Abstract
We sought to determine whether common intertidal and shallow subtidal zone grazers would consume extracts or fronds of three invasive Caulerpa spp., all of which are now resident in southern New South Wales, Australia. We examined the responses of herbivorous fishes, echinoderms and molluscs to C. filiformis. A subset of these organisms was tested with extracts of C. scalpelliformis and C. taxifolia. Polar (seawater) extracts of C. filiformis deterred a single herbivore, Aplysia sydneyensis, but confirmed that the biological activity reported from some Caulerpa spp. is not restricted to the lipophilic fractions. The large turbinid Turbo torquatus was deterred by an ethanol extract of C. filiformis, while the small congener T. undulatus demonstrated a significant preference for palatable agar discs containing ethanol extracts of C. filiformis. However, when T. undulatus were offered a choice of fronds from five algal species in the laboratory, they readily consumed Ulva spp. and Sargassum sp., showing the lowest preference for C. filiformis. Solvent extracts of C. scalpelliformis and C. taxifolia did not significantly deter any grazers. However, the overall trend was for reduced consumption of discs containing solvent extracts of these seaweeds. Indeed, for the large urchin Centrostephanus rodgersii and in the fish trials these effects were very near significant (P<0.06). We conclude that common herbivores associated with hard substrata are highly unlikely to intercede in the spread or control of these invasive algae.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Successful biological invasion is a two-part process; it requires translocation (usually mediated by humans) to a locality beyond the invaders normal range and then assimilation into an established assemblage of organisms (Carlton and Geller 1993). The degree to which the habitat is modified by humans, the invaders competitive ability and the response of natural enemies to the invader are all likely to be important determinants of success in the establishment phase (Keane and Crawley 2002). Consumers, in particular, may play a very important role in this process as it has been argued that the success of invaders is directly attributable to reduced consumer pressure in the invaded habitat (Williamson 1996). The directing of consumer attention to native competitors may also tip the balance in favour of invaders (Keane and Crawley 2002).
Many representatives of the green algal genus Caulerpa are highly invasive. Invasive populations of this genus have established themselves in the USA (Jousson et al. 2000), Australia (Creese et al. 2004), Japan (although unsuccessfully, Komatsu et al. 2003) and a number of Mediterranean countries (Meinesz et al. 1993, 2001; Piazzi et al. 1994). In some locations they have spread rapidly, dramatically altering the structure of natural assemblages (de Villele and Verlaque 1995; Davis et al. 1997). Caulerpa species contain a variety of biologically active secondary metabolites, notably sesquiterpenes, which may well mediate their interactions with consumers (Paul and Fenical 1986; Guerriero et al. 1992, 1993). Members of the Caulerpales show considerable variation in their palatability to tropical herbivores, and, in at least some cases, this is in response to their secondary chemistry (Paul and Fenical 1986; Paul et al. 1987). Whether these compounds have been instrumental in the success of Caulerpa spp. as invaders in temperate waters remains unclear. Features other than their secondary chemistry, such as an ability to fragment, disseminate and re-establish a viable alga almost certainly contribute significantly to their success as invaders (Sant et al. 1996; Smith and Walters 1999).
Biological control has been viewed as an important means of addressing the issue of invasive pests (Van Driesche and Bellows 1996). Indeed, some biological control programmes have met with spectacular success (Dodd 1940), but an equally large number have been a dismal failure, with far-reaching irreversible ecological effects (e.g. Clarke et al. 1984). Recently, authors have urged caution in the application of biological control in marine systems (e.g. Secord 2003). They have argued that the relative taxonomic diversity and complexity of marine versus terrestrial systems, combined with the fact that biological control is in its infancy in marine systems, necessitates extreme caution. Rather than seeking exotic biological control agents with their attendant risks, it has been argued that enhancing populations of native predators is a much less perilous approach (Chang and Karieva 1999). In order to adopt this approach, referred to as “augmentative biocontrol”, we need detailed information on the responses of native consumers to invasive species.
