Abstract
Fatty acid analyses are emerging as a powerful technique to probe trophic interactions between organisms. In this paper, the application of both this procedure and gonad index (GI) determination on two populations (intertidal and subtidal) of the echinoid Psammechinus miliaris is reported. The investigation spanned the 3-month spawning period of Scottish west coast populations. In both populations a progressive decrease in the GI was found, coupled with an increasing maturity stage (from mature to spent). Sexual maturation and decrease in GI was synchronous between the two populations. In conjunction, there were distinct changes in gonad biochemistry. Differences in the fatty acid composition of the gonad reflected the changes in sexual maturation. Mature males and females had significant differences in the fatty acid composition of their gonads, whereas post-spawned individuals showed no gender differences. Male urchins had higher levels of polyunsaturated fatty acids (PUFAs) compared to females, and there was a dramatic reduction in the fatty acids 22:6(n−3) and 20:5(n−3) with increasing maturity stage. Using multivariate statistical techniques, these changes in the fatty acid composition of the sea urchin gonad were linked to habitat related diet differences combined with gender differences. These changes in the fatty acid signatures clearly reflect the dual function of the gonad as both a nutrient store and a reproductive organ.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Understanding the trophic relationships that exist within and between species, and the way that these interactions vary along temporal and spatial scales has long been a principle component of marine ecological research (Elton 1927; Linderman 1942; Fretwell 1987; Menge 2000). Techniques have increased in variety and resolution to explore and quantify such interactions. At their simplest these include passive observations such as direct study of feeding behaviour (Zamon 2001) or examination of fecal pellets (Bonesi et al. 2004). Potentially powerful insights can be gained by invasive methods such as stomach content analysis (de la Moriniere et al. 2003) and biochemical sampling such as stable isotope analysis (Fry and Sherr 1984), carotenoid pigment analysis (Hudson et al. 2003) and fatty acid analysis (Pond et al. 1997).
Fatty acid analysis, the technique employed in the current study, has been used to study trophic interactions in a particularly wide range of habitats from terrestrial (McWilliams et al. 2004) to freshwater and marine (Go et al. 2002). It has been successfully applied to discriminate the trophic niches of co-occurring species (Howell et al. 2003) and diet changes within a species across a range of temporal and spatial scales (Cripps et al. 1999; Iverson et al. 1997; Phillips et al. 2003). It is also a useful method for examining the biochemical changes in marine invertebrates linked to their reproductive cycle. The link between the fatty acid signature of the gonad and the reproductive cycle has been demonstrated for the bivalves Pecten maximus (Pazos et al. 1997), Crassostrea gigas (Soudant et al. 1999), Ruditapes decussates (Ojea et al. 2004), the cephlapods Octopus vulgaris and O. defilippi (Rosa et al. 2004a), Eledone cirrhosa and E. moschata (Rosa et al. 2004b), and the crustaceans Nephrops norvegicus (Rosa and Nunes 2002) and Aristeus antennatus (Rosa and Nunes 2003). These studies have shown that there are major changes in the fatty acid signatures of the gonads during sexual maturation, and that two fatty acids, 20:5(n−3) and 22:6(n−3), are particularly important in this maturation cycle.
Our study focuses on a less frequent target taxon, the echinoderms, and specifically echinoids. Gonadal index (GI) studies have revealed that gonad mass relative to whole organism mass varies considerably along both spatial and temporal scales (McClanahan and Kurtis 1991; Kelly 2000). Echinoid gonads have a multifunctional role and are known to reflect both nutritive and reproductive status (Russell 1998). Psammechinus miliaris, a small and common coastal species, is known to be an opportunistic omnivore, which can occur at very high densities—upto 350 individuals per square meter (Kelly 2000) in Scottish sea lochs. It occurs from the low littoral to ~100 m depth (Kelly and Cook 2001) and in our study region, West Scotland, P. miliaris typically occurs in the littoral and sub-littoral of sheltered sea lochs. Here we investigate changes in the gonadal indices and biochemistry across the spawning period. The study was designed to address two specific hypotheses: that changes in the GI over the spawning period were consistent between the intertidal and subtidal populations; and that the fatty acid signature of the gonad would reflect changes in gonad maturation.
