Abstract
The structure, diversity and temporal distribution of the infaunal polychaetes associated with Cymodocea nodosa meadows were studied in Tenerife (Canary Islands). The samples were collected monthly throughout a year, to depths of 13–16 m. The sediment was extracted by means of PVC cores, in which four layers were separated (i.e. 0–5 cm, 5–10 cm, 10–20 cm and 20–30 cm). A total of 1,167 polychaete specimens, belonging to 69 taxa were collected, representing one of the most dominant groups in the benthic assemblage throughout the entire year. The most common families were Syllidae, Paraonidae and Spionidae, both in terms of abundance and species richness. The dominant species were Streptosyllis bidentata, Aricidea assimilis and Exogone parahomoseta mediterranea, representing also the only constant species throughout the year. The highest values of species richness, diversity, equitability and abundance of polychaetes occurred in September. The multifactorial analysis of abundances (i.e. cluster analysis and non-metric, multi-dimensional scaling) indicated temporal segregation of the samples from July, August and September (i.e. the warmest months) with respect to those from the rest of the year, due to structural differences in the assemblage. Polychaete species have been found to a depth of up to 30 cm in the sediment. Nevertheless, most of them (89%) occurred in the upper 5 cm of the sediment, with an increase of specimens in deeper layers in February (i.e. due to sporadic episodes of higher hydrodynamics). To compare the vertical distribution of polychaetes, additional core samples were collected in two seagrass meadows (i.e. C. nodosa and Ruppia cirrhosa) at Ebro’s Delta (NW Mediterranean); these were separated into five layers (i.e. 0–5 cm, 5–10 cm, 10–15 cm, 15–20 cm, 20–25 cm). The results obtained for the R. cirrhosa meadow (98% of the polychaetes within the upper 5 cm) agree with those for the Canarian C. nodosa meadow, while the polychaetes reached up to 15 cm depth in the Mediterranean C. nodosa meadow (i.e. ~39% between 0 and 5 cm, ~41% between 5 and 10 cm, ~20% between 10 and 15 cm). Our results indicated that the structural characteristics of the assemblages appeared to be more strongly controlled by the combined characteristics of the sediment (i.e. lack of oxygen, granulometry and degree of compaction) than by the seagrass species building the meadow.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most dominant and widely distributed seagrass in the Canary Islands is Cymodocea nodosa (Ucria) Ascherson. The species grows on sandy sublittoral bottoms, forming dense meadows (Afonso-Carrillo and Gil-Rodríguez 1980; Reyes 1993); these can be monospecific or constitute mixed populations with the green algae Caulerpa prolifera (Forskål) Lamouroux and C. racemosa (Forskål) Agardh, which replace C. nodosa on deeper bottoms and, sometimes, at sites affected by human activities. C. nodosa occurs both in protected and semi-exposed areas along the southeastern and southwestern coasts of almost all of the islands, from intertidal pools at sheltered sites to 35–40 m in depth. C. nodosa is considered a pioneer species in the colonization of soft substrata, being able to form meadows in the absence of other accompanying species (Molinier and Picard 1952). Studies on C. nodosa from the Canary Islands show that its biological cycle is very similar to that observed in the Mediterranean. However, the formation of fruit is earlier and the density of flowers and the number of fruit per square meter is higher in the Canary Islands (Reyes 1993).
Polychaete populations have been considered to be excellent descriptors of the faunal communities associated with seagrasses, due to the great diversification of species and life habits (Hutchings 1982; Somaschini et al. 1994), including small-sized species such as C. nodosa (Lanera and Gambi 1993; Gambi et al. 1998). In the Canary Islands, there have been several studies dealing with the taxonomy of polychaetes harbored in C. nodosa meadows (Brito et al. 2000a, 2000b, 2001; Brito and Núñez 2002). Nevertheless, little is known of the ecology and structure of the associated assemblages. In other geographic areas, numerous studies have been carried out concerning this topic, mainly on the polychaetes associated to Posidonia oceanica (L.) Delile (Colognola et al. 1984; Alós 1988; Somaschini et al. 1994; Gambi et al. 1995). All studies concur on the high diversity and species richness of these ecosystems (Young and Young 1977; Kikuchi 1980; Howard et al. 1989). Among the studies on small-sized seagrass beds [like C. nodosa or Ruppia cirrhosa Petagna (Grande)], those carried out in the Mediterranean are of special relevance for comparison with the present study (True-Schlenz 1965; Giangrande and Gambi 1986; Palacín et al. 1991; Lanera and Gambi 1993; Martin et al. 1993, 2000; Gambi et al. 1995, 1998).
