Abstract
Although there is a great deal of evidence to show that supplementary feeding by humans in terrestrial environments causes pronounced changes in the distribution and behaviour of wild animals, at present very little is known about the potential for such effects on marine fish. This study evaluated the consequences of feeding by snorkellers on fish assemblages in the no-take area of the Ustica Island marine protected area (MPA; western Mediterranean) by (1) determining if reef fish assemblage structure is affected in space and time by tourists feeding the fish; (2) assessing the effects of feeding on the abundance of the most common fish species; and (3) assessing the effects of feeding on the size structure of the two most numerically dominant ones. In particular, we hypothesised that both the abundance and the size structure of some fish species would increase at the study site following supplementary feeding, since the additional food provided by humans would make the site more appealing to them. Fish feeding influenced the fish assemblages within the Ustica MPA, and significant spatio-temporal changes occurred. While fish feeding appeared to have no effect on the ornate wrasse Thalassoma pavo, there was a noticeable increase in the number of Oblada melanura and Epinephelus marginatus in the impacted location after feeding. It is very likely that aggregations of fishes that evolve as a result of fish feeding by the public may have negative effects on local populations of fishes and invertebrates that make up their prey. Recreational use of coastal areas and MPAs is increasing elsewhere, making fish feeding a generalised human activity. Accurate information about its effect on the fish assemblage is essential to make responsible management decisions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the last few decades, a large body of literature has been produced on the effects that fishing bans inside marine protected areas (MPAs) have had on the abundance and size structure of fish assemblages (Garcia-Rubies and Zabala 1990; Polunin and Roberts 1993; Harmelin et al. 1995; Russ and Alcala 1996; Wantiez et al. 1997; Edgar and Barrett 1999; Willis et al. 2003). There is increasing evidence that, as well as target populations, other species may be indirectly affected through a cascade of trophic interactions (McClanahan 1994, 1995; Sala and Zabala 1996; Sala et al. 1998; Babcock et al. 1999; Pinnegar et al. 2000; Shears and Babcock 2002, 2003).
It has recently been demonstrated that the implementation of protection measures can make a marine area more attractive to tourists (Badalamenti et al. 2000). This has provided a great impetus to study the potential harm that visitors to MPAs around the world may cause (Davis and Tisdell 1995; Sala et al. 1996; Eckrich and Holmquist 2000; see Milazzo et al. 2002 for a review). The results of these studies have led to widespread concern that, when intensive and unregulated, human recreational activities may play a part in modifying marine ecosystems and their living organisms (Harriot et al. 1997; Creed and Amado-Filho 1999; Eckrich and Holmquist 2000).
Most of the research carried out on the effects of tourist activities within MPAs has focused on human trampling, scuba diving, and boat anchoring (Milazzo et al. 2002 and references therein). The biological consequences (e.g. changes in fish distribution and behaviour) of fish feeding by tourists has received very little attention (Cole 1994; Sweatman 1996; Hawkins et al. 1999), despite the fact that in terrestrial parks the impact of animal feeding is deemed to be important (Huestis 1951a, 1951b; Robinson and Cowan 1954; McDougal 1980; Manski et al. 1981; Manski 1982; Walpole 2001).
Studies carried out in both tropical and temperate waters have demonstrated that feeding can alter fish behaviour towards humans (Cole 1994; Sweatman 1996), with fishes actively following divers and snorkellers. It has also been suggested that when fish are fed, their behaviour towards humans may become aggressive (Perrine 1989; Quinn and Kojis 1990).
Fish density, size structure, and behaviour of three large carnivorous species have been analysed in a marine reserve in north-eastern New Zealand where fish feeding by humans was regularly carried out (Cole 1994). There were no significant differences in fish density at a number of locations within the reserve (i.e. feeding sites vs no-feeding sites). However, individuals of the most abundant species, the snapper Pagrus auratus, were larger in areas where feeding activity generally occurred (Cole 1994). Moreover, it was only in these areas that the macrocarnivores P. auratus and Parapercis colias showed positive reactions to the presence of divers (e.g. diver-oriented behaviour), while no differences at all were detected in the behaviour of Cheilodactylus spectabilis (Cole 1994), which feeds on small invertebrates. Another study carried out in the Bonaire marine park (Dutch Antilles) suggested that differences in fish density between dived sites (where fishes are regularly fed by dive guides) and reserve sites (where there is no feeding activity) were mainly due to habitat characteristics (e.g. differences in coral cover and structural complexity) rather than to supplementary feeding by humans (Hawkins et al. 1999).