Materials and methods
Study site
The study was conducted at two locations on the south shore of Loch Creran, west Scotland (Fig. 1). Loch Creran is a silled sea loch 12.8 km long, with four basins that reach a maximum depth of 49 m. It has been characterized as a well-mixed system with a flushing time of 3 days (Black et al. 2000). Two study locations separated by approximately 5 km, Rubha Garbh (RG) and South Shian (SS), were selected. The substratum nature, rugosity and profile were similar at both locations, being boulders, cobbles and pebbles on mixed sand and mud. The most obvious macroscopic biota in the intertidal zone was fucoid algae (Fucus vesiculosus and F. serratus) and in the shallow subtidal, Laminaria saccharina.
Field protocol
Five P. miliaris individuals of approximately equal test diameter were collected from each of two replicate sites, at each of two depths (intertidal and subtidal), and at each of two locations (Fig. 2). Those from the intertidal zone were collected from an area just above extreme low water springs (ELWS). By observation this level was determined to be exposed for approximately 2.5 h on three consecutive days on spring tides. P. miliaris individuals from the subtidal zone were collected (by snorkelling) from permanently submerged areas at depths of 2–3 m. The specimens from each replicate were collected from within a 1 m radius. The replicate sites were selected at each location to be approximately 10 m apart. The P. miliaris individuals were transferred to the laboratory and maintained in running sea water and without food until analysis (within 48 h). This was repeated each month, from July to September.
Laboratory analysis
Test diameter and wet mass for each individual P. miliaris was determined prior to the removal of the gonad. Test diameter was measured three times using adapted Vernier calipers, and the mean of the three measurements was used. The excised gonad was weighed, and a small sample was taken and used for microscopic determination of gender and maturity stage. The maturity stage was determined using a wet squash where a small section of fresh tissue was placed between a slide and cover slip and examined under a microscope. The gonad was scored on a six point scale: stage 1 recovery, stage 2 early growth, stage 3 premature, stage 4 mature, stage 5 partially spawned and stage 6 spent (Kelly 2001). The GIs were calculated for each animal using the formula:
After dissection of the gonad, it was placed in chloroform:methanol (vol. 2:1), and stored under nitrogen until ready for lipid extraction (<1 h).
Lipid extraction and fatty acid analysis
The gonad was homogenized in chloroform:methanol (2:1 10 cm3) and stored under nitrogen overnight at 4°C. Prior to storage, the fatty acid standard 23:0 (400 μl, 0.5 mg/g tissue) was added to each sample as an internal standard. The lipid was then extracted and transesterified to fatty acid methyl esters (FAMEs). The FAMEs were purified using thin layer chromatography (250 μm silica gel, Whitman®) and then stored in hexane under nitrogen at -16°C for no longer than a week prior to gas chromatography.
The purified FAMEs were separated using a Perkin Elmer 8320 gas chromatograph equipped with split injector (100:1), flame ionization detector (FID) and a Zebron ZB-WAX fused silica capillary column (30 m × 0.25 mm i.d., 0.25 μm film thickness). Helium was the carrier gas and the oven temperature was programmed to increase from 160–240°C at 4°C min−1, and then held for 10 min. In order to store and integrate the chromatograms, the detector output was coupled to a data system (Varian Star™). The FAMEs were identified by comparing their retention times with those of authentic standards with the exception of 16:0 dimethylacetal and 20:2 non-methylene interrupted dienes. These were identified using retention times from a previous study (Cook et al. 2000). All samples were run on the same column and under the same conditions. Individual fatty acids were identified and the relative content of each was determined using peak areas and expressed as the percentage by mass of the total fatty acids characterized. These values were then converted to relative mass per gram of wet mass of gonad tissue (mg g−1) using the response of the internal standard.
Statistical analysis
Prior to investigation of the GI variability, the relationships between urchin test diameter and whole wet-mass, gonad wet-mass and GI were examined using Pearson’s product–moment correlation. Prior to all parametric univariate analysis, data normality was tested using the Kolmogorov–Smirnov test; any data found to significantly differ was log transformed to meet assumptions of normality. Data was also checked for homogeneity of variance using Cochran’s test. Pearson product moment correlation values (rho statistic) and associated probabilities demonstrated a significant relationship between P. miliaris test diameter and (a) whole wet mass (ρ=0.834, p<0.001) and (b) gonad wet mass (ρ=0.416, p<0.001) as expected. There was no significant correlation between test diameter and GI (ρ=0.132, p=0.15) thus establishing GI as a size-independent metric.