Analysis of the benthos is very important to facilitate future monitoring studies of C. nodosa meadows, since benthic organisms are among the first responding to the stress caused by organic enrichment (Bilyard 1987). The variability of the distribution patterns of these organisms is an excellent tool allowing determination of the vulnerability to disturbance (Rizzo and Amaral 2000). Thus, the present study focused on polychaete taxocoenosis, which plays the most relevant role in structuring the assemblage harbored by C. nodosa. Temporal variability and vertical distribution in the sediment at a fixed station on Tenerife (Canary Islands) were analyzed. Furthermore, it was possible to compare the vertical distribution of the Canarian assemblages with some additional data obtained from the Bay of Els Alfacs (Ebro’s Delta, NW Mediterranean) for the infauna associated with C. nodosa and R. cirrhosa. The results were also compared with those obtained by other authors for C. nodosa and Zostera noltii Mediterranean meadows.
Materials and methods
Study sites
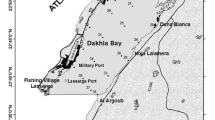
The present study was mainly carried out in the Ensenada de los Abades, southeast of Tenerife (Canary Islands) (Fig. 1a). The northern cove limits are defined by the volcano Punta de los Abades, which has partially been destroyed by marine abrasion, while several abrupt cliffs form the southern limits. The beach, located between two points (Los Abades and Los Jureles), is mainly formed by boulders and Holocene sand.
The water temperature during the study period ranged between 18.5°C in winter and 22.5°C in summer. This southeastern coastal region of Tenerife is protected leeward from the influence of the Canarian Current and the NE trade winds. These zones of the island allow the development of extensive Cymodocea nodosa meadows. Populations of Lobophora variegata, Cystoseira abies-marina, C. humilis, Sargassum vulgare and Asparagopsis armata form the shallow-water infralittoral photophylic algae of the beach, while more sciaphylic zones are inhabited by Codium intertextum. Down to 3 m depth, the bottoms are occupied by the community of “blanquizal”; these areas are characterized by the presence of bare rocky surface without seaweeds, due to the intense grazing of the sea urchin Diadema antillarum. Sandy bottoms start at 12 m depth with an assemblage characterized by the presence of the gardener-eel Heteroconger longissimus, and the C. nodosa meadow starts at 13 m depth. Although some C. nodosa meadows may have a system of dense interlacing rhizomes and roots, called “turf” (Buia et al. 1985; Terlizzi and Russo 1998), the meadows in Ensenada de los Abades are very sparse, due to the strong currents characterizing the hydrodynamics of the zone.
Additional samples to analyze the vertical distribution were obtained in the Bay of Els Alfacs, a semi-enclosed, shallow-water area located in Ebro’s Delta on the Catalan coasts of the NW Mediterranean (Fig. 1b). Els Alfacs is the southernmost bay of the delta. It has an area of about 50 km2, a mouth of 3 km connecting it to the sea and an average depth of 4 m (max.=6 m). The bay has a deeper central area and a littoral shelf 0–2 m deep. The bay is mainly characterized by a marine hydrographical regime, but is also influenced by freshwater inputs occurring during spring and summer (Palacín et al. 1991). In the absence of strong winds, there is still an exchange between the bay and the open sea, following either a periodicity of ca. 10 days or through typical estuarine circulation (Camp et al. 1991). As a result, the organic inputs coming from freshwater (i.e. about 62 mg C m−2 day−1) mainly affected the northern sections of the littoral shelf, where the Ruppia cirrhosa meadows were located, but they scarcely reached the southern sections of the shelf, where the C. nodosa meadows occurred, before they flowed out of the bay towards the open sea (Palacín et al. 1991; Martin et al. 1993, 2000).