We examined the responses of native herbivores to three Caulerpa spp. (C. filiformis, C. scalpelliformis and C. taxifolia) that have recently appeared near Sydney, in south-eastern Australia (Fig. 1). These species all form dense mono-specific stands in these waters. C. filiformis (Suhr) Hering was recorded in Botany Bay in the mid-1920s (Lucas 1927), and has continued to spread (May 1976). It now occurs over a 200-km stretch of rocky intertidal coastline north and south of this major port (Davis, personal observations). This species usually forms a thick mat on the lower intertidal or immediate subtidal zone (Sanderson 1997), but has been observed anchored to rocky substrata to depths of at least 25 m (Davis et al. 1997). Recent genetic evidence questions whether C. filiformis is indeed an invader (Pillman et al. 1997), but these conclusions are based on a relatively few samples and await confirmation. A range extension of C. scalpelliformis (R. Brown ex Turner) C. Agardh was observed in 1994, and, at its peak, an area of approximately 0.5 hectares of the southern entrance of Botany Bay was heavily infested by this alga (Davis et al. 1997). This species is restricted to the subtidal zone and has been observed on hard substrata to a depth of at least 25 m. Finally C. taxifolia (Vahl) C. Agardh was discovered in two estuaries near Sydney in 2000 (Creese et al. 2004). At the time of writing it has been confirmed that nine estuaries in southern and central New South Wales (NSW) now possess this invader, and it has also appeared in the state of South Australia (Creese et al. 2004). In NSW, C. taxifolia is restricted to relatively calm embayments, and has not been seen below 10 m in depth (Davis, personal observations). This contrasts with observations in the Mediterranean, where it forms a thick mat over a variety of substrata and grows successfully to at least 50-m depths (Meinesz 1999). Southern Australian waters host a large number of native Caulerpa species, and paradoxically it has been argued that this may be the centre for divergence of this predominantly tropical genus (Calvert et al. 1976). Nevertheless, the chemical ecology of temperate members of this genus remains virtually unstudied.
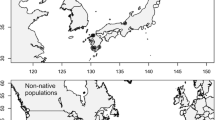
Caulerpa spp. Distribution of invasive populations of Caulerpa spp. in south-eastern Australia. Dark bar denotes extent of distribution of C. filiformis. Numbers denote the nine locations that C. taxifolia has invaded. C. scalpelliformis is restricted to Botany Bay (number 4) (numbered locations are: 1 Lake Macquarie; 2 Pittwater; 3 Port Jackson; 4 Botany Bay; 5 Port Hacking; 6 Sussex Inlet; 7 Lake Conjola; 8 Narrawallee Inlet; 9 Burrill Lake)
The aim of this study was to determine the response of native subtidal invertebrate and vertebrate megagrazers to solvent and aqueous extracts from these three invasive Caulerpa species and, where possible, to determine the response of grazers to algal tissue. To date, the interaction between herbivores and Caulerpa spp. has largely focussed on tropical waters (e.g. Hay 1984; Paul and Fenical 1986, 1987). This represents the first study to examine the response of a suite of vertebrate and invertebrate herbivores to Caulerpa spp. in the temperate zone. Our focus was on large, common, rocky reef animals, as these generalist consumers have a demonstrated role in structuring shallow subtidal algal assemblages on rocky substrata in temperate Australia (Fletcher 1987), and, with the exception of C. taxifolia, this is the habitat in which these algae are commonly found. Moreover, as has been observed in the Mediterranean, we expect C. taxifolia to eventually invade the exposed rocky coast of NSW. We used feeding trials to gauge the response of common molluscs, urchins and fish to extracts of each alga. We deemed it too risky to use living fronds of these algae in our feeding trials, as Caulerpa spp. have the ability to regrow from very small fragments of the frond or stolon and might establish new invasive populations. Instead, we used solvent and aqueous extracts incorporated into palatable agar discs, a technique we have previously employed in feeding trials (Wright et al. 1997). We used responses of consumers to extracts of Caulerpa spp. and then to the fronds of a Caulerpa species and some other common native algae to infer their ability to intercede in the persistence and spread of these algae.
Materials and methods
Sample collection and extraction
We made solvent extracts of each species and incorporated these extracts into agar discs containing the palatable algae Ulva spp. or Corallina officinalis. To produce these extracts Caulerpa scalpelliformis was collected from near Bare Island, on the northern side of Botany Bay (33°59′32″S; 151°13′50″E); C. taxifolia was collected in Gunnamatta Bay, Port Hacking (34°03′30″S; 151°09″E); and C. filiformis was collected from Flagstaff Point Wollongong (34°25′30″S; 150°54′30″E), as were the Ulva spp. and C. officinalis used in the feeding trials. Caulerpa species were usually frozen (−20°C) as soon after collection as possible and then thawed just prior to the production of extracts. Caulerpa spp. were collected from several sites within a location so as to account for individual variation in metabolites within species.
Solvent extracts were produced by thawing, then macerating each Caulerpa species in a blender (Waring model 32BL79). Only the upright fronds of each alga were used to make extracts as previous work has indicated that they contain the highest levels of secondary metabolites (Meyer and Paul 1992). The resultant slurry was steeped in ethanol (LR grade) for 3 h. This was done twice, and on the third occasion the macerated alga was steeped overnight (15 h). The resultant fractions were combined and evaporated to near dryness under vacuum and then dried under a stream of nitrogen. The resultant extract was then frozen (−20°C) until used in the feeding trials.