Differences in test diameter, and GI were tested using a four factor ANOVA with depth (two levels and fixed) being orthogonal to month (three levels and random) and location (two levels and random), and sites nested within location (two levels).
All multivariate analysis was carried out using the PRIMER v5 (Plymouth Routines In Multivariate Ecological Research) with the exception of the two-way SIMPER which used v6ß. Though this routine has no known issues connected with it, as it is a beta version of the software the results should be interrupted with a degree of caution (R. Gorley, personal communication) The data was left untransformed (Howell et al. 2003) and converted into similarity matrices using Euclidean distances as the metric. A single outlier from the third month which had very low quantities of all fatty acids, was removed from the analysis. If the procedure required a balanced data set, a dummy variable was created as the average of the other four fatty acid signatures from that replicate.
Permutation based analysis of similarity (ANOSIM) routines were used as the hypothesis testing framework. Differences in the fatty acid signatures with depth, gender, and maturity stage were explored using the similarity percentages routine (SIMPER), both one-way and crossed.
By matching the fatty acid signatures to the environmental/biological variables for each sample, it is possible to find which combination of the variables are best correlated (Spearman rank) with the fatty acid signatures (BIO-ENV). The five variables used were depth, site, month, gender, and maturity stage.
Results
From July to September, the gonadal indices of P. miliaris typically decreased across locations, and depths (Fig. 3). The largest magnitude of decline occurred in the intertidal at the SS site (which had the highest initial value), from a mean GI of 8.1% in July to 2.0% by September. The mean level of decrease between July and September across locations was 71% for both intertidal and subtidal. The maturity stage clearly progressed in both genders over the study months (Fig. 4). In July, the majority of male P. miliaris were at maturity stage 4 as opposed to 3 for females. This trend for males to be developmentally ahead continued for the remainder of the study, with a modal stage of 5 for males, and 4 for females during August, and 6 and 5, respectively, during September.
There was a significant difference between the GI for each maturity stage (df=3,116; f=22.81, p<0.001). Pair-wise comparison showed that stages 3 and 4 were similar and significantly different from stages 5 and 6. The highest mean GI was recorded for stage 4 with a mean of 5.95% and the lowest was stage 6 with a mean GI of 2.15% (Fig. 5).
Fatty acid analysis
The predominant fatty acid for all months and depths (Table 1) was 20:5(n−3) being highest in the subtidal in July (17.88%), followed by 20:4(n−6) and 16:0. Polyunsaturated fatty acids [PUFAs: 18:2(n−6), 18:2(n−3), 18:3(n−6), 18:3(n−3), 18:4(n−3), 20:2(n−6), 20:4(n−6), 20:3(n−3), 20:4(n−3), 20:5(n−3), 22:2(n−6), 22:5(n−3), 22:6(n−3)] formed the largest class ranging between 35.9 to 45.5%. The saturated fatty acids (SFAs: 14:0, 15:0, 16:0, 17:0, 18:0, 20:0, 21:0) were the second largest class ranging between 17.1 and 19.9%. Mono-unsaturated fatty acids [MUFAs: 16:1(n−7), 18:1(n−9), 18:1(n−7), 20:1(n−9), 20:1(n−7), 22:1(n−9)] were the smallest class. The total FAMEs (mg/g tissue) varied little between habitats but decreased between July and August, and remained static after that. The relationship between the fatty acid signatures of each month shows a clear segregation in the signature, confirmed by the ANOSIM results (Table 2). Post hoc pair-wise testing showed differences between all pairs of months.
In order to examine the role that the recorded change in maturity stage had on the fatty acid signature, a crossed ANOSIM was used to test for significance differences in the fatty acid signature of each gender at each maturity stage, averaged over the months (Table 3, 4). The test confirmed that there was a significant difference between the months when averaged across gender and maturity stages (r=0.157, p=0.014), and a significant difference between gender/maturity stage when averaged across months (r=0.269, p<0.001). Post hoc pair-wise testing was used to examine the relation between the genders at each maturity stage, when averaged over the months. Only at maturity stage 4, was there a significant difference in the fatty acid signatures between the genders. A two-way SIMPER was used to see which specific fatty acids were contributing to this difference. It was shown that 20:5(n−3), 20:4(n−6), and 22:6(n−3) were all found in higher proportions in the males, and contributed a total of 59% of the difference between them.