Field sampling and sediment analyses
In the Ensenada de los Abades, the samples were collected from 13 to 16 m depth from January to December 1994 by direct sampling. We used PVC corers of 4.5 cm inner diameter, which were pushed into the substrate to 30 cm in depth to provide sediment volumes of 450 cm3 per corer. The habitual depth in studies dealing with vertical distribution of infauna based on manual sampling oscillates between 6 cm for a muddy beach (Cruz and Vargas 1987) and 40 cm for the infauna of other environments (Bloom et al. 1972). However, this last depth seems to be excessive, and the one used most is 20 cm (Whitlatch 1977). Therefore, to prevent possible loss of information, we used 30-cm depth corers, because the high hydrodynamics linked to the island environment favors increased oxygenation of the sediment, thus resulting in a deeper anoxic layer and potentially increased depth penetration of the infauna. A similar rationale formed the basis for collecting samples only up to 25 cm into the sediment in the Bay of Els Alfacs.
In the Ensenada de los Abades, five core replicates were obtained monthly. One of them was used to determine the granulometry and the structural and chemical variables of the sediment. All 48 samples dedicated to the faunal study were subdivided into four layers (i.e. 0–5 cm, 5–10 cm, 10–20 cm, 20–30 cm), to analyze the vertical distribution of polychaetes. The samples were fixed with a 4% formaldehyde/seawater mixture prior to sieving though a 100-µm-pore-size mesh. The polychaetes were separated from the rest of the fauna, including all larval, juvenile and adult stages, then preserved in 70% ethanol. In the Bay of Els Alfacs, four replicates were obtained from each seagrass meadow in early summer. All of them were subdivided into five layers (i.e. 0–5 cm, 5–10 cm, 10–15 cm, 15–20 cm, 20–25 cm) prior to being fixed with a 4% formaldehyde/seawater mixture. All infaunal organisms were sorted and preserved in 70% ethanol.
In the Ensenada de los Abades, the granulometry of the sediment was obtained from subsamples of 100 g. The sediment was first dried at ambient temperature and then sieved through a graded series of sieves following the Wentworth procedure (Buchanan and Kain 1971; Buchanan 1984). The percentage of organic matter was estimated according to the method by Walkley (1947; adapted and modified by Jackson 1960). The carbonate content was estimated according to the method by Allison and Moodie (1965), and the analysis of nitrogen followed the Kjeldahl method (Marr et al. 1990).
The analytical methods employed to define the sediment variables of the two stations in the Bay of Els Alfacs have been fully described in Palacín et al. (1991) and Martín et al. (1993, 2000).
Statistical analyses
In the Ensenada de los Abades, the structure of the polychaete assemblage was analyzed by means of PRIMER 5.2.2 (PRIMER-2000) software (Clarke and Warwick 1994) using two multivariate methods, i.e. cluster analysis and non-metric, multi-dimensional scaling (MDS). Cluster analysis was based on the Bray–Curtis similarity index (Bray and Curtis 1957) and used the group average group method. The similarity matrix was based on fourth-root transformed average abundances. No reductions in species number were applied. Sample segregation was perfectly explained by a two-dimensional MDS ordination. The ANOSIM routine (Clarke 1993; Clarke and Warwick 1994) based on replicate abundances was used to analyze the temporal variability. The polychaete species responsible for the observed patterns were identified by means of the SIMPER routine (Clarke and Warwick 1994). The indices of species diversity (H’, Shannon and Weaver 1949), evenness (J, Pielou 1969) and Simpson dominance were calculated. Soyer’s dominance and frequency indexes were calculated, and the species were classified as dominant (D, dominance ≥1%) or non-dominant (d, dominance <1%), and as constant (C, frequency ≥50%), accessory (A, 25% <frequency <50%), or accidental (a, frequency ≤ 25%) (Soyer 1970).
The temporal trends shown by the biological descriptors of the polychaete assemblages from the Ensenada de los Abades, and their differences when grouped according to the results of the multivariate analysis, were analyzed by means of parametrical one-way analysis of variance (ANOVA), and the differences in vertical distribution in the different meadows were analyzed by two-way ANOVA. To compare the Canarian and Catalan meadows, sediment layers were grouped into three depth ranges (i.e. 0–5 cm, 5–10 cm and the remaining layers pooled all together). Post hoc multiple comparisons were carried out by means of Tukey’s honestly significance difference test (Tukey HSD) (Zar 1984). The data were either ranked (temporal trends and groups) or square-root transformed (vertical distribution) to meet the assumptions of normality and homoscedasticity required for parametrical analyses (Zar 1984).
