Abstract
The functional response of the aspects of reproductive success of a southwestern Baltic population of Acartia tonsa (Copepoda: Calanoida) was quantified in the laboratory using wide ranges in temperatures and salinities. Specifically, daily egg production (EP, # female−1 day−1) was determined for 4 or 5 days at 18 different temperatures between 5 and 34°C and the time course and success of hatching were evaluated at 10 different temperatures between 5 and 23°C. The effect of salinity (0 to 34 psu) on egg hatching success was also examined. The highest mean rates of EP were observed between 22 and 23°C (46.8–50.9 eggs female−1 day−1). When studied at 18 psu, hatching success of eggs increased with increasing temperature and was highest (92.2%) at 23°C. No hatching was observed for eggs incubated at low temperatures (≤12°C) that were produced by females acclimated to temperatures ≤10°C indicating a possible thermal threshold between 10.0 and 13.0°C below which only the production of diapause (or low quality) eggs exists in this population. When tested at 18°C, the hatching success of eggs incubated at 15 different salinities increased asymptotically with increasing salinity and was maximal (81.4–84.5%) between 17 and 25 psu. The high reproductive success observed over wide ranges in temperatures and salinities in this Baltic population demonstrates one of the mechanisms responsible for the cosmopolitan distribution of this species within productive, estuarine and marine habitats.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calanoid copepods play a key role in the cycling of nutrients and energy in marine ecosystems by forming a trophodynamic link between primary (phytoplankton) and tertiary (e.g., planktivorous fish) production (DeYoung 2004). The widespread distribution and abundance of members of this family result, in part, from adaptation of life history traits to match specific environmental (physical and chemical) conditions and/or constraints. For example, diapause eggs have been developed by some calanoid species inhabiting relatively shallow temperate habitats (e.g., Marcus 1984; Lindley 1990; Viitasalo and Katajisto 1994) to cope with intolerable annual ranges in biotic (e.g., seasonal primary production) and/or abiotic (e.g., temperature) factors within these areas.
Within the Baltic Sea, hydrographic changes in recent decades have been correlated with trophodynamic changes in terms of zooplankton and fish (e.g., Möllmann et al. 2000). Specifically, Möllmann et al. (2000) suggested that decreasing salinity might be one of the causal mechanism behind a regime shift in the dominant calanoid copepod species in the Baltic from Pseudocalanus elongatus and Pseudocalanus acuspes to Acartia spp. (mostly Acartia longiremis and Acartia bifilosa) since the former species may require higher salinities for high reproductive success than the latter ones. These changes in species abundance help demonstrate that the wide ranges in salinities and temperatures of the Baltic Sea often exceed those of the preferred niche of the calanoid species found there. Unfortunately, the functional response of reproductive success (i.e., egg production and hatching) to salinity and/or temperature in many calanoid species is not well known, having been studied in only a handful of species such as Eurytemora affinis (e.g., Gonzalez and Bradley 1994) and a number of Acartia congeners (e.g., Tester and Turner 1991; Chinnery and Williams 2004). Moreover, within estuaries and brackish enclosed waters, the considerable temporal and spatial variation in abundance and distribution of calanoid copepods has not been explained merely by variations in abiotic factors such as salinity and temperature (Bradley 1991; Wellershaus and Soltanpour-Gargari 1991) but also by the dynamics of biotic variables such as food concentration and predation pressure (Paffenhöfer and Stearns 1988).
Acartia tonsa (Dana) is easily maintained in the laboratory culture (Støttrup 2000) and hence it is one of the most intensively studied calanoid species (Mauchline 1998). Previous studies have quantified the effect of temperature and/or feeding on A. tonsa vital rates including growth and egg production (e.g., Heinle 1969; Miller et al. 1977; Klein Breteler and Gonzales 1986; White and Roman 1992; Broglio et al. 2003). However, relatively little attention has been paid to the effect of salinity on vital rates (Heinle 1981; Cervetto et al. 1999; Gaudy et al. 2000). Chinnery and Williams (2004) found a significant effect of both temperature and salinity on egg hatching success in four Acartia species including A. tonsa. However, it is clear that studies on this (and other) calanoid species often have not covered sufficiently wide ranges in temperatures and or salinities to develop complete functional responses of vital rates to these factors. For this reason, attempts to understand and model the life history dynamics of A. tonsa within the Baltic Sea (and other calanoid species in other systems, i.e., Norberg and DeAngelis 1997; DeYoung 2004) may be met with limited success.
The present study examined the effects of temperature (5–34°C) and salinity (0–34 psu) on aspects of the reproductive success of the southwestern Baltic population of A. tonsa. Specifically, the effect of temperature on egg production, hatching success and the time course of hatching and the influence of salinity on hatching success were examined. These experiments, conducted at unlimited feeding levels, were designed to generate more complete functional responses of A. tonsa reproductive success to these environmental factors.