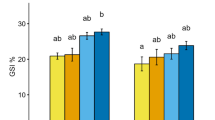
The different fatty acid classes varied as much as 5% in their mean levels for each maturity stage and gender (Fig. 6). The total of the PUFA decreased over the maturity stages for both sexes, whilst the SFA showed only a reduction for the males. The levels of 20:5(n−3) reduced with increasing maturity stage in both male and female (Fig. 7). A rapid reduction in this fatty acid occurred in females between stages 4 and 5, and in males between stages 5 and 6. In the fatty acid 20:4(n−6) there was a dramatic reduction between stage 3 and 4 in females followed by a slight increase, but only slight changes in males. An inverse pattern to that of 20:4(n−6) is seen for 22:6(n−3), with an increase for females between stages 3 and 4 followed by a reduction between stages 4 and 6. For the males, there was an increase from stages 4 to 5, and a reduction from stages 5 to 6.
Of the five variables measured in P. miliaris individuals (depth, gender, maturity stage, month and location), depth and gender had the highest correlation (ρ=0.305) with the fatty acid signatures.
Discussion
The findings of the current study supported the hypothesis that changes in the GI of the study species, P. miliaris, over the spawning period were consistent between intertidal and subtidal populations. Although clear and significant differences were found between individuals at these two depths, both populations showed similar reductions over the study months. Comparable differences between the nutritional state of urchins from different habitats have been recorded for other echinoid species elsewhere. For example, Anthocidaris crassispina from sargassum beds had a higher GI when compared to those from areas dominated by coralline algae (Yatsuya and Nakahara 2004), and Centrostephanus rodgersii from barren areas had a lower GI compared to those from algal dominated areas (Byrne et al. 1998). Both studies found that gonad development was synchronous between the habitats, as described in the current study. The reduction in GI was coupled with a progressive increase in maturity stage of P. miliaris individuals from the study populations. A similar pattern was suggested for this species by Kelly (2000), who noted a dramatic reduction in GI during the month of August, when all of the females had reached maturity stage 5. Although GI has been questioned as a reliable predictor of maturity stage (Lozano et al. 1995), the study population showed a significant difference in GI between pre-spawned (stages 3 and 4), and post-spawned (stages 5 and 6). This difference is very likely to be due to the loss of reproductive material as a result of spawning.
Fatty acid analysis
Studies to date have suggested that echinoids have similar fatty acid compositions, from which this study found P. miliaris differs little. Using all soft body parts of Paracentrotus lividus, Serrazanetti et al. (1995) found the dominant fatty acids to be 14:0, 16:0 and 20:5(n−3). Similarly Floreto et al. (1996), using all the soft tissue of Tripneustes gratilla, found PUFA to be the dominant class, and 16:0, 20:4(n−6) 20:5(n−3) to be the primary fatty acids. The testis of Echinus esculentus collected from southern England again showed a similar pattern but showed much higher levels of 22:6(n−3) than those of P. miliaris in our study (Allen 1968). The study by Cook et al. (2000) of the fatty acid composition of west coast Scottish P. miliaris found similar overall patterns with those observed in this study except that their wild populations had lower values of total PUFA. This is probably due to habitat differences between the sample populations of the two studies.
Temporal variation
Crucially, all temporal variation observed in the fatty acid signature of wild populations of urchins can be confounded by the dual role of the urchin gonad. Because the organ is both the reproductive and nutritive store, any observed differences in the fatty acid signature over time may be a result of both changes in diet and changes in the reproductive maturity of the animal. The current study showed clear differences over the experiment duration in the fatty acid signature and this was consistent over depth and location. An unknown proportion of the difference can be attributed to diet variation, as when averaged over the maturity stage there were still significant differences between the months.