In the Mediterranean sea, both public (i.e. policy and tourism) and research interest in MPAs are rapidly growing (Juanes 2001). Fish feeding—widely practised in many Mediterranean MPAs since they were first established—is a powerful tourist attraction (e.g. Medes Island, Spain; Lavezzi, France; La Maddalena Archipelago and the Island of Ustica, Italy; S. Riggio, personal communication), yet the biological consequences of this activity on fish assemblages have never been investigated (Milazzo et al. 2002).
The Ustica marine reserve is one among the few real functioning MPAs in Italy and was one of the first to be established in the Mediterranean Sea (Badalamenti et al. 2000). With the main objective of increasing visitor awareness of marine wildlife, since 1991 the MPA management has organised guided snorkelling tours in a small area of the integral reserve (no-take area), where human access is restricted. During these twice-daily visits, snorkellers can feed fishes with frozen cephalopods and shrimps provided by the MPA staff. In addition, several times a day a large number of visitors feed the fish bread in the same location (M. Milazzo, personal observation).
The objectives of this study were to evaluate the potential consequences of feeding by snorkellers on fish assemblages in the no-take area of the Ustica MPA by (1) determining if reef fish assemblage structure is affected in space and time by tourists feeding the fish, (2) assessing the effects of feeding on the abundance of the most common fish species, and (3) assessing the effects of feeding on the size structure of the two most numerically dominant ones: Thalassoma pavo and Oblada melanura. In particular, we hypothesised that both the abundance and the size structure of some fish species would increase at the study site following supplementary feeding, since the additional food provided by humans would make the site more appealing to them.
Materials and methods
Study area
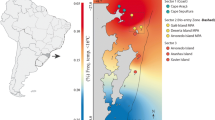
Ustica is a small volcanic island situated 36 miles off the north-west coast of Sicily (southern Tyrrhenian Sea, Italy; 10°43′43″E, 38°42′20″N). Its marine reserve is a protected area, destined for biodiversity conservation and educational and research activities. Established in 1986 and effectively running since 1991, it is divided into three zones with different levels of protection (Fig. 1). Zone A (about 60 ha), in the western part of the island, is a no-take area (or integral reserve) where only scientific research is permitted. Tourist swimming is restricted to two bays at the southernmost (Cala Acquario) and northernmost limits (Cala Sidoti) of the integral reserve (Fig. 1).
Local commercial fishing is permitted in zone B (about 8,000 ha, stretching along both sides of zone A) and zone C (about 8,000 ha, in the southern part of the island from E–NE to SW) only. There are no restrictions on recreational activities (i.e. SCUBA diving, boat anchoring, swimming, and angling) in these zones. The MPA management (Ustica Town Council) has recently banned spear fishing in all parts of the island.
Fish feeding occurs from late June to early September, around a basaltic outcrop at Cala Sidoti (I; Fig. 1). Two similar outcrops within the integral reserve were selected as control locations (no impact): Sbarramento (C1), and Cala Acquario (C2), which are about 400 m and 800 m from Cala Sidoti, respectively (Fig. 1). Control locations were selected as far as possible from each other within the logistic limitations of the study area (Underwood 1993). The integral reserve is too small for selecting proper controls at larger distances. In the other zones of the MPA, in fact, fishing restrictions are different and this could cause experimental confounding.
Each outcrop extends about 100 m2 and rises up to the surface from a 5-m-depth flat basaltic sea bottom. In these locations the seascape is characterised by a narrow sublittoral fringe dominated by brown algae (Cystoseira spp.), which ends up in a homogeneous coralline barrens habitat.
Sampling procedures
Visual census techniques were used for sampling. Two sampling surveys (i.e. times) were carried out in each location, before and after seasonal fish feeding by tourists (i.e. periods). This was necessary to demonstrate that potential temporal trends are not caused by short-term fluctuations. Three replicates were performed for each time (e.g. late May/June and October 2000). A total of 36 independent censuses were carried out. Each replicate was gathered on a different day to achieve independence of data (Stewart-Oaten et al. 1986) and to be sure that potential fish aggregations, as a result of diver positive behaviour (Cole 1994; Sweatman 1996), do not confound the results.