Materials and methods
Acartia tonsa used in this study were the progeny of adults collected from two WP2 seawater samples (12 m to surface, 55 μm mesh size) taken in August 2003 (S=14 psu, T=18°C) in Kiel Bight in the southwestern Baltic Sea (54°N; 10°E). In the laboratory, zooplankton samples were acclimated to S=18 psu and T=18°C over the course of 4 days after which A. tonsa was isolated from each field sample in a ratio of 3:1 (females:males) and placed into each of the two cylindrical 8 l tanks (density ~23 ind l−1; 180 ind tank−1). Cultures were provided daily rations of a cryptophyte (Rhodomonas sp.) at concentrations (>50,000 cells ml−1) providing unlimited growth and egg production in A. tonsa (Kiørboe et al. 1985; Støttrup and Jensen 1990). Cultures were maintained on a 13L:11D light regime and received gentle aeration for mixing. Eggs were collected every 2 days by removing the aeration, letting the eggs settle and siphoning the bottom of the tank. Collected eggs were stored at 4°C and hatched later within six, 350 l “starter culture” tanks. Cohorts of A. tonsa were maintained at 30–50 ind l–1 in these tanks and fed Rhodomonas sp. at ≥50,000 cells ml−1 each day. The copepods used in experiments described in later sections were the progeny of the aforementioned starter cultures that were maintained for approximately eight generations in the laboratory at 18 psu and 18–20°C. Three different experiments were conducted in this study within a controlled-environment room having a 12L:12D light regime with a daytime water surface light intensity of 1–5 μE (μmol m−2 s−1).
Experiment 1: temperature and egg production
The effect of temperature on egg production (EP, # female−1 day−1) was quantified at 10 temperatures between 5 and 23°C (trial 1) and between 21 and 34 °C (trial 2) with two common temperatures (21 and 23°C) used in each trial. Copepods were acclimated to different temperatures prior to the trials due to the influence of temperature history on temperature tolerance in this species (González 1974). A total of ~40 ind l−1 (nauplii to adults) was loaded into each of five 8-l (trial 1) and three 250-l acclimation tanks (trial 2) containing filtered (1 μm) seawater and acclimated at a rate of ~0.6°C day−1 to one of the seven different temperatures (6, 9, 13, 17 and 22°C in trial 1; 22, 24, and 28°C in trial 2). Rhodomonas sp. was also acclimated to and grown at three different temperatures (6, 12 and 20°C).
Both EP trials were conducted using a thermal gradient table (Thomas et al. 1963), an aluminium block that was heated and cooled by pumping temperature-controlled water through holes drilled in both ends. Copepod EP was measured in three replicate 250 ml glass beakers at each temperature. Thermal stratification within beakers was avoided by conducting trials at relatively cold air temperatures (6°C in trial 1 and 18°C in trial 2). Temperatures were maintained between ±0.15°C (at low temperatures) and ±0.7°C (at two highest temperatures).
To avoid egg cannibalism, five females and one male were held within mesh-bottom sieves (8.4 cm height, 4.5 cm diameter, 130 μm mesh size) suspended in each container. Adults used in the trials had been previously acclimated between 1 and 2°C of the test temperature for at least 2 days. Since developmental rates are temperature dependent, C5 stage A. tonsa were initially loaded into containers at temperatures ≥18°C to minimize differences in the amounts of temperature–time (i.e., degree–days) individuals were within the adult stage at the different test temperatures. Every 24 h, the adults were placed into a new container by carefully transferring the sieve. The new container had filtered seawater (same temperature) containing >50,000 cells ml−1 Rhodomonas sp. The contents of the old container were collected (35 μm sieve), rinsed into a Bogorov dish, and the number of eggs counted under a Leica MZ 95 dissecting scope. Using these methods, data were collected from each of the 30 replicate containers each day for 4 days (trial 2) or 5 days (trial 1). Containers were checked daily for mortalities and any dead individuals were replaced with individuals acclimated to a similar (±1–2°C) temperature. At the end of the trials, adults were videotaped and prosome lengths measured using computer image analysis (Optimas 6.51).
Experiment 2: temperature and egg hatching
Eggs were collected from the adult cultures acclimated and maintained at either 6, 9, 13, 17 or 22°C (±0.2°C) and then loaded (n=30) into each of the three replicate 150 ml containers at each of 10 different temperatures (Table 1) within the thermal gradient table (conditions were the same as in Experiment 1, trial 1). The number of unhatched eggs was counted periodically until no further hatching was noted over a 2-day period (total time course of experiment was 168 h). The frequency of observations depended upon the temperature. During the first 48 h, containers incubating eggs at 14–23°C were checked every hour and the number of unhatched eggs was recorded. Containers between 8 and 12°C were checked every 4 h while those at 5, 7 and 8°C were examined every 12 h. Additionally, the prosome length of 20 adults within each of the five acclimation temperatures was measured. The cumulative egg hatch (H CUM) versus time (h) and the total hatch success (HST, %) of eggs were calculated.
Experiment 3: salinity and hatching success
Egg hatching success (HSS, %) was quantified at 15 different salinities from 0 to 34 psu by conducting four separate trials. In each trial, due to technical limitations, seven or eight different salinities were tested (Table 1). Egg hatching among the four trials was compared at three common salinities (6, 17 and 25 psu).
In each trial, a known number of eggs (59–65) was loaded into a 250 ml culture flask containing 200 ml of gently aerated, 1 μm filtered seawater. Three replicate flasks were used at each salinity. All flasks were incubated for 48 h within a controlled-environment room at 18°C (range ±0.5°C). After 48 h, the contents of the flasks were gently poured through a 35 μm sieve and rinsed into a Bogorov dish. Unhatched eggs were counted with the aid of a Leica MZ 95 dissecting scope. A duration of 48 h was based upon the time course of hatching in previous salinity hatching trials conducted at the same temperature (M.A. Peck and L. Holste, submitted) and results of Experiment 1.