Results
Abiotic characteristics of sediments
The granulometry of the sediment inside the Cymodocea nodosa meadows is characterized by the presence of fine sand in the upper 20 cm and medium-sized sand from 20 to 30 cm deep. The most abundant grain size fractions are fine sands (42.4%) and medium-sized sand (31.5%). The very fine and fine fractions decrease with depth in the sediment, while coarse fractions increase. The median range is between Q50=0.25–0.21 (fine sand) from 0 to 20 cm and Q50=0.33–0.43 (medium-sized sand) from 20 to 30 cm. The sorting is moderate, S0=1.77–1.58, with a low pelitic content (0.73%), as is usual in open zones with high hydrodynamics.
The organic matter content is low, as is common in oligotrophic oceanic waters, ranging between 0.54% and 0.16%. The carbonate content is also quite low, with an average of 4.14%, decreasing with depth into the sediment. Nitrogen content is low too, ranging from 0.03% in the superficial layer to 0.02% in the deepest layer. These values are similar to those obtained in other areas of the Canary Islands, which are characterized by clean waters, with very little terrigenous runoff and far away from urban areas.
The sandy areas from the study site show differences with respect to the meadow sediments. The granulometry is characterized by coarse sand in the upper 10 cm and medium-sized sand from 10 to 30 cm deep. The sorting is poor (S0=1.95). The organic matter reaches much lower average values (0.03%), as do the pelites (0.29%), carbonates (2.60%) and nitrogen (0.02%). These results demonstrate that, by reducing flow, the C. nodosa meadows allow the settlement of fine particles of sediment and favor the increase of organic matter inside and surrounding the meadows.
The particular morphology and water circulation in the Bay of Els Alfacs generates two different environmental conditions at the two study areas. Consequently, the Ruppia cirrhosa meadows are characterized by sediments with low Redox potential (less than −200 mE), high silt contents (23–37%) and relatively high organic contents (1.3–6.3%), while the C. nodosa meadows show sediments with high Redox potential (−8.3 to 16.5 mE), low silt contents (0.05–0.06%) and relatively low organic contents (0.6–0.7%) (Palacín et al. 1991; Martin et al. 1993, 2000).
Systematics and faunistics
From a total of 3,985 specimens collected in the sediments associated with C. nodosa throughout the year, 21 higher taxa were identified: Amphipoda, Anthozoa, Copepoda, Cumacea, Decapoda, Gastropoda, Gastrotricha, Halacaridae, Isopoda, Kinorhyncha, Nematoda, Nemertina, Oligochaeta, Ophiuroidea, Ostracoda, Polychaeta, Pycnogonida, Rhizopoda, Sipuncula, Tanaidacea and Turbellaria. Among them, nematodes and polychaetes were dominant, including 34.8% and 29.9% of the abundance and 31.3% and 23.7% of the species richness, respectively.
A total of 1,167 polychaete specimens (including larval phases, juveniles and adults) belonging to 24 families were collected. Of these, 69 taxa have been identified, 57 at species level, 3 at genus level and 9 forms of larval phases and juveniles. Syllidae and Paraonidae were the most abundant families throughout the year. Syllidae was also the richest family, followed by Spionidae, Paraonidae and Opheliidae. The assemblage showed a high diversity, with 16 dominant species. Among them, the most abundant were Streptosyllis bidentata, Aricidea assimilis, Exogone parahomoseta mediterranea and Cirrophorus perdidoensis (Table 1). Especially interesting is the high number of Streptosyllis spp. juveniles, which made this genus dominant in the assemblage. Their abundances clearly indicate a high reproductive potential of the species of this genus throughout the year. As for the frequency, three species were constant (A. assimilis, S. bidentata and E. parahomoseta mediterranea) and eight species were accessory (C. perdidoensis, Streptosyllis campoyi, Periquesta canariensis, Exogone breviantennata, Streptosyllis websteri, Capitomastus minimus, Streptosyllis templadoi and Exogone verugera).