Due to the particular morphology and structure of the study sites (i.e. basaltic outcrops surrounded by extensive barren platforms), we adopted a circular transect technique (Jennings et al. 2001) to estimate fish density and species composition. This technique could be considered a modified version of the standard linear transect method (Brock 1954; Harmelin-Vivien et al. 1985). Similar approaches have been adopted in particular case studies (Sale and Douglas 1981 and references therein) involving the analysis of fish assemblages associated with topographically distinct features, such as artificial reefs (Russell et al. 1974; D’Anna et al. 1999; Relini et al. 2002), fish attracting devices (FADs; D’Anna et al. 1999), or isolated natural patch reefs (Williams 1980; but see Sale and Douglas 1981).
Fish counts were performed by a diver swimming a circle of 10 m radius from the centre of each outcrop. The transect was 60 m long and 5 m wide (300 m2). The diver covered the area in 10 min corresponding to an average rate of coverage of 30 m2 min−1, which is considered a fast swimming rate for Mediterranean fish assemblage assessment (De Girolamo and Mazzoldi 2001). This avoided biases due to diver-oriented behaviour of fishes (Cole 1994), potentially interfering with the density estimates. All transects were carried out at depths of 2–5 m on basaltic platforms of homogeneous coralline barren.
Fish size was assessed as reported in Garcia-Rubies (1999). Estimated sizes were grouped into size classes and the mean size of each class was taken for subsequent data analysis.
Experimental design and data analysis
A potential impact on the fish assemblage structure was checked by means of a beyond-BACI (before-after-control-impact) experimental design (Underwood 1992, 1993, 1994). The factors involved were: location (L), fixed, with three levels, one impact (I) and two controls (C); period (BA), fixed, with two levels, before (B) and after (A); time (T), random, nested in BA, with two levels.
The damselfish Chromis chromis—very common in Mediterranean shallow rocky assemblages (Reñones et al. 1997; La Mesa and Vacchi 1999)—was not included in the species data set, as the considerable shift in its spatial distribution during the reproductive season could have biased the analysis. One of the sampling periods (before feeding) coincided with its reproductive season (i.e. from May to August; Verginella et al. 2000), when adults move towards the spawning–nesting areas, remaining there to patrol the nests until the eggs hatch (Verginella et al. 1999). For the rest of the year, they aggregate in large schools in the water column (Fishelson 1998).
An asymmetrical non-parametric multivariate analyses of variance (NP-MANOVA) based on Bray–Curtis dissimilarities was used to test for differences both before and after feeding and differences in the short temporal variability on the whole fish assemblage. Canonical analysis on principal coordinates (CAP) was performed to visualise the impact pattern in the fish assemblage data set (Anderson and Willis 2003).
In addition, NP-ANOVAs were performed on the abundances of single species that occurred in more than ten samples. For these tests, P-values were calculated after 4,999 permutations under the reduced models (Anderson 2001). A significance level of 0.1 was selected, following the rationale for environmental impact assessment described in Underwood (1997). Homogeneity of variances was tested by Cochran’s C test.
Data were transformed as y′=(y+1)½ to avoid over-dominance of the most abundant species (Clarke and Warwick 1994; Legendre and Legendre 1998), to centre the means of the abundance intervals (García Charton and Pérez Ruzafa 1998), and to attain homogeneity of variances (Snedecor and Cochran 1989).
The above analyses were performed using CAP11 and DISTLM2 (Anderson 2003) and GMAV 5.0 (University of Sydney) software.
The size structure of the two most numerically dominant species was analysed using the Kolmogorov–Smirnov two-sample test (Sokal and Rohlf 1981). This analysis was performed on these two species since only their high abundances in the study site make reliable comparisons possible (La Mesa and Vacchi 1999).
Results
Effects of feeding on fish assemblage
There were 19 fish species belonging to six families recorded in the study area. At all locations, there was a marked dominance of the Labridae (8 species) and Sparidae (5 species) in the fish assemblages.
The mean abundance of fish species in the three sampling locations over time (i.e. before and after feeding) is reported in Table 1. Twelve of the 19 species were constantly present. Chromis chromis, Thalassoma pavo, and Oblada melanura were the most abundant species in both sampling periods (Table 1).
Epinephelus marginatus, Spondyliosoma cantharus, and Labrus merula were recorded at almost all sampling locations, although in limited numbers. Diplodus spp., the labrid Symphodus rostratus, and Muraena helena were only occasionally seen. Red mullet Mullus surmuletus juveniles were found at all locations only before feeding by the public (Table 1).
A significant effect of feeding activity on the multivariate fish assemblage was detected by the NP-MANOVA (P=0.017; Table 2). The observed differences coincided with visitor disturbance, since impact was detected in the largest temporal scale (before vs after), whereas no variations in the short term (i.e. sampling times) were revealed (Table 2).