Statistics
Data collected in this study were analysed by linear and non-linear regression analysis. Predictive regressions were used and parameter estimates were obtained by the least-squares method. The functional form of regressions was chosen based upon several statistical criteria (significance level, coefficient of determination (r 2), sum of squared errors (SSE) and residual trend analysis). A one-way ANOVA tested for differences in adult size (prosome length) among the different acclimation temperatures was used in Experiments 1 and 2. A two-way ANOVA used in Experiment 1 (EP, trial × temperature) and Experiment 3 (arcsin transformed percent hatch [arcsin×(%/100)0.5], trial × salinity). Q 10 values were calculated for data collected in this (Experiment 1) and other studies from a linear regression of lnEP versus T (lnEP = lna +bT, where Q 10 = eb×10). EP data from other studies were taken directly from the published text, tables, or figures. Data from figures were collected after digitization of the images (MATLAB 5.3, Mathworks-Inc, Natick, MA, USA; DIGIREAD shareware). All statistical tests were performed using SAS software (SAS 1989) and were considered significant at P≤0.05.
Results
Experiment 1: egg production and temperature
An increasing trend in the EP rate observed during the first 2 days (trial 2) or 3 days (trial 1) was considered to denote an acclimation period to the test chambers. Only EP data collected after this period were averaged (n=2 days) and used in subsequent analyses. Mean(±SE) EP at common temperatures (EP at 21 and 23°C, trial 1=29.7(±7.7) and 32.7(±8.7), trial 2=29.6(±9.8) and 32.9(±5.6), respectively) was not significantly different between trials and data from the two trials were combined and analysed together.
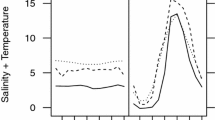
Under unlimited feeding conditions, mean EP increased with increasing temperature (T) from 0 (zero) at 5.2°C to a maximum (EPMAX) of 50.9 eggs female−1 day−1 at 22.9°C and declined at higher temperatures (Fig. 1a). Between temperatures of 5.2 and 22.9°C, mean EP was related to T based upon:
A. tonsa egg production rate (EP, panel A) and hatching success (HS, panel B) as affected by temperature (trial 1, squares; trial 2, circles). In panel A, the observed mean value for each replicate (n=2 days) is provided. In panel B, each datum represents the percentage hatch of 30 eggs. The mean(±SE) EP and HS at each temperature in each trial (n=3) is also given (triangles). Arrows indicate female acclimation temperatures. Total mortality of adults was observed at the highest test temperature (34°C). Parameter estimates for predicted O`Neill (1968) function (panel A) and linear regression (panel B) are indicated within the text
where mean(±SE) parameter estimates are provided (P<0.001). The slope estimate in Eq. 1 (0.28±0.02) corresponds to a Q10 value of 16.6(±2.8). At the warmest water temperature used in the present study (34°C), 100% mortality occurred. This temperature was considered the thermal maximum (TMAX) for this population. Observed values for EPMAX and TMAX, and the estimated Q10 value were used within a slightly modified version of an equation developed by O’Neill (1968) to estimate the functional relationship of mean EP versus T and the optimal temperature (TOPT):
where x is equal to:
and W is a function of the Q10:
TOPT and α were estimated parameters equal to mean(±SE) 24.78(±0.28) and 0.216(±0.010), respectively (P<0.01). When plotted against T, no trend in the residuals of Eq. 2 was noted.
Experiment 2: egg hatching and temperature
No hatching was observed over the course of 168 h for eggs produced by females acclimated to the two lowest temperatures (6 and 9°C) and incubated at temperatures between 5 and 10.5°C (5.4, 6.9, 8.7 and 10.4°C). However, for eggs produced at temperatures ≥13°C and incubated at 12.4, 14.0, 15.9, 18.0, 20.4 and 22.4°C, hatching was observed within 1 h of the start of observations and the total hatch success (HST, %) increased in a linear fashion with increasing T:
where mean(±SE) parameter estimates are provided (P<0.0001) (Fig. 1B). Hatching was completed within 24 h at the highest temperature tested (22.4°C), within 40 h at an intermediate temperature (18.0°C) but occurred over a time course of 120 h for eggs incubated at 12.3°C. At time = 0, the average age of eggs was approximately 6 h.
Between 12.3 and 22.4°C, the cumulative percent hatch (H CUM) versus time was best described by a non-linear function:
where H CUM was expressed in percent (%), t = time (h) and A, B, and C were estimated parameters. Two of the parameters in Eq. 6 were significantly influenced by T according to:
Thus, the effect of T on the cumulative time-course of hatching (HCUM+T) was:
Parameter estimates for A0, A1, B, C0, and C1 were 10.16(±4.04), 3.66(±0.25), 62.61(±1.49), −0.0537(±0.0247) and −0.0023(±0.0015), respectively, r2=0.87, n=385, P<0.01 (Fig. 2).
Cumulative hatching percent (%) versus time (h) for A. tonsa eggs within three replicate containers (triangles, squares, circles) at each of the six different temperatures (panels a–f). Within each panel, the regression line denotes predicted HCUM based upon Eq. 9 in the text
According to Eq. 9, the time to 50% hatch was 29.5, 19.8, 13.6, 9.2, 5.8 and 3.8 h at 12.4, 14.0, 15.9, 18.0, 20.4 and 22.4°C, respectively. Variability existed in the time course of hatching among replicates at some temperatures, particularly at the intermediate temperatures (15.9 and 18.0°C). Interestingly, the replicate with the most rapid increase in cumulative hatch (%) was usually, but not always, the replicate with the highest total hatch at each temperature.