Feeding experiments
Artificial diets
Treatment disks in the majority of trials were made up of the green algae Ulva spp. embedded in agar and Caulerpa extract dissolved in ethanol. Control disks contained Ulva, agar and ethanol only. The Ulva was collected from intertidal rock pools on the day of the trials to ensure that it was fresh. Feeding trials for the turbinid Turbo torquatus used the red alga C. officinalis in producing palatable discs for both aqueous- and solvent-extract feeding trials (see Table 1), as this turbinid showed a strong preference for this alga. We followed the same procedure as Wright et al. (1997) in producing discs. The alga was blended and then strained using a kitchen strainer (1 mm mesh). Agar (oxoid code no. CM3) was dissolved in distilled water at 5% (w/v) and boiled in a microwave at least three times until it was clear. When the agar had cooled to 50°C, 3.5 g wet weight of macerated alga was added per disk and the mixture stirred. The agar mixture was allowed to cool further to 45°C, and the extract was dissolved in ethanol, or ethanol only was added. The agar was stirred to ensure that the solvent and extract were evenly spread throughout the mixture. The mixture was poured into 57-mm glass petri dishes to a thickness of approximately 10 mm. Numbered stainless steel washers (~13 g) were incorporated into the disks to weight them down and to allow identification, and the agar was allowed to cool and harden around them in the petri dish.
To prepare the water extracts a set volume of Caulerpa fronds (or C. officinalis or Ulva spp. as a control) was macerated in an equal volume of seawater. The extract was then filtered through a coarse (1 mm) sieve to remove any remaining frond fragments. The water extract was then used directly to prepare feeding disks by dissolving agar (oxoid code no. CM3) in half of the available volume at 10% (w/v). The agar mixture was then cooled to ~60°C before the remaining half of the water extract was added and thoroughly stirred before being poured into petri dishes (as above).
All extracts were tested at natural concentrations; the volume of agar discs was equivalent to the volume of Caulerpa extracted. These extracts were tested against a range of invertebrate consumers (Table 1) in laboratory-based feeding experiments, with the exception of the urchins Centrostephanus rodgersii and Heliocidaris tuberculata, which did not feed well in aquaria. The invertebrates were all collected from either Bellambi Point (34°22′20″S; 150°55′45″E ) or Bass Point (34°35′45″S; 150°53′20″E) near Wollongong, NSW. Each feeding trial used different individual consumers, thus making them independent replicates. The taxonomic affinities of a number of SE Australian molluscs are currently under review (Dr W. Ponder, Australian Museum, personal communication) and here we follow the identifications adopted by Edgar (1997). Consumers used in experimental feeding trials in other studies have been starved for various lengths of time: 4 days (Ayling 1978) and from 36 to 48 h (Steinberg 1988; Steinberg and van Altena 1992). In view of the potential to introduce artefacts in feeding studies (e.g. Cronin and Hay 1996), we sought to minimise the period of starvation, but to allow animals sufficient time to acclimate to field or laboratory enclosures. Hence, in this study, consumers were deprived of food for 2 days and then presented with the two pre-weighed disks, one treatment and one control; the trial was allowed to run for 24 h.
Laboratory experiments were done in 23-l tanks in a recirculating seawater system at 21°C. The size of animals dictated the number used in each replicate. With the exception of Astralium tentoriformis, Turbo torquatus and T. undulatus a single individual consumer was housed in each of 14–16 replicate tanks and offered 1 treatment and 1 control disk. Experiments with A. tentoriformis and T. undulatus were done with groups of ten individuals per tank, while two to three individuals were used per tank in feeding trials with T. torquatus. A further 6–14 tanks housed feeding disks without consumers to measure autogenic change. Field-based experiments with Centrostephanus rodgersii and Heliocidaris tuberculata were done by placing individual consumers into 23-l plastic tubs (Nally Plastic catalogue number 1H049; dimensions 448 mm×391 mm×200 mm) covered with plastic mesh (mesh size≈40 mm). The plastic bins were weighted down with large pieces of scrap metal and placed in water 3–4 m deep at Bass Point (34°35′45″S; 150°53′20″E), 40 km to the south of Wollongong (Fig. 1). Control tubs containing agar disks, but no consumers were used in each trial to measure autogenic changes in the disks. At the completion of the trials, the disks were collected, blotted dry with paper towel and then weighed. Generally, the weight of these disks increased by 1.5–2%. These figures were used to correct for changes in the weights of the disks used in the trials.
The response of fishes to the discs was assessed by placing treatment and control discs randomly over an area of shallow reef in an urchin-grazed barren habitat of approximately 400 m2 (20×20 m), at 3–4 m in depth in daylight hours. Between 22 and 38 discs were left for up to 1 h and then recovered. Individual bite marks in the discs were counted in the laboratory and converted into the number of bites per minute each disc was exposed.