The permutation tests computed via CAP showed a significant effect of both location (δ2=0.347; P=0.0196) and period (δ2=0.5026; P=0.0002; Table 3), with the canonical axes corresponding to these two main effects clearly separating assemblages in the plot (Fig. 2). Before feeding, the fish assemblages in impacted and control locations (open symbols) grouped close together, whereas in the period after feeding (black symbols), the fish assemblage in the impacted location (black circles) was well separated from control samples (black triangles and quadrates; Fig. 2).
Constrained ordination plot obtained by canonical analysis on principal coordinates (CAP) on the fish assemblage abundances after square root transformation. Open symbols represent samples in the period before (B), and black symbols correspond to samples in the period after (A). Quadrates indicate samples from control 1 (C1), triangles samples from control 2 (C2), and circles samples from the impacted location (I).
The correlations of individual species with the canonical axis corresponding to the ‘location effect’ (I vs Cs) are shown in Table 4, where a positive correlation indicates an association with impact and a negative correlation indicates an association with controls. The species mostly associated with the impacted location was Oblada melanura. The ornate wrasse Thalassoma pavo and the dusky grouper Epinephelus marginatus also exhibited a correlation with impact (Table 4).
The correlations of individual species with the canonical axis corresponding to the ‘period effect’ (before vs after) indicated that many species were associated with the period before (Table 5). Only E. marginatus showed a negative correlation indicating an association with the period after (Table 5).
Among the individual species considered for further analyses, the abundance of O. melanura (P=0.0002) and E. marginatus (P=0.064) showed a significant response to disturbance; no statistical evidence was recorded for the remaining species (Table 2).
Effects of feeding on size structure of the ornate wrasse and the saddled bream
There was very little variation in size class distribution of ornate wrasse Thalassoma pavo between different locations. Overall, small fishes (i.e. class I, 6–9 cm) were more abundant than medium-sized fishes (class II, 10–13 cm), and there was always a lower number of large fishes (class III, 14–17 cm; Fig. 3). No differences were detected in size distributions between impacted and control locations sampled before and after feeding (Table 6).
A greater number of larger saddled bream Oblada melanura individuals (especially those belonging to 20–24, 25–29, and 30–35 size classes) after feeding is clearly evident (Fig. 4). This was confirmed with Kolmogorov–Smirnov analysis (Table 7). Size distribution differs significantly between C1 and both C2 and I, in the period before feeding (Table 7). This is because smaller fishes are present at C1 prior to feeding (Fig. 4). After feeding, the larger fishes are found at I, which significantly differs from both C1 and C2. Moreover, the saddled bream individuals censused in the impacted location after feeding are notably larger than those recorded in the same location in the period before feeding (Table 7).
Discussion and conclusions
We found the species composition of the shallow water fish assemblage of the integral zone of the Ustica MPA to be similar to that reported in previous research conducted in the same area (La Mesa and Vacchi 1999) and in other Mediterranean MPAs (Garcia-Rubies and Zabala 1990; Harmelin et al. 1995).
Feeding influences the fish assemblage within the Ustica MPA, and considerable changes occur within a spatio-temporal scale of hundreds of meters and months. Periods before and after feeding are clearly separated in the constrained ordination plot. Slight differences in fish assemblage before and after feeding in the control locations can be attributed to normal seasonal variation within the integral reserve (La Mesa and Vacchi 1999). The separation of the impacted location in the period after feeding, however, depends on changes in the abundance of Oblada melanura primarily, and secondarily of ornate wrasse Thalassoma pavo and dusky grouper Epinephelus marginatus as revealed by the correlation coefficients.
Univariate analyses on abundance and size distribution somewhat confirm these results. The ornate wrasse appeared to be unaffected by fish feeding, since no significant differences in abundance and size distribution were detected between locations before or after feeding. It is possible that the Thalassoma pavo population in Ustica is habitat limited rather than food limited; therefore the surplus of food available in the impacted location does not affect its distribution.
In contrast, the density of O. melanura and E. marginatus individuals in the impacted location increased significantly after feeding. This could reasonably be attributed to human intervention, as no such changes in the pattern of differences before or after feeding occurred in the controls. This result is probably due solely to changes in the distribution of these species, as overall densities at this location show little temporal variability (La Mesa and Vacchi 1999). In particular, we observed a slight decrease in the mean abundance of O. melanura between the two sampling periods (before and after feeding) within the control locations, where average abundance dropped from 6.2±4.7 to 2.7±1.2 individuals in C1 and from 9.2±3.5 to 2.8±2.1 individuals in C2.