Differences in fatty acid profile between testes and ovaries have been previously discussed for this population of urchins (Hughes et al. 2005). However, it appears that these gender specific differences are transient and may be the result of the annual gametogenic cycle. Significant differences existed between genders prior to spawning but not for the post spawning stages. Thus the difference in the fatty acid signature between male and female gonads at stage 4 would seem likely to be the result of the presence of high levels of gametes, and that these gametes have different fatty acid compositions. There were distinct changes in the fatty acid composition of gonads with increased maturity stage. PUFAs have been associated with reproductive capacity in shrimps (Rosa and Nunes 2003) and more specifically with structural elements in the phospholipid membranes that have been shown to be prominent components in spermatozoa of marine invertebrates (Cook and Gabbott 1972). PUFAs were found at higher levels in the gonad of male limpets compared to the females (Morais et al. 2003) as also reported in the current study. We suggest that this was as a result of a reduction in male gametes in the gonad during spawning. In contrast MUFAs have been linked to energy reserves necessary for vitalogenesis, and significantly higher concentrations have been reported for female gonads (Brazao et al. 2003). The current study found no clear pattern in P. miliaris—similar levels occurred in both males and females and there was no noticeable reduction with maturity stage. It is possible that the multifunctional role of the sea urchin gonad may lead to a more complicated interruption of the variation in the fatty acid signature of the gonad. The fatty acids 20:5(n−3) and 22:6(n−3) have been linked to energy storage in teleost eggs (Fraser et al. 1988), and as an energy source during embryogenesis (Xu et al. 1994). It has also been hypothesised that 22:6(n−3) plays an important role in the membrane of eggs (Pazos et al. 1997). There was a rapid reduction in these fatty acids from stage 4 to 5 in the female gonads of P. miliaris. This reduction may be as a result of the release of oocytes rich in 20:5(n−3) and 22:6(n−3).
Further studies are required to differentiate between the role of diet and sexual maturation in influencing the biochemical composition of the echinoid gonad. Ojea et al (2004) concluded that changes in the fatty acid composition of the clam Ruditapes deccusatus appeared to be more closely related to diet than to endogenous factors. The current study showed that the variables of depth and gender were best correlated with that of the fatty acid signature. Variation in the fatty acid signature as related to depth has been linked to differences in diet between the intertidal and subtidal populations with intertidal populations having a diet richer in invertebrates and ephemeral green algae (Hughes et al. 2005). As such, depth differences in fatty acid signatures can be attributed to nutritional sources, while gender differences are likely to be due to the presence of gametes. This suggests that the duality of function of the gonad is strongly reflected in its fatty acid signature; habitat related diet differences combined with gender differences give the best correlation with the fatty acid signature.
References
Allen WV (1968) Fatty-acid synthesis in the echinoderms:Asterias rubens and Echinus esculentus and Holothuria forskali. J Mar Biol Assoc of UK 48:521–533
Black KD, Hughes DJ, Provost PG, Pereira PMF (2000) Broad scale survey and mapping of seabed biota in Loch Creran, Argyll. Report no. F98AA408., Scottish Natural Heritage
Bonesi L, Chanin P, Macdonald DW (2004) Competition between Eurasian otter Lutra lutra and American mink Mustela vison probed by niche shift. Oikos 106:19–26
Brazao S, Morais S, Boaventura D, Re P, Narciso L, Hawkins SJ (2003) Spatial and temporal variation of the fatty acid composition of Patella spp. (Gastropoda: Prosobranchia) soft bodies and gonads. Comp Biochem Physiol B-Biochem Mol Biol 136:425–441