Algal consumption
The responses of a turbinid mollusc to a selection of common algae was tested in small aquaria in the laboratory. Living algae were used in two trials: the first offered a choice of algae simultaneously and the second a single algal species (i.e. no choice). In the first trial, approximately 2 g of each of five common algal species: Caulerpa filiformis, Corallina officinalis, Padina crassa, Sargassum sp. and Ulva spp. were presented to Turbo undulatus. The pre-weighed algae were positioned equidistant in a circle around ten individuals of this small turbinid in 17 small aquaria. Algae were then removed from aquaria after 24 h, blotted dry and weighed. Mean change in the six autogenic controls for each alga was then subtracted, and the change in weight was then converted to a percentage of each alga consumed during the 24-h period. The analysis of multiple-choice feeding trials such as this can be problematic, as many statistical tests assume independence and the consumption of one food type may be dependent on the presence of others (Peterson and Renaud 1989). We used Quade’s test, which does not make this assumption (Roa 1992).
Three species were offered in the second trial: C. filiformis, Sargassum sp. and Ulva spp. Six aquaria, each with ten T. undulatus, were used for each alga. Mean change in six autogenic controls was subtracted, and percent algal consumption over 24 h was calculated.
Statistical analyses
The feeding trials were analysed using two-tailed, paired-sample t-tests or the nonparametric equivalent (Wilcoxon signed-rank test) (Zar 1999). Fish feeding trials were analysed with a standard t-test. We measured the amount of each disk consumed as a percentage of the initial weight (corrected for autogenic changes) and completed the analysis by comparing the proportion consumed of control disks to the proportion consumed of treatment disks. Trials where <5% of either disk had been consumed were excluded from the analysis. Some herbivores failed to eat during the trials, and their responses could not be assessed. Where trials were repeated on different days, we tested for the presence of heterogeneity in feeding deterrence between days. For all analyses, the assumption of normality was examined visually and homogenous variances were determined with a Cochran’s C-test. As data in the multiple-choice experiment examining algal consumption was non-independent, we employed Quade’s test (Conover 1999).
Results
Feeding experiments
Artificial diets
We detected little evidence of Caulerpa species deterring invertebrates or fish from feeding. Overall, in 12 of the 16 sets of feeding trials the consumption of treatment discs, that is those containing Caulerpa extracts, was lower than in the controls. In only three cases did we observe a significant depression in feeding, all with extracts of C. filiformis. Turbo torquatus and Heliocidaris tuberculata were deterred from consuming solvent extract of C. filiformis (Figs. 2, 3), while aqueous extracts of this alga discouraged feeding by Aplysia sydneyensis (Fig. 4). It is also noteworthy that two additional sets of trials were very close to significant (P<0.06); these trials involved extracts of C. scalpelliformis on fish assemblages (Fig. 5) and extracts of C. taxifolia on the urchin Centrostephanus rodgersii (Fig. 6). Conversely, in the four remaining sets of feeding trials, we observed increased consumption of discs containing Caulerpa extracts. In only one instance were these effects significant; T. undulatus consumed significantly more of the treatment discs containing solvent extract of C. filiformis than the controls (Fig. 2).
Feeding responses of turbinid molluscs to palatable agar discs containing solvent (ethanol) extracts (treatment) of Caulerpa filiformis (A) and Caulerpa taxifolia (B). Control discs contained solvent, but no extract. Bars are mean percent consumed (±1 SE). Statistical significance determined with a paired sample t-test; *P<0.05, n is the sample size
Feeding responses of a mollusc and echinoderm to palatable agar discs containing solvent (ethanol) extracts (treatment) of Caulerpa filiformis. Control discs contained solvent, but no extract. Bars are mean percent consumed (±1 SE). Statistical significance determined with a paired sample t-test; *P<0.05, n is the sample size
Feeding responses of molluscs and echinoderms to palatable agar discs containing aqueous (seawater) extracts (treatment) of Caulerpa filiformis. Control discs contained seawater, but no extract. Bars are mean percent consumed (±1 SE). Statistical significance determined with a paired sample t-test; *P<0.05, n is the sample size
Feeding responses of a natural assemblage of fish to palatable agar discs containing solvent (ethanol) extracts (treatment) of Caulerpa filiformis (A), Caulerpa scalpelliformis (B) and Caulerpa taxifolia (C). Control discs contained solvent, but no extract. Bars are mean number of bite marks per minute (±1 SE). Statistical significance determined with a t-test. Note that two trials were completed with C. filiformis, and both are presented
Feeding responses of the echinoid Centrostephanus rodgersii to palatable agar discs containing solvent (ethanol) extracts (treatment) of Caulerpa filiformis (A), Caulerpa scalpelliformis (B) and Caulerpa taxifolia (C). Control discs contained solvent, but no extract. Bars are mean percent consumed (±1 SE). Statistical significance determined with a paired sample t-test
Algal consumption
On providing T. undulatus with a suite of algae to consume, the apparent preference for C. filiformis was no longer evident. Within 24 h these molluscs had consumed all of the Ulva spp. and Sargassum sp. Consumption of C. filiformis was the lowest of the six algal species tested, and was significantly lower than Ulva spp. and Sargassum sp. (T1=26.6, df=4, 64, P<0.001, Fig. 7A). When offered algae in isolation (as opposed to extracts in palatable discs), T. undulatus consumed a high proportion of Ulva spp. and Sargassum sp., but <2% of C. filiformis (Fig. 7B). These differences were statistically significant (F=230.4, df=2, 15, P<0.001) following square-root transformation [√(x+3/8), Zar 1999] to remove heterogeneous variances. Post hoc Tukey’s tests confirmed that differences in the consumption of each species were statistically significant.