This suggests that, at least on a spatial scale of hundreds of metres, fish feeding can attract saddled bream and dusky grouper in the impacted location, producing larger aggregations.
Bohnsack (1996) suggested that distribution can change when fish are attracted to or aggregated by a man-made structure (i.e. artificial reefs and FADs) and thus vacate adjacent areas. Similarly, feeding by humans can—at least for some species such as O. melanura and E. marginatus—lead to a temporary (i.e. short-term) reshuffling of individuals within an area, with a higher concentration in the feeding site. Previous research carried out within zone A of the Ustica Island emphasised a behavioural change of the dusky grouper in terms of spatial distribution and this was related to the recovery of original habitats (i.e. shallower) in the absence of fishing activity (La Mesa et al. 2002). In our case, the MPA visitor activity may have facilitated this process.
The impacted location therefore proves to be a more suitable patch (i.e. because of supplementary food provided by visitors) for saddled bream and dusky grouper and, as a result, their abundances increase there. This was first highlighted in terrestrial parks (see Walpole 2001 and references therein), where artificial means (i.e. providing supplementary feeding and drinking water, or pumping water to enhance herbivore density) have often been used to attract wildlife to areas where they can be easily observed by visitors (Potts et al. 1996).
The Kolmogorov–Smirnov test gave controversial results. Data showed, however, that one month after feeding ceased, very large individuals of O. melanura (belonging to the 30–35 cm size class especially) were extremely abundant in the feeding site. Similarly, in north-east New Zealand larger individuals of another sparid species (the snapper Pagrus auratus) were recorded as a result of fish feeding by MPA visitors (Cole 1994). On Ustica, La Mesa and Vacchi (1999) investigated the length distribution of Oblada melanura in the whole island between 1994 and 1997 and reported that larger specimens were found at Cala Sidoti (integral reserve). However, their findings could have been influenced by the fact that fish feeding had been going on at Cala Sidoti since 1991, attracting and resulting in the redistribution of some species and of bigger individuals, as in the case of O. melanura.
Ecological consequences could rise from the non-natural aggregations of predator species that result from human disturbance (Sutherland 1996). For example, the changes recorded on the dusky grouper E. marginatus, a top-level predator, may play an important role in the whole shallow ecosystem (Parrish 1987).
Negative feedback forms, like interference and depletion, could affect local populations of invertebrate and fish species, both predators and prey. Fighting, kleptoparasitism, and prey disturbance are common forms of interference that occur amongst vertebrates (Gross-Custard 1980) as consumer density increases. Although such patterns have still to be clearly demonstrated in the marine environment (Sweatman 1996), it is likely that in impacted locations the increased number of individuals of certain species, like O. melanura and E. marginatus, may affect at least the abundance of their preys.
An increase in the density of these predators could, in the long term, lead to a decrease in the number of other fish species, as a result of direct competition for food. Increase in number of consumers can also lead to habitat changes due to the removal, up to depletion, of certain prey species (Babcock et al. 1999; Willis and Anderson 2003) and to the increase of excretions, which might modify habitat features (Mazzola et al. 1999). It is also possible that fish feeding could have the opposite effect, by releasing prey species from predation. In a short period of time (e.g. summer period), it is possible that supplementary feeding might decrease the predation rate on prey populations. Moreover, after being fed by humans, fish may show some behavioural deficits (Olla et al. 1994) that make them easier to be caught outside the no-take zone.
Since fish feeding is becoming increasingly popular in several MPAs, further investigation into the direct and indirect consequences of this recreational activity is urgently required. Due to this lack of information, there is still much controversy over the issue (Sweatman 1996). Some MPA management bodies argue that fish feeding can be used to re-channel detrimental recreational pursuits (e.g. snorkelling and scuba diving) away from vulnerable sites. While some MPAs have banned the activity altogether (e.g. Saba marine park in the Dutch Antilles, Leigh marine reserve in New Zealand and Ras Mohammed marine park in Egypt; Hawkins et al. 1999; T. Willis, personal communication), others have based their policy and legislation on animal feeding in terrestrial parks (e.g. the Great Barrier Reef Marine Park), on which there is more documentation (H. Sweatman, personal communication).
At Ustica Island, the management goals of the MPA should be explicitly stated. If it is desired only to promote marine conservation to the public, activities such as fish feeding may be regarded as desirable. However, this activity causes evident biological alterations that may reduce the effectiveness of the reserve for scientific purposes.