Byrne M, Andrew NL, Worthington DG, Brett PA (1998) Reproduction in the diadematoid sea urchin Centrostephanus rodgersii in contrasting habitats along the coast of New South Wales, Australia. Mar Biol 132:305–318
Cook EJ, Bell MV, Black KD, Kelly MS (2000) Fatty acid compositions of gonadal material and diets of the sea urchin, Psammechinus miliaris: trophic and nutritional implications. J Exp Mar Biol Ecol 255:261–274
Cook PA, Gabbott PA (1972) Seasonal changes in the biochemical composition of the adult barnacles, Balanus balanoides, and the possible relationships between biochemical composition and cold-tolerance. J Mar Biol Assoc of UK 52:805–825
Cripps GC, Watkins JL, Hill HJ, Atkinson A (1999) Fatty acid content of Antarctic krill Euphausia superba at South Georgia related to regional populations and variations in diet. Mar Ecol Prog Ser 181:177–188
de la Moriniere EC, Pollux BJA, Nagelkerken I, Hemminga MA, Huiskes AHL, van der Velde G (2003) Ontogenetic dietary changes of coral reef fishes in the mangrove-seagrass-reef continuum: stable isotopes and gut- content analysis. Mar Ecol Prog Ser 246:279–289
Elton C (1927) Animal ecology. Sidgwick & Jackson Ltd., London
Floreto EAT, Teshima S, Ishikawa M (1996) The effects of seaweed diets on the growth, lipid and fatty acids of juveniles of the white sea urchin Tripneustes gratilla. Fish Sci 62:589–593
Fraser AJ, Gamble JC, Sargent JR (1988) Changes in lipid-content, lipid class composition and fatty-acid composition of developing eggs and unfed larvae of Cod (Gadus-Morhua). Mar Biol 99:307–313
Fretwell SD (1987) Food-chain dynamics—the central theory of ecology. Oikos 50:291–301
Fry B, Sherr EB (1984) Delta-C-13 Measurements as indicators of carbon flow in marine and fresh-water ecosystems. Contrib in Mar Sci 27:13–47
Go JV, Rezanka T, Srebnik M, Dembitsky VM (2002) Variability of fatty acid components of marine and freshwater gastropod species from the littoral zone of the Red Sea, Mediterranean Sea, and Sea of Galilee. Biochem Syst Ecol 30:819–835
Howell KL, Pond DW, Billett DSM, Tyler PA (2003) Feeding ecology of deep-sea seastars (Echinodermata: Asteroidea): a fatty-acid biomarker approach. Mar Ecol Prog Ser 255:193–206
Hudson IR, Wigham BD, Billett DSM, Tyler PA (2003) Seasonality and selectivity in the feeding ecology and reproductive biology of deep-sea bathyal holothurians. Prog Oceanogr 59:381–407
Hughes AD, Catarino AI, Kelly MS, Barnes DKA, Black KD (2005) Gonad fatty acids and trophic interactions of the echinoid Psammechinus miliaris. Mar Ecol Prog Ser (in press)
Iverson SJ, Frost KJ, Lowry LF (1997) Fatty acid signatures reveal fine scale structure of foraging distribution of harbor seals and their prey in Prince William Sound, Alaska. Mar Ecol Prog Ser 151:255–271
Kelly MS (2000) The reproductive cycle of the sea urchin Psammechinus miliaris (Echinodermata: Echinoidea) in a Scottish sea loch. J Mar Biol Assoc UK 80:909–919
Kelly MS (2001) Environmental parameters controlling gametogenesis in the echinoid Psammechinus miliaris. J Exp Mar Biol Ecol 266:67–80
Kelly MS, Cook EJ (2001) The ecology of Psammechinus miliaris. In: Lawrence JM (ed) Edible sea urchins: biology and ecology. Elsevier, Amsterdam, pp 217–224
Linderman RL (1942) The trophic-dynamic aspect of ecology. Ecology 23:399–418
Lozano J, Galera J, Lopez S, Turon X, Palacin C, Morera G (1995) Biological cycles and recruitment of Paracentrotus-Lividus (Echinodermata, Echinoidea) in 2 contrasting habitats. Mar Ecol Prog Ser 122:179–191
McClanahan TR, Kurtis JD (1991) Population regulation of the rock-boring sea-urchin Echinometra-Mathaei (Deblainville). J Exp Mar Biol Ecol 147:121–146
McWilliams SR, Guglielmo C, Pierce B, Klaassen M (2004) Flying, fasting, and feeding in birds during migration: a nutritional and physiological ecology perspective. J Avian Biol 35:377–393
Menge BA (2000) Top-down and bottom-up community regulation in marine rocky intertidal habitats. J Exp Mar Biol Ecol 250:257–289