Feeding responses of the turbinid mollusc Turbo undulatus to fronds of Caulerpa filiformis and other common sympatric algae in laboratory aquaria over a 24-h period. Algae were offered simultaneously (A) or in isolation (B) in separate feeding trials. Bars are mean percent consumed (±1 SE). The same letter above the bars denotes values that did not differ significantly (P>0.05), as determined by Quade’s test for the simultaneous trial and a one-factor ANOVA when algae were offered in isolation
Discussion
Overall, the three species of Caulerpa we examined were relatively ineffective in deterring the suite of temperate-zone consumers tested. We observed significant anti-feedant effects in just 3 of the 16 feeding trials and then only for extracts of C. filiformis. Solvent extracts of C. filiformis deterred the turbinid mollusc Turbo torquatus and the urchin Heliocidaris tuberculata, while aqueous extract deterred Aplysia sydneyensis. It should also be noted that extracts of C. taxifolia and C. scalpelliformis yielded probabilities in the analysis of feeding trials that were very close to significant (<0.06) with the large urchin Centrostephanus rodgersii and the fish assemblage, respectively. Although the consumers examined here were rarely significantly deterred from feeding on extracts of Caulerpa, we hesitate to equate this to them exerting top-down control over these invasive algae. In the majority of feeding trials consumers showed a clear tendency to avoid the discs containing Caulerpa extracts, consuming smaller quantities of the treatment discs. The low probability that consumers will control these invasive algae is underscored by the outcome of the trials with T. undulatus. Solvent extracts of C. filiformis stimulated feeding in this abundant mollusc, although when T. undulatus was offered a choice of five algal species simultaneously, C. filiformis was consumed at the lowest rate. It was also apparent that these molluscs responded differently to the tissue of C. filiformis when offered in isolation as opposed to extracts of this alga (compare Fig. 2 with Fig. 7B). We did not see evidence of the turbinid snail enhancing fragmentation of C. filiformis during feeding, as has been observed for a mollusc on C. taxifolia in the Mediterranean (Zuljevic et al. 2001). It has been argued that for generalist herbivores to limit the establishment of invaders, they must consume the invader when it is at very low densities or biomass (Maron and Vilà 2001); we found no evidence in support of this. Taken together these data indicate that the generalist consumers considered here are highly unlikely to intercede in the establishment or persistence of these invaders. Based on a similar approach, much the same conclusion was reached by Trowbridge (1995) for the invasive siphonous alga Codium fragile on temperate rocky shores in New Zealand.
The presence of biologically active secondary metabolites is well established for members of the genus Caulerpa (Paul and Fenical 1986, 1987; Guerriero et al. 1992, 1993). Much of the activity is attributed to the sesquiterpene Caulerpenyne, although it rapidly forms degradation products, which may lead to underestimates of its biological activity (Dumay et al. 2002). Other minor compounds can also produce significant biological effects (e.g. Paul et al. 1987) and some may be water soluble (Lemée et al. 1993), as observed for our feeding trials with A. sydneyensis. Extracts or pure compounds from these algae have demonstrated toxicity or deterrent activity against bacteria, fungi, mammalian cell lines, invertebrates, their larvae and vertebrates (Paul et al. 1987). Despite this host of biological activity the relatively inconsistent anti-feedant activity that we observed in our trials is very similar to that for feeding trials with extracts or pure compounds for tropical members of this algal genus (see Table 6 in Meyer and Paul 1992). A number of tropical fish species, for example, readily consume Caulerpa racemosa, despite the presence of relatively high concentrations of sesquiterpenes (Paul and Hay 1986; Meyer and Paul 1992). Other Caulerpa spp. are also consumed at moderate to high levels by tropical fishes (Hay 1984). Despite the poor correlation between the presence of secondary metabolites and the consumption of algae by herbivores, it is apparent that C. taxifolia may well have an impact on generalist consumers in the Mediterranean (Boudouresque et al. 1996), prompting Paul and co-workers (2001) to conclude that the secondary chemistry of C. taxifolia may well contribute to its invasive success.
To date, the ability of Caulerpa spp. to deter consumers has been focussed almost exclusively on tropical fishes (Hay 1984; Paul and Hay 1986; Meyer and Paul 1992). Given that fish exert significant grazing pressure in the tropics (Hay et al. 1983) this approach is appropriate. However, as a number of the tropical Caulerpa spp. extend their ranges into temperate waters, we urgently need to assess their impact on the structure and dynamics of the temperate assemblages they are invading. Invertebrates, particularly urchins, often play a key role in structuring shallow subtidal habitats in the temperate zone (e.g. Fletcher 1987). In SE Australia, for example, the urchin Centrostephanus rodgersii assumes very high densities where appropriate shelter is available, creating and maintaining extensive areas of urchin-grazed barrens (Andrew 1993; Davis et al. 2003). Fish also play a role in structuring algal assemblages in SE Australia (Andrew and Jones 1990), but it is usually limited. We have reported the responses of generalist herbivores to algal extracts and in one case to algal tissue in a laboratory setting. The challenge will be examining the ability of native herbivores to suppress invasive algal populations in the field.