No significant differences were found in the mean length of females acclimated to the different temperatures (P=0.2). The mean(±SE) prosome length of females used in Experiments 1 and 2 acclimated to 6, 9, 13, 17, 22 (trial 1), 22 (trial 2), 24 and 28°C was 0.82(±0.03), 0.86(±0.03), 0.85(±0.01), 0.87(±0.04), 0.86(±0.02), 0.83(±0.04), 0.83(±0.01) and 0.84(±0.01) mm, respectively.
Experiment. 3: hatching success and salinity
The percent (%) hatch of A. tonsa eggs was lowest at 0 psu (11.4%), increased asymptotically with increasing salinity and was highest (84.5%) at 25 psu (Fig. 3). A modified logistic equation best described the effect of salinity (S) on the percent hatch (HSS):
where mean(±SE) parameter estimates are provided (P<0.001). There were no significant differences in HSS among the four trials for the HSS at each of the common salinities (6, 17 and 25 psu, P=0.91).
The mean(±SE) hatching success (HSS, %) of A. tonsa eggs incubated for 48 h at 14 different salinities. For visual clarity, each datum represents the mean of three replicates. Different symbols denote different trials. The equation and parameter estimates for the regression are indicated in the text. For comparison, the predicted hatching success of a Kattegat population of A. tonsa (reared at 25–30 psu) tested over the same range in salinities is shown (dashed line) (M.A. Peck and L. Holste, submitted)
At low salinities, results of a previous study (Holste 2004) indicated that A. tonsa eggs ruptured or burst (hypo-osmotic effect) within 48 h and that burst eggs were identified as hatched eggs in trials. Based upon those results, a correction value (CR) was calculated and used to modify all observed hatch values in this study at S≤10 psu (CR=0.3192+0.0608×S). The data used to parameterise Eq. 10 were corrected values.
Discussion
Temperature and egg production
Acartia tonsa is considered a typical warm water copepod species and is often most abundant during the summer months in temperate coastal environments (Arndt and Heidecke 1973; Hirche 1974; Behrends and Schneider 1995). Therefore, finding an observed EPMAX and a predicted TOPT for EP at 22.9 and 24.8°C, respectively, was not unexpected since these temperatures would be commonly encountered during the summer months in shallow coastal estuaries. Interestingly, although EP rapidly decreased at temperatures ≥25°C, it was still relatively high at 32°C (11.2 eggs female−1 day−1), a temperature that is likely to be rarely experienced in nature by this Baltic population and is close to its upper lethal temperature (González 1974, this study). A. tonsa is one of the most cosmopolitan calanoid copepod species and its widespread distribution in low- to mid-latitude waters from the Indo-Pacific to northern Atlantic is likely due, in part, to the capacity of this species to successfully reproduce over large ranges in temperatures as indicated in the present study.
A large range in temperature-specific values of EP has been reported for A.tonsa in previous studies which is not unexpected since EP in this (and other) species results from not only the effect of temperature (Castro-Longoria 2003; this study) but from the interplay of a number of different factors including the difference between in situ and experimental temperature (Kim 1995), salinity (M.A. Peck and L. Holste, submitted; this study), female age (e.g., Parrish and Wilson 1978) and food concentration and quality (e.g., Kiørboe et al. 1985; Broglio et al. 2003). Due to differences in one or more of these factors, EP values in different studies are often difficult to compare. For example, at a common temperature of 18°C and using the same algal species and concentration, Kiørboe et al. (1985) observed an EP of 48 eggs female−1 day−1 in a Kattegat population cultured at 27 psu, a rate that is twice that (24 eggs female−1 day−1) measured in the present study using the Baltic population of A. tonsa maintained at 18 psu. In this case, differences in female ages, water salinities, as well as inter-population differences may have contributed to the different results. Our EP results between 17.6 and 20.1°C (17.7 to 32.7 eggs female−1 day−1, respectively) agree well with those observed by Broglio et al. (2003) at 17 and 20°C (25–27 eggs female−1 day−1) using slightly lower concentrations of Rhodomonas sp.
Egg production is often used as a growth proxy for adults and populations (e.g., Kiørboe et al. 1985) since it represents the difference between energy inputs and metabolic costs. Differences in reproductive modes and body sizes preclude direct comparison of EP among different copepod species. However, an interspecific comparison of Q10 values for EP provides one method of identifying species-specific patterns in how the balance between metabolic costs and energy gains changes with increasing temperature (Table 2). Depending upon the species, EP can respond weakly to increasing temperature (e.g., Q10=1.8 for A. bifilosa between 4 and 24°C, Koski and Kuosa 1999) or strongly (Q10=4.6 for Calanus finmarchicus between −2 and 8°C, Hirche et al. 1997; Q10=5.8 for Temora longicornis between 2 and 10°C, Maps et al. 2005; Q10=11.1 for A. margalefi between 5 and 20°C, Castro-Longoria 2003). Not only species- but also population-specific differences in EP Q10 values and temperature optima likely exist due to adaptations to local conditions. These differences between species and populations may contribute to the consistently low Q10 values for EP (i.e., 1.33–1.93) derived from data sets containing mixtures of copepod species (Ikeda 1985; Hirst and Bunker 2003) that have different (and perhaps contrasting) temperature optima. The strategy of analysing the mixtures of species is useful when community-level effects of temperature are desired, but should not be applied to single species. This discussion on calanoid EP and Q10 appears to be especially germane for copepod modelling efforts since (1) thermal effects on growth are often depicted using a Q10 parameter, and (2) twofold differences can exist in the Q10 parameter applied within models constructed for the same copepod species (e.g., C. finmarchicus: Carlotti and Slagstad 1997; Carlotti and Wolf 1998; Hansen et al. 2003).