Multivariate analyses
The structure of the polychaete assemblage throughout the year showed a very uniform pattern, with few differences between the groups of monthly samples (40% of similarity). Cluster analysis corresponding to the 12 months of the year (averaged means) produced a dendrogram with four clear groups (Fig. 2a), which were also revealed by the MDS (Fig. 2b). The samples clustered in group I (58% of similarity) corresponded to the colder months of the year, while those clustered in group II (55% similarity) corresponded to the three summer months. Group III corresponded to samples from a single month (June), while samples from February and May were included in group IV, which cannot be considered a strong cluster because of the linkage of the 2 months under a very low level of similarity (48%).
The ANOSIM gave rise to a Global R of 0.221 (number of used permutations=20,000; number of permutations equal or superior to a Global R of 35; significance level=0.2%). The population structure of group I significantly differed from that of group II and did not differ significantly from that of group IV (significance level of 17.4% and 12.2%, respectively, for February and May), while group II differed significantly from group IV (significance level of 0.3%). In turn, groups I and II differed significantly from group III (significance level of 3.9% and 0.7%, respectively) and, although less robust due to the low sample number and number of permutations used (i.e. 35), significant differences also occurred between groups III and IV.
In agreement with the rather uniform pattern shown by the structure of the polychaete assemblage throughout the year, a few significant differences were found between the groups identified in the multivariate analyses when analyzing the main biological descriptors of the assemblage (Fig. 3). In fact, only the density and the evenness showed significant differences (F=3.23, P<0.03 and F=4.52, P<0.007, respectively). The Tukey test indicated that group III (P<0.04) and group II (P<0.02) were the main contributors to the observed differences.
The species with the greatest similarity among all samples studied were A. assimilis, S. bidentata, E. parahomoseta mediterranea, C. perdidoensis, P. canariensis and E. verugera. The greatest dissimilarity between groups I and II was caused by S. bidentata, C. perdidoensis, A. assimilis and E. verugera (more abundant in the former than in the latter group) and by P. canariensis and E. parahomoseta mediterranea (more abundant in the latter group) (Table 2). A. assimilis was the species mainly responsible for the differences between groups II and IV. S. bidentata was both the second most important contributor to the similarity throughout the year (except for group II) and the major species responsible for the dissimilarity between group I and groups II and IV. E. parahomoseta mediterranea was the major contributor to the dissimilarity between both groups I and IV and groups II and III. C. perdidoensis, P. canariensis and E. verugera caused great dissimilarity between group I, group II and group IV and the respective remaining groups.
The samples from group I showed an average similarity of 33.8% and were characterized by S. bidentata, A. assimilis, E. parahomoseta mediterranea, C. perdidoensis, C. minimus and E. breviantennata (81.6% of the similarity), with the first two being the most abundant and P. canariensis showing a remarkably low abundance (Table 2). Samples from group II showed an average of 31.8% similarity and were characterized by E. parahomoseta mediterranea, P. canariensis, C. perdidoensis, S. campoyi, S. bidentata, A. assimilis and S. templadoi (90.7% of the similarity), the first two being the most abundant (Table 2). Among the characteristic species of group IV, only three (i.e. A. assimilis, S. bidentata and E. verugera) coincided in the two sampling months. February and May were, respectively, characterized by C. minimus, S. websteri, Grubeosyllis clavata and by Microspio mecznikovianus, larvae of Opheliidae and Schroederella laubieri, while P. canariensis again showed a very low abundance in all samples (Table 2). The most characteristic species of group III (June) were A. assimilis, S. bidentata, S. laubieri, Spiochaetopterus costarum, E. verugera, Armandia intermedia and S. templadoi, while the structure of their polychaete assemblages appeared as a transition between the colder (groups I and IV) and warmer periods (groups II).
The highest average dissimilarity occurred between groups III and II (78.4%), with the major species responsible being E. parahomoseta mediterranea (less abundant in group III and more abundant in group II), P. canariensis and C. perdidoensis (present in group II and absent in group III) and S. laubieri (very abundant in group III and scarce in group II). The average dissimilarity between groups I and III was also high (74.0%), with the major contributors being S. laubieri (absent in group I), C. perdidoensis (absent in group III) and E. parahomoseta mediterranea and S. bidentata (highly abundant in group I).