Several workers have attributed the success of invaders to the lower consumer pressure they experience in the invaded habitat (Crawley 1996; Williamson 1996). The so-called “enemy-release hypothesis” assumes that generalist consumers will have more impact on native competitors (Keane and Crawley 2002). Although the reasons for the success of these three Caulerpa species in SE Australia are unknown, our findings that C. filiformis was consumed at the lowest rate when a variety of common algal species were offered to T. undulatus offers support to this hypothesis. Nevertheless, recent data have challenged the applicability of the enemy-release hypothesis, at least in terrestrial environments (Agrawal and Kotanen 2003). Specialist molluscan herbivores, sacoglossans, commonly feed on siphonous algae, including Caulerpa spp. (Trowbridge and Todd 2001) and have been of interest in the Mediterranean as potential agents of biological control (Meinesz 1999; Thibaut and Meinesz 2000; Thibaut et al. 2001). In SE Australia, the sacoglossans Oxynoe viridis, Placida dendritica and Elysia cf. australis have all been observed including invasive Caulerpa spp. in their diets (Edwards 2002; authors’ personal observations). Such dietary shifts appear to be a common response of stenophagous herbivores to introduced algae that are closely related to their original hosts (Trowbridge 2004). We maintain that the utility of specialist herbivores as control agents, particularly through “augmentative biocontrol” is worthy of closer examination, but requires rigorous scientific assessment (Trowbridge 2004).
References
Agrawal AA, Kotanen PM (2003) Herbivores and the success of exotic plants: a phylogenetically controlled experiment. Ecol Lett 6:712–715
Andrew NL (1993) Spatial heterogeneity, sea urchin grazing, and habitat structure on reefs in temperate Australia. Ecology 74:292–302
Andrew NL, Jones GP (1990) Patch formation by herbivorous fish in a temperate Australian kelp forest. Oecologia 85:57–68
Ayling AL (1978) The relation of food availability and food preferences to field diet of an echinoid Evechinus chloroticus (Valenciennes). J Exp Mar Biol Ecol 33:223–235
Boudouresque CF, Lemee R, Mari X, Meinesz A (1996) The invasive algae Caulerpa taxifolia is not a suitable diet for the sea urchin Paracentrotus lividus. Aquat Bot 53:245–250
Calvert HE, Dawes CJ, Borowitzka MA (1976) Phylogenetic relationships of Caulerpa (Chlorophyta) based on comparative chloroplast ultrastructure. J Phycol 12:149–162
Carlton JT, Geller JB (1993) Ecological roulette: the global transport of nonindigenous marine organisms. Science 261:78–82
Chang GC, Kareiva P (1999) The case of indigenous generalists in biological control. In: Hawkins BA, Cornell HV (eds) Theoretical approaches to biological control. Cambridge University Press, Cambridge, pp 13–115
Clarke B, Murray J, Johnson MS (1984) The extinction of endemic species by a program of biological control. Pacif Sci 38:97–104
Conover WJ (1999) Practical nonparametric statistics. Wiley, New York
Crawley MJ (1996) Plant ecology. Blackwell, Oxford
Creese RG, Davis AR, Glasby TM (2004) Eradicating and preventing the spread of the invasive alga Caulerpa taxifolia in NSW. Final report to the Natural Heritage Trust’s coast and clean seas introduced marine pests program, project no. 35593. NSW Fisheries Final Report no. 64 (available at http://www.fisheries.nsw.gov.au/sci/outputs/aqua_sust/466_Crease.htm)
Cronin G, Hay ME (1996) Susceptibility to herbivores depends on recent history of both the plant and aninmal. Ecology 77:1531–1543
Davis AR, Roberts DE, Cummins SP (1997) Rapid invasion of a sponge-dominated deep-reef by Caulerpa scalpelliformis (Chlorophyta) in Botany Bay, New South Wales. Aust J Ecol 22:146–150
Davis AR, Fyfe SK, Turon X, Uriz MJ (2003) Size matters sometimes: wall height and the structure of subtidal benthic invertebrate assemblages in southeastern Australia and Mediterranean Spain. J Biogeogr 30:1797–1807
de Villele X, Verlaque M (1995) Changes and degradation in a Posidonia oceanica bed invaded by the introduced tropical alga Caulerpa taxifolia in the north western Mediterranean. Bot Mar 38:79–87
Dodd AP (1940) The biological campaign against prickly-pear in Australia. Commonwealth Prickly Pear Board, Brisbane