References
Anderson MJ (2001) Permutation tests for univariate or multivariate analysis of variance and regression. Can J Fish Aquat Sci 58:626–639
Anderson MJ (2003) DISTLM v.2: a FORTRAN computer program to calculate distance-based multivariate analysis for a linear model. Department of Statistics, University of Auckland
Anderson MJ, Willis TJ (2003) Canonical analysis of principal coordinates: a useful method of constrained ordination for ecology. Ecology 84:511–525
Babcock RC, Kelly S, Shears NT, Walker JW, Willis TJ(1999) Large-scale habitat change in temperate marine reserves. Mar Ecol Prog Ser 189:125–134
Badalamenti F, Ramos-Esplà A, Voultsiadou E, Sanchez-Lisazo JL, D’Anna G, Pipitone C, Mas J, Ruiz-Fernandez JA, Whithmarsh D, Riggio S (2000) Cultural and socio-economic impacts of Mediterranean marine protected areas. Environ Conserv 27:1–16
Bohnsack AJ (1996) Maintenance and recovery of reef fishery productivity. In: Polunin NVC, Roberts CM (eds) Reef fisheries. Chapman and Hall, London, pp 283–313
Brock VE (1954) A preliminary report on a method of estimating reef fish populations. J Wildl Manage 18:297–308
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Cole RG (1994) Abundance, size structure, and diver-oriented behaviour of three large benthic carnivorous fishes in a marine reserve in northeastern New Zealand. Biol Conserv 70:93–99
Creed JC, Amado-Filho GM (1999) Disturbance and recovery of the macroflora of a seagrass (Halodule wrightii Ascherson) meadow in the Abrolhos Marine National Park, Brazil: an experimental evaluation of anchor damage. J Exp Mar Biol Ecol 235:285–306
D’Anna G, Lipari R, Badalamenti F, Cuttitta A (1999) Questions arising from the use of visual census techniques in natural and artificial habitats. Nat Sicil 23:187–204
Davis D, Tisdell C (1995) Recreational scuba diving and carrying capacity in marine protected areas. Ocean Coast Manage 26:19–40
De Girolamo M, Mazzoldi C (2001) The application of visual census on Mediterranean rocky habitats. Mar Environ Res 51:1–16
Eckrich CE, Holmquist JG (2000) Trampling in a seagrass assemblage: direct effects, response of associated fauna, and the role of substrate characteristics. Mar Ecol Prog Ser 201:199–209
Edgar GJ, Barrett NS (1999) Effects of the declaration of marine reserves on Tasmanian reef fishes, invertebrates and plants. J Exp Mar Biol Ecol 242:107–144
Fishelson L (1998) Behaviour, socio-ecology and sexuality in damselfishes (Pomacentridae). Ital J Zool 65:387–398
García Charton JA, Pérez Ruzafa A (1998) Correlation between habitat structure and rocky reef fish assemblage in the southwest Mediterranean. Mar Ecol 19:111–128
Garcia-Rubies A (1999) Effects of fishing on community structure and on selected populations of Mediterranean coastal reef fish. Nat Sicil 23:59–81
Garcia–Rubies A, Zabala M (1990) Effects of total fishing prohibition on the rocky fish assemblages of Medes Island marine reserve (NW Mediterranean). Sci Mar 54:317–328
Gross-Custard JD (1980) Competition for food and interference amongst waders. Ardea 68:31–52
Harmelin JG, Bachet F, Garcia F (1995) Mediterranean marine reserve: fish indices as tests of protection efficiency. Mar Ecol 16:233–250
Harmelin-Vivien ML, Harmelin JG, Chauvet C, Duval C, Galzin R, Lejeune P, Barnabe G, Blanc F, Ghevalier R, Duclerc J, Lasserre G (1985) Evaluation visuelle des peuplements et populations de poissons: méthodes et problèmes. Rev Ecol (Terre Vie) 40:468–539
Harriot VJ, Davis D, Banks SA (1997) Recreational diving and its impact in marine protected areas in eastern Australia. Ambio 26:173–179
Hawkins JP, Roberts CM, Van’t Hof T, De Meyer K, Tratalos J, C Aldam (1999) Effects of recreational scuba diving on Caribbean coral and fish communities. Conserv Biol 13:888–897
Huestis RR (1951a) The golden-mantled squirrels in Crater Lake National Park. Crater Lake Nature Notes 1:5–15
Huestis RR (1951b) Report on the trapping and marking of golden-mantled squirrels at Crater Lake National Park, 1939. Crater Lake Nature Notes 1:16–22