Univariate measurements of community structure
The temporal patterns of the main variables describing the structure of the polychaete assemblages tended to be both rather uniform over the entire year and to have high within-replicate variability, thus making it difficult to assess the significance of the observed trends. In fact, only abundance, evenness and dominance showed significant differences (Table 3).
The maximum polychaete densities occurred in September, November and December (461.5, 451.8 and 438.8 ind. m−2, respectively), with secondary maxima in March and April (380.3 and 338.0 ind. m−2, respectively) and minimum values in May and June (139.8 and 175.5 ind. m−2, respectively) (Fig. 4a). The main factor responsible for the significant differences in the ANOVA (Table 3) was the high density occurring in September (Tukey test, P<0.05).
Annual trends of mean values of the biological descriptors of the polychaete assemblages from the Ensenada de los Abades: a density (ind. m−2), b number of species per sample, c Margalef’s species richness, d Shannon–Weaver diversity, e Pielou’s evenness and f Simpson’s dominance. Error bars: standard deviations
The number of species per sample showed an annual trend, which was more uniform than that of density (Fig. 4b), with a maximum in September (11.0 species) and minima in May and July (5.5 and 6.0 species, respectively). The global average of the number of species per month was 7.6. Margalef species richness was also uniform throughout the year, the highest and lowest values being 2.99 and 1.59 in September and July, respectively (Fig. 4c).
The Shannon–Weaver diversity index was relatively constant throughout the year, with the highest and lowest values being 2.11 (September) and 1.37 (December) and 1.42 (July) (Fig. 4d). Unusually low values of the Pielou’s evenness index were observed in one sample during December, due to the high number of individuals of C. perdidoensis and E. parahomoseta mediterranea. Nevertheless, this index showed uniform values throughout the year, ranging between 0.79 (April and August) and 0.94 (May and June) (Fig. 4e), with the main factor responsible for the observed differences being the low evenness in August and the high evenness in May/June (Tukey test, P<0.02).
Values of the Simpson dominance index were very low (Fig. 4f), as can be inferred from the high evenness registered throughout the year. Highest dominance values occurred in May (0.34), with only 43 specimens and 11 species being collected. A secondary dominance maximum occurred in July (0.3), when only 67 specimens and 12 species were found. The lowest dominance values occurred in September and December (0.18), when high density, species richness and diversity were observed. In this case, the main contributor to the observed differences was the low dominance in September and December (Tukey test, P<0.05).
Vertical distribution
In the Ensenada de los Abades, most polychaete specimens inhabited the most superficial layer of sediment, between 0 and 5 cm depth (89% of specimens), which represented about 30% of the total infauna found in this layer. A value of 9% occurred between 5 and 10 cm depth, 1.3% between 10 and 20 cm depth and the remaining 0.7% between 20 and 30 cm depth, which represented from about 30% to somewhat <20% of the total infauna (Fig. 5). Not all polychaete species were able to inhabit the deeper layers of sediment. Several species of Paraonidae, Syllidae, Capitellidae and Questidae were the most frequent: A. assimilis, S. bidentata, P. canariensis, C. minimus, C. perdidoensis, Cirrophorus armatus and E. breviantennata (between 5 and 10 cm depth); P. canariensis, A. assimilis, S. bidentata, E. verugera, S. campoyi, C. perdidoensis, C. armatus and C. minimus (between 10 and 20 cm depth); and Syllides japonicus, Grubeosyllis vieitezi and Polyophtalmus pictus (between 20 and 30 cm depth) (Table 4).
The maximum abundance in the deep layers of sediment (20–30 cm) occurred in February (33.3%), with 7.4% of the total polychaetes. A secondary maximum occurred in August, with 17.7% of the specimens in the layer between 5 and 20 cm depth. Both maximum abundances in deep sediment layers coincided with storms in the study zone. Thus, these particular environmental conditions (closely related to episodes of strong hydrodynamics) probably forced some species to migrate into deeper sediment layers. In contrast, no relevant differences were observed when analyzing the vertical distribution of the four groups obtained in the multivariate analyses, with the average percentage of density between 5 and 30 cm depth ranging between 10% and 12%.