Due to the positive relationship between body size and EP (Mauchline 1998), the suggestion was previously made to normalize EP data from field and laboratory studies to female length (McLaren and Leonard 1995). In the present study, the mean prosome length of females acclimated to and tested at the different temperatures was not significantly different. However, if not taken into account, body size may be a confounding variable in the field studies examining the effect of temperature on EP since, in many temperate calanoid species, adult body size is smallest during the warmest months (Viitasalo et al. 1995). Naturally, in situ EP can also be influenced by a variety of other, uncontrolled factors such as food quality that may vary seasonally, explaining, in part, the higher Q 10 estimates obtained in this study (with controlled conditions and ad libitum feeding) compared to most field-based estimates.
Temperature and egg hatching:
Temperature not only affects EP but also hatching success (HST) (Chinnery and Williams 2003; this study). The present study quantified the hatching of eggs produced by adults acclimated to five temperatures (6, 9, 13, 17, and 22°C) and incubated between 1 and 2°C of acclimation T. The most conspicuous result of Experiment 2 was the lack of egg hatching at relatively cold temperatures. Eggs produced by adults acclimated to T≤9.0°C did not hatch within 168 h when incubated at T≤10.5°C, whereas eggs produced by adults acclimated to T≥13°C initiated hatching within 1 h of the start of the experiment and finished hatching 115 h later when incubated at 12.3°C. Tester and Turner (1991) observed poor hatching when incubating A. tonsa subitaneous eggs at temperatures below 10°C. Furthermore, no hatching was found by Castro-Longoria (2003) when eggs of A. tonsa and three other Acartia congeners were incubated at 5 and 10°C. The values of HST at warmer temperatures in this study agree with those in other laboratory studies. For example, the high HST observed at 20°C in the present study (~92%) agrees well with that (85.4%) obtained by Chinnery and Williams (2004) for A. tonsa and other congeners at the same temperature. Moreover, commencement of hatching was similar between the two studies (i.e., in both studies hatching was observed within 1 h of the start of observations at all incubation temperatures). Field data for several Acartia congeners collected in the Bornholm Basin, Baltic Sea suggested that egg hatching success was low in cold months (January–April) but increased rapidly and was highest (80%) in warm months (May–August) (Dutz et al. 2004).
The results of previous studies conducted in the Baltic Sea (Arndt and Schnese 1986; Madhupratap et al. 1996) and elsewhere (e.g., Sullivan and McManus 1986; Marcus 1996) indicated that A. tonsa produces normal eggs, subitaneous eggs and resting eggs. Normal eggs hatch rapidly within the water column. Subitaneous eggs have been described as “quiescent eggs” that may forego hatching in unfavourable conditions but can hatch as soon as improved environmental conditions are experienced. Resting eggs or “diapause eggs” have an obligatory refractory phase that may span several years (Watson and Smallman 1971; Grice and Marcus 1981; Marcus et al. 1994). For members of the Acartia genus, temperature, photoperiod and oxygen concentration seem to be the major environmental cues influencing the production of eggs that are considered to be diapause eggs (e.g., Castro-Longoria and Williams 1999; Chinnery and Williams 2003; Katajisto 2004). In recent laboratory trials conducted at 17°C (M.A. Peck and L. Holste, submitted), the 48 h percent hatch of eggs produced by A. tonsa reared from nauplii to adults at 8, 12, 16 and 20 h photoperiods was 25, 55, 85 and 78% respectively, indicating a strong influence of photoperiod on the 48-h hatching success.
Depending upon the species, diapause eggs can be morphologically distinct from subitaneous eggs. Although no differences in egg morphology of hatched and unhatched eggs were noted in the present study (magnification 96×), a recent study on a congener suggested that differences between the two egg types could be difficult to recognize without scanning electron microscopy (SEM) (Castellani and Lucas 2003). The strategy of diapause egg production may be more strongly influenced by abiotic factors such as T, light and oxygen concentration when feeding levels are high. But poor food quality and quantity may override these abiotic effects.
Temperature-specific diapause egg production would explain the lack of hatching observed at cold temperatures in the present study, although other interpretations are also possible (i.e., temperature effect on egg quality). The relationships observed between temperature, EP and HST in the present experiments suggest that the temperature affected the proportion of either diapause eggs, poor quality eggs (that do not hatch and die), or both that was produced each day (i.e., 100% diapause eggs at T≤10, decreasing proportions of diapause eggs with increasing T≥13°C). The results of the present study offer no direct evidence for the presence of diapause egg production. Future hatching trials conducted after long-term storage of eggs produced at different temperatures combined with SEM should help resolve whether the results of the present study can be explained solely by differences in diapause egg production.