Jennings S, Kaiser MJ, Reynolds JD (2001) Marine fisheries ecology. Blackwell Science, Oxford
Juanes F (2001) Mediterranean marine protected areas. Trends Ecol Evol 16:169–170
La Mesa G, Vacchi M (1999) An analysis of the coastal fish assemblage of the Ustica Island Marine Reserve (Mediterranean Sea). Mar Ecol 20:147–165
La Mesa G, Louisy P, Vacchi M (2002) Assessment of microhabitat preferences in juvenile dusky grouper (Epinephelus marginatus) by visual sampling. Mar Biol 140:175–185
Legendre P, Legendre L (1998) Numerical ecology, 2nd edn. Elsevier Science, Amsterdam
Manski DA (1982) Management of grey squirrels and people in a downtown national park. Park Sci 2:8–9
Manski DA, VanDruff LW, Flyger V (1981) Activities of grey squirrels and people in a downtown Washington, D.C. park: management implications. Transactions of the 46th North American wildlife natural resource conference. Wildlife Management Institute, Washington D.C., pp 439–454
Mazzola A, Mirto S, Danovaro R (1999) Initial fish-farm impact on meiofaunal assemblages in coastal sediments of the western Mediterranean. Mar Pollut Bull 38:1126–1133
McClanahan TR (1994) Kenyan coral reef lagoon fish: effects of fishing, substrate complexity, and sea urchins. Coral Reefs 13:231–241
McClanahan TR (1995) Fish predators and scavengers of the sea urchin Echinometra mathaei in Kenyan coral-reef marine parks. Environ Biol Fish 43:187–193
McDougal C (1980) Some observations on tiger behaviour in the context of baiting. J Bombay Nat Hist Soc 77:476–485
Milazzo M, Chemello R, Badalamenti F, Camarda R, Riggio S (2002) The impact of human recreational activities in marine protected areas: what lessons should be learnt in the Mediterranean sea? Mar Ecol 23:280–290
Olla BL, Davis MW, Ryer CH (1994) Behavioral deficits in hatchery-reared fish: potential effects on survival following release. Aquacult Fish Manage 25:19–34
Parrish JD (1987)The trophic biology of snappers and groupers. In: Polovina JJ, Ralston S (eds)Tropical snappers and groupers: biology and fisheries management. Westview, Boulder, Colo., pp 405–463
Perrine D (1989) Reef fish feedings: amusement or nuisance? Sea Frontiers 35:272–279
Pinnegar JK, Polunin NVC, Francour P, Badalamenti F, Chemello R, Harmelin-Vivien M, Hereu B, Milazzo M, Zabala M, D’Anna G, Pipitone C (2000) Trophic cascades in benthic marine ecosystems: lessons for fisheries and protected-area management. Environ Conserv 27:159–178
Polunin NVC, Roberts CM (1993) Greater biomass and value of target coral-reef fishes in two small Caribbean marine reserves. Mar Ecol Prog Ser 100:167–176
Potts FC, Goodwin HJ, Walpole MJ (1996) People, wildlife and tourism in and around Hwange National Park, Zimbabwe. In: Price MF (ed) People and tourism in fragile environments, Wiley, Chichester, pp 199–219
Quinn NJ, Kojis BL (1990) Are divers destroying the Great Barrier Reef’s cod hole? Diving Sci 5:303–309
Relini G, Relini M, Torchia G, Calandri G (2002) Ten years of censuses of fish fauna on the Loano artificial reef. ICES J Mar Sci 59:132–137
Reñones O, Moranta J, Coll J, Morales-Nin B (1997) Rocky bottom fish communities of Cabrera Archipelago National Park (Mallorca, western Mediterranean). Sci Mar 61:495–506
Robinson DJ, Cowan IM (1954) An introduced population of the grey squirrel (Sciurius carolinensis Gmelin) in British Columbia. Can J Zool 32:261–282
Russ GR, Alcala AC (1996) Marine reserves: rates and patterns of recovery and decline of large predatory fish. Ecol Appl 6:947–961
Russell BC, Talbot FH, Domm S (1974) Patterns of colonisation of artificial reefs by coral reef fishes. Proc 2nd Int Coral Reef Symp 1:207–215
Sala E, Zabala M (1996) Fish predation and the structure of the sea urchin Paracentrotus lividus populations in the NW Mediterranean. Mar Ecol Prog Ser 140:71–81
Sala E, Garrabou J, Cabala M (1996) Effects of diver frequentation on Mediterranean sublittoral populations of the bryozoan Pentapora fascialis. Mar Biol 126:451–459