Morais S, Boaventura D, Narciso L, Re P, Hawkins SJ (2003) Gonad development and fatty acid composition of Patella depressa Pennant (Gastropoda : Prosobranchia) populations with different patterns of spatial distribution, in exposed and sheltered sites. J Exp Mar Biol Ecol 294:61–80
Ojea J, Pazos AJ, Martinez D, Novoa S, Sanchez JL, Abad M (2004) Seasonal variation in weight and biochemical composition of the tissues of Ruditapes decussatus in relation to the gametogenic cycle. Aquaculture 238:451–468
Pazos AJ, Roman G, Acosta CP, Sanchez JL, Abad M (1997) Lipid classes and fatty acid composition in the female gonad of Pecten maximus in relation to reproductive cycle and environmental variables. Comp Biochem Physiol B-Biochem Mol Biol 117:393–402
Phillips KL, Jackson GD, Nichols PD (2003) Temporal variations in the diet of the squid Moroteuthis ingens at Macquarie Island: stomach contents and fatty acid analyses. Mar Ecol Prog Ser 256:135–149
Pond DW, Dixon DR, Bell MV, Fallick AE, Sargent JR (1997) Occurrence of 16:2(n−4) and 18:2(n−4) fatty acids in the lipids of the hydrothermal vent shrimps Rimicaris exoculata and Alvinocaris markensis: nutritional and trophic implications. Mar Ecol Prog Ser 156:167–174
Rosa R, Costa PR, Nunes ML (2004a) Effect of sexual maturation on the tissue biochemical composition of Octopus vulgaris and O. defflippi (Mollusca : Cephalopoda). Mar Biol 145:563–574
Rosa R, Costa PR, Pereira J, Nunes ML (2004b) Biochemical dynamics of spermatogenesis and oogenesis in Eledone cirrhosa and Eledone moschata (Cephalopoda: Octopoda). Comp Biochem Physiol B-Biochem Mol Biol 139:299–310
Rosa R, Nunes ML (2002) Biochemical changes during the reproductive cycle of the deep-sea decapod Nephrops norvegicus on the south coast of Portugal. Mar Biol 141:1001–1009
Rosa R, Nunes ML (2003) Tissue biochemical composition in relation to the reproductive cycle of deep-sea decapod Aristeus antennatus in the Portuguese south coast. J Mar Biol Assoc UK 83:963–970
Russell MP (1998) Resource allocation plasticity in sea urchins: rapid, diet induced, phenotypic changes in the green sea urchin, Strongylocentrotus droebachiensis (Muller). J Exp Mar Biol Ecol 220:1–14
Serrazanetti GP, Pagnucco C, Conte LS, Cattani O (1995) Hydrocarbons, Sterols and fatty-acids in Sea-Urchin (Paracentrotus-Lividus) of the Adriatic Sea. Chemosphere 30:1453–1461
Soudant P, Van Ryckeghem K, Marty Y, Moal J, Samain JF, Sorgeloos P (1999) Comparison of the lipid class and fatty acid composition between a reproductive cycle in nature and a standard hatchery conditioning of the Pacific Oyster Crassostrea gigas. Comp Biochem Physiol B-Biochem Mol Biol 123:209–222
Xu XL, Ji WJ, Castell JD, Odor RK (1994) Influence of dietary-lipid sources on fecundity, egg hatchability and fatty-acid composition of Chinese Prawn (Penaeus-Chinensis) Broodstock. Aquaculture 119:359–370
Yatsuya K, Nakahara H (2004) Density, growth and reproduction of the sea urchin Anthocidaris crassispina (A. Agassiz) in two different adjacent habitats, the Sargassum area and Corallina area. Fish Sci 70:233–240
Zamon JE (2001) Seal predation on salmon and forage fish schools as a function of tidal currents in the San Juan Islands, Washington, USA. Fish Oceanogr 10:353–366
Acknowledgements
The authors would like to thank Dr. E. Cook for her help and Miss H. Muir for her technical support with the fatty acid analysis. They would also like to thank M.V. Bell for his helpful comments on an earlier draft of this manuscript. We would also like to acknowledge the Leonardo da Vinci scholarship for facilitating the visit of AIC to SAMS. This research was funded through the NERC Ph.D. studentship NER/S/A/2002/1055.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by J. P. Thorpe
Rights and permissions
About this article
Cite this article
Hughes, A.D., Kelly, M.S., Barnes, D.K.A. et al. The dual functions of sea urchin gonads are reflected in the temporal variations of their biochemistry. Marine Biology 148, 789–798 (2006). https://doi.org/10.1007/s00227-005-0124-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0124-0