Dumay O, Pergent G, Pergent-Martini C, Amade P (2002) Variations in caulerpenyne contents in Caulerpa taxifolia and Caulerpa racemosa. J Chem Ecol 28:343–352
Edgar GJ (1997) Australian marine life: the plants and animals of temperate waters. Reed, Victoria, Australia
Edwards A (2002) Fauna associated with Caulerpa spp.; potential biological control of C. taxifolia. Honours thesis, Department of Biological Sciences, University of Wollongong, Wollongong, Australia
Fletcher WJ (1987) Interactions among subtidal Australian sea urchins, gastropods and algae: effects of experimental removals. Ecol Monogr 57:89–109
Guerriero A, Meinesz A, D’Ambrosio M, Pietra F (1992) Isolation of toxic and potentially toxic sesqui- and monoterpenes from the tropical green seaweed Caulerpa taxifolia which has invaded the region of Cap Martin and Monaco. Helv Chim Acta 75:689–695
Guerriero A, Marchetti F, D’Ambrosio M, Senesi S, Dini F, Pietra F (1993) New ecotoxicologically and biogenetically relevant terpenes of the tropical green seaweed Caulerpa taxifolia which is invading the Mediterranean. Helv Chim Acta 76:855–864
Hay ME (1984) Predictable spatial escapes from herbivory: How do these affect the evolution of herbivore resistance in tropical marine communities? Oecologia 64:396–407
Hay ME, Colburn T, Downing D (1983) Spatial and temporal patterns in herbivory on a Caribbean fringing reef: the effects on plant distribution. Oecologia 58:299–308
Jousson O, Pawlowski J, Zaninetti L, Zechman FW, Dini F, Di Guiseppe G, Woodfield R, Millar A, Meinesz A (2000) Invasive alga reaches California. Nature 408:157
Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends Ecol Evol 17:164–170
Komatsu T, Ishikawa T, Yamaguchi N, Hori Y, Ohba H (2003) But next time? Unsuccessful establishment of the Mediterranean strain of the green seaweed Caulerpa taxifolia in the Sea of Japan. Biol Invasions 5:275–278
Lemée R, Pesando D, Durand-Clément M, Dubreuil A, Meinesz A, Guerriero A, Pietra F (1993) Preliminary survey of toxicity of the green alga Caulerpa taxifolia introduced into the Mediterranean. J Appl Phycol 5:485–493
Lucas AHS (1927) Notes on the Australian marine algae. V. Proc Linn Soc NSW 52:555–562
Maron JL, Vilà M (2001) When do herbivores affect plant invasion? Evidence for the natural enemies and biotic resistance hypotheses. Oikos 95:361–373
May V (1976) Changing dominance of an algal species (Caulerpa filiformis (Suhr) Hering). Telopea 1:135–138
Meinesz A (1999) Killer algae. University of Chicago Press, Chicago
Meinesz A, de Vaugelas J, Hesse B, Mari X (1993) Spread of the introduced tropical green alga Caulerpa taxifolia in northern Mediterranean waters. J Appl Phycol 5:141–147
Meinesz A, Belsher T, Thibaut T, Antolic B, Mustapha KB, Boudouresque CF, Chiaverini D, Cinelli F, Cottalorda J-M, Djellouli A, El Abed A, Orestano C, Grau Antoni M, Ivesa L, Jaklin A, Langar H, Massuti-Pascual E, Peirano A, Tunesi L, de Vaugelas J, Zavodnik N, Zuljevic A (2001) The introduced green alga Caulerpa taxifolia continues to spread in the Mediterranean. Biol Invasions 3:201–210
Meyer KD, Paul VJ (1992) Intraplant variation in secondary metabolite concentration in three species of Caulerpa (Chlorophyta: Caulerpales) and its effects on herbivorous fishes. Mar Ecol Prog Ser 82:249–257
Paul VJ, Fenical W (1986) Chemical defense in tropical green algae, order Caulerpales. Mar Ecol Prog Ser 34:157–169
Paul VJ, Fenical W (1987) Natural products chemistry and chemical defence in tropical marine algae of the phylum Chlorophyta. In: Scheuer PJ (ed) Bioorganic marine chemistry. Springer, Berlin, pp 1–29
Paul VJ, Hay ME (1986) Seaweed susceptibility to herbivory, chemical and morphological correlates. Mar Ecol Prog Ser 33:255–264
Paul VJ, Littler MM, Littler DS, Fenical W (1987) Evidence for chemical defense in tropical green algae Caulerpa ashmeadii (Caulerpaceae: Chlorophyta): isolation of bioactive sesquiterpenoids. J Chem Ecol 13:1171–1185
Paul VJ, Cruz-Rivera E, Thacker RW (2001) Chemical mediation of macroalgal–herbivore interactions: ecological and evolutionary perspectives. In: McClintock JB, Baker BJ (eds) Marine chemical ecology. CRC Press, Boca Raton, Fla., USA, pp 227–265
Peterson CH, Renaud PE (1989) Analysis of feeding preference experiments. Oecologia 80:82–86