Salinity effect on egg hatching
Few studies have examined the effect of salinity on hatching success of calanoid copepod eggs, which is surprising given the abundance of many members of this family within estuarine and brackish waters. The present study using a Baltic population demonstrated that hatching success (HSS) increased with increasing salinity and was maximal at 25 psu. The non-linear relationship suggested that HSS markedly declined with decreasing salinity after a threshold of ~17 psu. For a North Sea population of A. tonsa, salinity had an even stronger impact on hatching (Chinnery and Williams 2004). In that study, only 55% of eggs hatched at a salinity of 15 psu, whereas in this study hatching success of eggs from the Baltic population at 14 psu was 1.5 times greater (78%). Recent egg hatching trials performed on a Kattegat (27 psu) population using similar methods and salinity ranges as the present study indicated nearly the same non-linear response of HSS to salinity except that the Kattegat population had a higher HSS at salinities ≥15 psu (M.A. Peck and L. Holste, submitted). Not only hatching success but also EP can be affected by salinity and a recent study using the same Baltic population as the present study indicated significantly higher EP at 14 psu compared to 30 psu (M.A. Peck and L. Holste, submitted). These studies and field observations indicating population persistence during warm periods at very low salinities (i.e., 4 psu, northeastern Baltic) suggest a high degree of phenotypic plasticity in the response to salinity among populations of A. tonsa in the North and Baltic Seas.
The reproductive characteristics of A. tonsa examined in the present study showed clear functional responses to temperature and salinity when these abiotic factors were studied separately. However, the interaction between temperature and salinity (T×S) was not examined, a limitation of the present research. The T×S interaction can be important especially with regard to physiological tolerances affecting vital rates. For example, pelagic invertebrates often have higher tolerances to lower salinities at higher temperatures (Kinne 1970), a finding inferred for A. tonsa from seasonal field distributions (Jeffries 1962). Moreover, the effect of the T×S interaction on vital rates can be species-specific in copepods. For example, when the effect of temperature on rates of energy loss (respiration, R, and excretion, E) was compared at different salinities, Gaudy et al. (2000) observed no significant differences for A. clausi, whereas the Q 10 (10–20°C) at 15 psu for both R and E in A. tonsa was significantly lower (1.5 and 1.21) compared to 35 psu (4.79 and 2.2). Interestingly, these results (direct measurements of energy loss) agree well with the finding at 18°C of higher EP (proxy for surplus energy) in A. tonsa at an intermediate salinity (14 psu) compared to a higher salinity (30 psu) more characteristic of coastal marine habitats (M.A. Peck and L. Holste, submitted).
Conclusions
Within the Baltic Sea, seasonal temperature differences spanning 15–20°C are often observed in waters having surface salinities of ~4 psu (northeast) to 22 psu (southwest). Populations of A. tonsa normally exist within shallow, coastal areas of the Baltic Sea, areas likely to experience larger seasonal (and daily) ranges in temperatures compared to the deeper basins. In this regard, laboratory experiments were conducted using the southwestern Baltic population to evaluate the functional response of factors associated with reproductive success (egg production and hatching) to wide ranges in temperatures (5–34°C) and salinities (0–34 psu). The results of this and other studies suggest several reasons for the numerical abundance and cosmopolitan distribution of this species in productive near-shore estuarine and marine environments including: (1) an increase in egg production rate with increasing temperature that was far stronger than that estimated from studies of other calanoid copepod species, (2) a considerable phenotypic plasticity in the effect of salinity on egg hatching success, and (3) the development of a diapause life strategy that may be triggered in response to either (or both) abiotic (decreasing temperatures and photoperiods) and biotic (feeding resources) factors.
References
Arndt AE, Heidecke D (1973) Investigations on zooplankton in the coastal areas of the Bay of Mecklenburg. Wiss Z Univ Rostock, Math Naturwiss Reihe 22:599–616
Arndt AE, Schnese W (1986) Population dynamics and production of Acartia tonsa (Copepoda: Calanoida) in Darss-Zingst estuary, southern Baltic. Ophelia supplement 4:329–334
Ban S (1994) Effect of temperature and food concentration on post-embryonic development, egg production and adult body size of calanoid copepod Eurytemora affinis. J Plank Res 16:721–735
Behrends G, Schneider G (1995) Impact of Aurelia aurita medusae (Cnidaria, Scyphozoa) on the standing stock and community composition of mesozooplankton in the Kiel Bight (western Baltic Sea). Mar Ecol Prog Ser 127:39–45
Bradley BP (1991) Seasonal succession in Chesapeake Bay. Bull Plank Soc Jap Spec Vol 129–131