Sala E, Boudouresque CF, Harmelin-Vivien M (1998) Fishing, trophic cascades, and the structure of algal assemblages: evaluation of an old but untested paradigm. Oikos 82:425–439
Sale PF, Douglas WA (1981) Precision and accuracy of visual census technique for fish assemblages on coral patch reefs. Environ Biol Fish 6:333–339
Shears NT, Babcock RC (2002) Marine reserves demonstrate top-down control of community structure on temperate reefs. Oecologia 132:131–142
Shears NT, Babcock RC (2003) Continuing trophic cascade effects after 25 years of no-take marine reserve protection. Mar Ecol Prog Ser 246:1–16
Snedecor GW, Cochran WG (1989) Statistical methods, 8th edn. Iowa State University Press, Ames
Sokal RR, Rohlf FJ (1981) Biometry. Freeman, New York
Stewart-Oaten A, Murdoch WW, Parker KR (1986) Environmental impact assessment: “pseudoreplication” in time? Ecology 67:929–940
Sutherland WJ (1996) From individual behaviour to population ecology. Oxford University Press, Bath
Sweatman HPA (1996) Impact of tourist pontoons on fish assemblages on the Great Barrier Reef. CRC Reef Research Centre, Townsville
Underwood AJ (1992) Beyond BACI: the detection of environmental impacts on populations in the real, but variable world. J Exp Mar Biol Ecol 161:145–178
Underwood AJ (1993) The mechanisms of spatially replicated sampling programmes to detect environmental impacts in a variable world. Aust J Ecol 18:99–116
Underwood AJ (1994) On beyond BACI: sampling designs that might reliably detect environmental disturbances. Ecol Appl 4:3–15
Underwood AJ (1997) Experiments in ecology: their logical design and interpretation using analysis of variance. Cambridge University Press, Cambridge
Verginella L, Spoto M, Ciriaco S, Ferrero EA (1999) Ethogram of the reproductive territorial male of the damselfish Chromis chromis L. (Pisces: Pomacentridae). Boll Soc Adr Sci 78:437–454
Verginella L, Spoto M, Ferrero EA (2000) Spawning behaviour sequence analysis of the Mediterranean damselfish Chromis chromis (Pomacentridae) in the field. Z Fischk 5:3–10
Walpole MJ (2001) Feeding dragons in Komodo National Park: a tourism tool with conservation complications. Anim Conserv 4:67–73
Wantiez L, Thollot P, Kulbicki M (1997) Effects of marine reserves on coral reef fish communities from five islands in New Caledonian lagoon. Coral Reefs 16:215–224
Williams DM (1980) Dynamics of the pomacentrid community on small patch reefs in One Tree Lagoon (Great Barrier Reef). Bull Mar Sci 30:159–170
Willis TJ, Anderson MJ (2003) Structure of cryptic reef fish assemblages: relationships with habitat characteristics and predator density. Mar Ecol Prog Ser 257:209–221
Willis TJ, Millar RB, Babcock RC, Tolimieri N (2003) Burdens of evidence and the benefits of marine reserves: putting Descartes before des horse? Environ Conserv 30:97–103
Acknowledgements
The authors would like to thank Paolo Guidetti (University of Lecce), Trevor J. Willis (University of Bologna), Giulia Ceccherelli (University of Sassari), Antonio Terlizzi (University of Lecce), and anonymous reviewers for their comments that helped improve the manuscript; Hugh Sweatman (James Cook University, Townsville) for his useful suggestions; Raffaele Camarda (University of Palermo) for invaluable help during field work; Ustica Municipality and Prof. Antonio Gianguzza (C.I.R.I.T.A., University of Palermo) for support and logistic assistance. Special thanks are due to Daniela Bilello, Gaetano Caminita, Tonino Licciardi, Giacomo Lo Schiavo, and Maria Concetta Natale (MPA staff). This study was part of a Ph.D. thesis by M.M. and was funded by MIUR ‘Progetto Giovani Ricercatori’ (grant/2000) to M.M.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Milazzo, M., Badalamenti, F., Vega Fernández, T. et al. Effects of fish feeding by snorkellers on the density and size distribution of fishes in a Mediterranean marine protected area. Marine Biology 146, 1213–1222 (2005). https://doi.org/10.1007/s00227-004-1527-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1527-z