Piazzi L, Balestri E, Cinelli F (1994) Presence of Caulerpa racemosa in the north-western Mediterranean. Cryptogam Algol 15:183–189
Pillman A, Woolcott GW, Olsen JL, Stam WT, King RJ (1997) Inter- and intraspecific genetic variation in Caulerpa (Chlorophyta) based on nuclear rDNA ITS sequences. Eur J Phycol 32:379–386
Roa R (1992) Design and analysis of multiple-choice feeding-preference experiments. Oecologia 89:509–515
Sanderson JC (1997) Subtidal macroalgal assemblages in temperate Australian coastal waters, Australia. State of the Environment Technical Paper Series (Estuaries and the Sea), Department of the Environment, Canberra
Sant N, Delgado O, Rodriguez-Prieto C, Ballesteros E (1996) The spreading of the introduced seaweed Caulerpa taxifolia (Vahl) C. Agardh in the Mediterranean Sea: testing the boat transportation hypothesis. Bot Mar 39:427–430
Secord D (2003) Biological control of marine invasive species: cautionary tales and land-based lessons. Biol Invasions 5:117–131
Smith CM, Walters LJ (1999) Fragmentation as a strategy for Caulerpa species: fates of fragments and implications for management of an invasive weed. Mar Ecol 2:307–319
Steinberg PD (1988) Effects of quantitative and qualitative variation in phenolic compounds of feeding in three species of marine invertebrate herbivores. J Exp Mar Biol Ecol 120:221–237
Steinberg PD, van Altena I (1992) Tolerance of marine invertebrate herbivores to brown algal phlorotannins in temperate Australasia. Ecol Monogr 62:189–222
Thibaut T, Meinesz A (2000) Are the Mediterranean ascoglossan molluscs Oxynoe olivacea and Lobiger serradifalci suitable agents for a biological control against the invading tropical alga Caulerpa taxifolia? C R Acad Sci Ser III Life Sci 323:477–488
Thibaut T, Meinesz A, Amade P, Charrier S, DeAngelis K, Ierardi S, Mangialajo L, Melneck J, Vidal V (2001) Elysia subornata (Mollusca) a potential control agent of the alga Caulerpa taxifolia (Chlorophyta) in the Mediterranean Sea. J Mar Biol Assoc UK 81:497–504
Trowbridge CD (1995) Establishment of the green alga Codium fragile ssp. tomentosoides on New Zealand rocky shores: current distribution and invertebrate grazers. J Ecol 83:949–965
Trowbridge CD (2004) Emerging associations on marine rocky shores: specialist herbivores on introduced macroalgae. J Anim Ecol 73:294–308
Trowbridge CD, Todd CD (2001) Host–plant change in marine specialist herbivores: ascoglossan sea slugs on introduced macroalgae. Ecol Monogr 71:219–243
Van Driesche RG, Bellows Jr TS (1996) Biological control. Chapman and Hall, New York
Williamson M (1996) Biological invasions. Chapman and Hall, New York
Worthington DG, Fairweather PG (1989) Shelter and food interactions between Turbo undulatum (Archaeogastropoda, Turbinidae) and coralline algae on rocky seashores in New South Wales, Australia. J Exp Mar Biol Ecol 129:61–80
Wright JT, Benkendorff K, Davis AR (1997) Habitat associated differences in temperate sponge assemblages: the importance of chemical defence. J Exp Mar Biol Ecol 213:199–213
Wright JT, de Nys R, Steinberg PD (2000) Geographic variation in halogenated furanones from the red alga Delisea pulchra and associated herbivores and epiphytes. Mar Ecol Prog Ser 207:227–241
Zar JH (1999) Biostatistical analysis, 4th edn. Prentice Hall, Engelwood Cliffs, N.J., USA
Zuljevic A, Thibaut T, Elloukal H, Meinesz A (2001) Sea slug disperses the invasive Caulerpa taxifolia. J Mar Biol Assoc UK 81:343–344
Acknowledgements
We acknowledge the assistance of D. Barker at NSW Fisheries for allowing access to their seawater facility. S. Fyfe and J. Wright improved early drafts of this manuscript. All algae and invertebrates for this study were collected under NSW Fisheries permit F265. This work was undertaken with financial assistance from the Institute for Conservation Biology, University of Wollongong. This is contribution number 252 from the Ecology and Genetics Group, University of Wollongong.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by M.S. Johnson, Crawley
Rights and permissions
About this article
Cite this article
Davis, A.R., Benkendorff, K. & Ward, D.W. Responses of common SE Australian herbivores to three suspected invasive Caulerpa spp.. Marine Biology 146, 859–868 (2005). https://doi.org/10.1007/s00227-004-1499-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1499-z