Broglio E, Jonasdottir SH, Calbet A, Jakobsen HH, Saiz E (2003) Effect of heterotrophic versus autotrophic food on feeding and reproduction of the calanoid copepod Acartia tonsa: relationship with prey fatty acid composition. Aquat Microb Ecol 31:267–278
Carlotti F, Slagstad D (1997) Population dynamics model of interacting copepod species coupled with a 1-D model of phytoplankton dynamics in the Greenland Sea Gyre. Environ Model Assess 2:29–36
Carlotti F, Wolf K-U (1998) A Lagrangian ensemble model of Calanus finmarchicus coupled with a 1-D ecosystem model. Fish Oceanogr 7:191–204
Castellani C, Lucas IAN (2003) Seasonal variation in egg morphology and hatching success in the calanoid copepods Temora longicornis, Acartia clausi and Centropages hamatus. J Plank Res 25:527–537
Castro-Longoria E (2003) Egg production and hatching success of four Acartia species under different temperature and salinity regimes. J Crust Biol 23:289–299
Castro-Longoria E, Williams JA (1999) The production of subitaneous and diapause eggs: a reproductive strategy for Acartia bifilosa (Copepods: Calanoida) in Southampton water, UK. J Plank Res 21:65–84
Cervetto G, Gaudy R, Pagano M (1999) Influence of salinity on the distribution of Acartia tonsa (Copepoda, Calanoida). J Exp Mar Biol Ecol 239:33–45
Chinnery FE, Williams JA (2003) Photoperiod and temperature regulation of diapause egg production in Acartia bifilosa from Southampton water. Mar Ecol Prog Ser 263:149–157
Chinnery FE, Williams JA (2004) The influence of temperature and salinity on Acartia (Copepoda: Calanoida) nauplii survival. Mar Biol 145:733–738
DeYoung B, Heath M, Werner F, Chai F, Megrey B, Monfray P (2004) Challenges of modeling ocean basin ecosystems. Science 304:1463–1466
Dutz J, Mohrholz V, Peters J, Renz J, Alheit J (2004) A strong impact of winter temperature on spring recruitment of a key copepod species in the Bornholm Basin: potential linkages to climate variability. International GLOBEC Newsletter 10.1:9–10
Gaudy R, Cervetto G, Pagano M (2000) Comparison of the metabolism of Acartia clausi and A. tonsa: influence of temperature and salinity. J Exp Mar Biol Ecol 247:51–65
González JG (1974) Critical thermal maxima and upper lethal temperatures for the calanoid copepods Acartia tonsa and A. clausi. Mar Biol 27:219–223
Gonzalez CRM, Bradley BP (1994) Salinity stress proteins in Eurytemora affinis. Hydrobiol 292/293:461–469
Grice GD, Marcus NH (1981) Dormant eggs of marine copepods. Oceanogr Mar Biol Ann Rev 19:125–140
Halsband-Lenk C, Hirche H-J, Carlotti F (2002) Temperature impact on reproduction and development of congener copepod populations. J Exp Mar Biol Ecol 271:121–153
Hansen BW, Marker T, Andreassen P, Arashkewich E, Carlotti F, Lindeque P, Tande KS, Wagner M (2003) Differences in Life cycle traits of Calanus finmarchicus originating from 60°N and 69°N, when reared in mesocosms at 69°N. Mar Biol 142:877–893
Heinle DR (1969) Temperature and zooplankton. Chesapeake Sci 10:186–209
Heinle DR (1981) Zooplankton In: Vernberg FJ, Vernberg WB (eds) Functional adaptations of marine organisms. Academic Press, New York, pp 85–145
Hirche H-J (1974) The copepods Eurytemora affinis Poppe and Acartia tonsa Dana and their infestation by the stalked ciliate Myoschiston centropagidarum Precht (Peritricha) in the Schlei. Kiel Meeresforsch 30:43–64
Hirche H-J, Meyer U, Niehoff B (1997) Egg production of Calanus finmarchicus: effects of temperature, food and season. Mar Biol 127:609–620
Hirst AG, Bunker AJ (2003) Growth of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnol Oceanogr 48:1988–2010
Holste L (2004) The influence of temperature, salinity and feeding history on population characteristics of Baltic Acartia tonsa: egg production, hatching success and cohort development. Masters Thesis, Institute for Hydrobiology and Fisheries Research, University of Hamburg, Hamburg, Germany, p 79
Ikeda T (1985) Metabolic rates of epipelagic marine zooplankton as a function of body mass and temperature. Mar Biol 85:1–11
Jeffries HP (1962) Succession of two Acartia species in estuaries. Limnol Oceanogr 7:354–364
Jónasdóttir SH (1994) Effects of food quality on the reproductive success of Acartia tonsa and Acartia hudsonica: laboratory observations. Mar Biol 121:97–81
Kim WS (1995) The effect of temperature on the egg production rates of Acartia tonsa (calanoid copepod) in Long Island Sound. Ocean Res 17:1–7
Kinne O (1970) Temperature: animals: invertebrates. In: Kinne O (ed) Marine Ecology, vol 1. Environmental factors. Part 1. Wiley-Interscience, London, pp 407–514
Kiørboe T, Møhlenberg F, Hamburger K (1985) Bioenergetics of the planktonic copepod Acartia tonsa: relation between feeding, egg production and respiration, and composition of specific dynamic action. Mar Ecol Prog Ser 26:85–97
Klein Breteler WCM, Gonzales SR (1986) Influence of temperature and food concentration on body size, weight and lipid content of two calanoid copepod species. Hydrobiol 167/168:201–210
Koski M, Kuosa H (1999) The effect of temperature, food concentration and female size on the egg production of the planktonic copepod Acartia bifilosa. J Plank Res 21:1779–1789
Lee H-W, Ban S, Ikeda T, Matsuishi T (2003) Effect of temperature on development, growth and reproduction in the marine copepod Pseudocalanus newmani at a satiating food concentration. J Plank Res 25:261–271
