecol Prog Ser 167:137–148
Vacelet J (1981) Éponges hypercalcifiées (Pharétronides, Sclérosponges) des cavités des récifs coralliens de Nouvelle-Calédonie. Bull Mus Nat Hist Natur (Paris), Sec A 3:313–351
Watkins RF, Beckenbach AT (1999) Partial sequence of a sponge mitochondrial genome reveals sequence similarity to Cnidaria in Cytochrome Oxidase Subunit II and the large ribosomal RNA subunit. J Mol Evol 48:542–554
Wood R (1987) Biology and revised systematics of some late Mesozoic stromatoporoids. Spec Pap Palaeontol 37:1–89
Wörheide G, Degnan BM, Hooper JNA, Reitner J (2002) Phylogeography and taxonomy of the Indo-Pacific reef cave dwelling coralline demosponge Astrosclera willeyana - new data from nuclear ITS sequences. In: Moosa KM, Soemodihardjo S, Soegiarto A, Romimohtarto K, Nontji A, Soekarno Suharsono (eds) Proceedings of the 9th International coral reef symposium, vol 1. Ministry for Environment, Indonesian Institute of Sciences, International Society for Reef Studies, Jakarta, pp 339–346
Wörheide G, Degnan BM, Hooper JNA (2000) Population phylogenetics of the common coral reef sponges Leucetta spp. and Pericharax spp. (Porifera: Calcarea) from the Great Barrier Reef and Vanuatu. Abstracts, 9th international coral reef symposium, Bali, 23 October 2000
Wörheide G, Hooper JNA, Degnan BM (2002) Phylogeography of western Pacific Leucetta ‘chagosensis’ (Porifera: Calcarea) from ribosomal DNA sequences: implications for population history and conservation of the Great Barrier Reef World Heritage Area (Australia). Mol Ecol 11:1753–1768
Wörheide G (1998) The reef cave dwelling ultraconservative coralline demosponge Astrosclera willeyana Lister 1900 from the Indo-Pacific. Micromorphology, ultrastructure, biocalcification, isotope record, taxonomy, biogeography, phylogeny. Facies 38:1–88
Yue GH, Orban L (2001) Rapid isolation of DNA from fresh and preserved fish scales for polymerase chain reaction. Mar Biotechnol 3:199–204
Acknowledgements
Funding from the BMB+F Juniorprofessor-Programme and from the Deutsche Forschungsgemeinschaft (DFG) is highly acknowledged. I wish to thank Dr. John Hooper (Brisbane), Dr. Gustav Paulay (Gainesville, FL) and Dr. Tomoki Kase (Tokyo) for their help in obtaining samples, Laura Epp, Luciana Macis and Martin Dohrmann for assistance in the lab, and Dr. Dirk Erpenbeck, Oliver Voigt (both Department of Geobiology, Göttingen) and two anonymous reviewers for valuable comments on earlier drafts of this manuscript. I would also like to thank Fernando Parra (Universidad Nacional de Colombia) for sharing his unpublished results on COI variation in the genus Agelas. I would like to thank the Egyptian Environmental Affairs Agency (EEAA) for permission to carry out field work in Egypt and the staff of the Red Sea Environmental Centre (RSEC) for their support. All experiments carried out comply with current German laws.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Wörheide, G. Low variation in partial cytochrome oxidase subunit I (COI) mitochondrial sequences in the coralline demosponge Astrosclera willeyana across the Indo-Pacific. Marine Biology 148, 907–912 (2006). https://doi.org/10.1007/s00227-005-0134-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0134-y