Lindley JA (1990) Distribution of overwintering calanoid copepod eggs in seabed sediments around southern Britain. Mar Biol 104:209–217
Madhupratap M, Nehring S, Lenz J (1996) Resting eggs of marine zooplankton (Copepoda and Cladocera) from Kiel Bay and adjacent waters (southwestern Baltic). Mar Biol 125:77–87
Maps F, Runge JA, Zarardjian B, Joly B (2005) Egg production and hatching success of Temora longicornis (Copepoda, Calanoida) in the southern Gulf of St. Lawrence. Mar Ecol Prog Ser 285:117–128
Marcus NH (1984) Recruitment of copepod nauplii into the plankton: importance of diapause eggs and benthic processes. Mar Ecol Prog Ser 15:47–54
Marcus NH (1996) Ecological and evolutionary significance of resting eggs in marine copepods: past, present, and future. Hydrobiol 32:141–152
Marcus NH, Lutz R, Burnett W, Cable P (1994) Age, viability and vertical distribution of zooplankton resting eggs from an anoxic basin: evidence of an egg bank. Limnol Oceanogr 39:154–158
Mauchline J (1998) The biology of calanoid copepods. Elsevier, Oxford, p 710
Mclaren IA, Leonard A (1995) Assessing the equivalence of growth and egg production of copepods. ICES J Mar Sci 52:397–408
Miller CB, Johnson JK, Heinle DR (1977) Growth rules in the marine copepod genus Acartia. Limnol Oceanogr 22:326–335
Möllmann C, Kornilovs G, Sidrevics L (2000) Long-term dynamics of main mesozooplankton species in the central Baltic Sea. J Plank Res 22:2015–2038
Norberg J, DeAngelis D (1997) Temperature effects on stocks and stability of a phytoplankton–zooplankton model and the dependence on light and nutrients. Ecol Model 95:75–86
O’Neill RV (1968) Population energetics of a millipede, Narceus americanus. Ecology 49:803–809
Paffenhöfer GA, Stearns DE (1988) Why is Acartia tonsa (Copepoda: Calanoida) restricted to nearshore environments? Mar Ecol Prog Ser 42:33–38
Parrish KK, Wilson DF (1978) Fecundity studies on Acartia tonsa (Copepoda: Calanoida) in standardized culture. Mar Biol 46:65–81
Runge JA (1984) Egg production of the marine, planktonic copepod, Calanus pacificus Brodsky: laboratory observations. J Exp Mar Biol Ecol 74:53–66
SAS Institute Inc. (1989) SAS/STAT® User’s Guide Version 6, Fourth Edition Vol. 2. SAS Institute Inc, Cary, NC, p 846
Sekiguchi H, McLaren IA, Corkett CJ (1980) Relationship between growth rate and egg production in the copepod Acartia clausi hudsonica. Mar Biol 58:133–138
Støttrup JG (2000) The elusive copepods: their production and suitability in marine aquaculture. Aquacult Res 31:703–711
Støttrup JG, Jensen J (1990) Influence of algal diet on feeding and egg production of the calanoid copepod Acartia tonsa Dana. J Exp Mar Biol Ecol 141:87–105
Sullivan BK, McManus LT (1986) Factors controlling seasonal succession of the copepods Acartia hudsonica and A. tonsa in Narragansett Bay, Rhode Island: temperature and resting egg production. Mar Ecol Prog Ser 28:121–128
Tester PA (1985) Effects of parental acclimation temperature and egg incubation temperature on egg-hatching time in Acartia tonsa (Copepoda: Calanoida). Mar Biol 89:45–53
Tester PA, Turner JT (1991) Why is Acartia tonsa restricted to estuarine habitats? In: Proc 4th Internat Copepod Conference, Bull Plank Soc Jap Spec Vol 603–611
Thomas WH, Scotten HL, Bradshaw JS (1963) Thermal gradient incubators for small aquatic organisms. Limnol Oceanogr 8:357–360
Watson NHF, Smallman BW (1971) The role of photoperiod and temperature in the induction and termination of an arrested development in two species of freshwater cyclopoid copepods. Can J Zool 49:855–862
Wellershaus S, Soltanpour-Gargari A (1991) Planktonic copepods in the very low salinity region in estuaries. Bull Plank Soc Jap Spec Vol 133–142
White JR, Roman MR (1992) Egg production by the calanoid copepod Acartia tonsa in the mesohaline Chesapeake Bay: the importance of food resource and temperature. Mar Ecol Prog Ser 86:239–249
Viitasalo M, Katajisto M (1994) Mesozooplankton resting eggs in the Baltic Sea: identification and vertical distribution in laminated and mixed sediments. Mar Biol 120:455–465
Viitasalo M, Koski M, Pellikka K, Johansson S (1995) Seasonal and long-term variations in the body size of planktonic copepods in the northern Baltic Sea. Mar Biol 123:241–250
Acknowledgements
We are grateful for the help of Philipp Kanstinger, Bianca Ewest, Meike Martin and Gudrun Bening with laboratory rearing and data collection. We would also like to thank Dr. Mike A. St.John and Dr. Axel Temming and two anonymous reviewers for helpful comments and suggestions on earlier drafts of this manuscript. This research was funded by the Global Ocean Ecosystem Dynamics (GLOBEC, Germany) program by the German Federal Ministry for Education and Research (BMBF 03F0320E) and the German Science Foundation (DFG) AQUASHIFT program cluster Resolving Trophodynamic Consequences of Climate Change (“RECONN”, DFG # JO556/1-1).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Holste, L., Peck, M.A. The effects of temperature and salinity on egg production and hatching success of Baltic Acartia tonsa (Copepoda: Calanoida): a laboratory investigation. Marine Biology 148, 1061–1070 (2006). https://doi.org/10.1007/s00227-005-0132-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0132-0