In the Bay of Els Alfacs, the most evident result when observing the vertical distribution of polychaetes (Table 5; Fig. 5) was the absence of organisms below 15 cm depth; thus, the two corresponding layers (i.e. 15–20 cm and 20–25 cm) were not taken into account in analyses. The total number of polychaete individuals and species found in the assemblages was 5,400 and 76,700 ind. m−2 and 14 and 7 species in C. nodosa and R. cirrhosa meadows, respectively. In terms of abundance, the polychaetes represented about 30% and 64% of the total infauna of the C. nodosa and R. cirrhosa meadows, respectively.
When comparing the vertical distribution in the C. nodosa meadow from the Ensenada de los Abades with those of the C. nodosa and R. cirrhosa meadows in the Bay of Els Alfacs (Fig. 5), the differences in number of species, abundance and diversity of polychaetes were always significant for the two analyzed factors (i.e. meadow and sediment layer) used in the two-way ANOVA (Table 6). However, there were also significant combined effects of both factors, thus resulting in different interpretations of the obtained results.
In terms of number of species and diversity, the main factor responsible for the differences was the 0–5 cm sediment layer in the Ensenada de los Abades (Tukey test, P<0.0001 and P<0.001, respectively), in which both values were significantly higher than those for the remaining sediment layers at the same locality as well as those for all meadows and layers in the Bay of Els Alfacs (Fig. 5). Additionally, a Tukey test also revealed significant differences in number of species and diversity between the 5–10 cm and the remaining sediment layers in the Ensenada de los Abades. There was, however, a divergence in the results of the Tukey test for the number of species with respect to that based on diversity, as the differences between the 0–5 cm layer in the two C. nodosa meadows differed in terms of species number (being higher in the Canarian assemblage), but did not differ in terms of diversity.
As for the number of individuals, the Tukey test revealed that the 0–5 cm layers from the Ensenada de los Abades and from the R. cirrhosa meadow in the Bay of Els Alfacs (Tukey test, P<0.0001 and P<0.0003, respectively) were responsible for the observed variation among the two locations (highest abundances in the R. cirrhosa meadow and intermediate in the Canarian C. nodosa meadow) and for the difference to all other sediment layers from the two localities (with clearly low abundances) (Fig. 5).
In summary, the differences between the three studied meadows may be attributable to a combination of the two distinct factors. The local differences in the species pool when comparing the Ensenada de los Abades with the Bay of Els Alfacs and the differences in the structure of the C. nodosa and R. cirrhosa meadows as habitats for the polychaete assemblage, particularly because the later was mainly inhabited by small, highly abundant opportunistic species, which usually characterize highly disturbed environments.
Discussion
The structure of the polychaete assemblage inhabiting the Cymodocea nodosa meadows in the Ensenada de los Abades (Tenerife) was rather uniform throughout the year. Most of the polychaete fauna corresponded to interstitial forms typical of soft bottoms, particularly belonging to the families Syllidae, Paraonidae and Spionidae. The Syllidae family was the most common of the assemblage, with a higher abundance and species richness during the colder months. The most representative species all year round belonged to the subfamilies Eusyllinae, genus Streptosyllis (S. bidentata, S. campoyi, S. websteri, S. templadoi), and Exogoninae, genus Exogone (E. parahomoseta mediterranea, E. breviantennata, E. verugera). The Paraonidae were represented mainly by Aricidea assimilis and Cirrophorus perdidoensis. These species also showed maxima in abundance during the colder months and decreased remarkably during the warmer ones, while the species richness was similar over the entire year. The Spionidae were represented by six species throughout the year, although none of them was clearly dominant in the assemblage. The Capitellidae were more frequent during winter, Capitomastus minimus, a frequent species on soft bottoms covered with vegetation, being the most abundant species.
The families Syllidae, Capitellidae and Spionidae (Gambi et al. 1998), together with the Paraonidae (Giangrande and Gambi 1986; Lanera and Gambi 1993), have also been considered the most abundant taxa among the polychaete fauna from the foliar and rhizome layers of C. nodosa and Zostera noltii meadows in the Mediterranean. Nevertheless, there are some differences in the dominant species between the assemblages of the Canary Islands and those of the Mediterranean. In the Canary Islands, four species of the genus Streptosyllis are very important, while, in the Mediterranean Sea, only S. websteri was present and fairly scarce in the assemblage (Gambi et al. 1998). The Nereididae is one of the most abundant families in Mediterranean C. nodosa meadows, almost exclusively represented by Neanthes caudata (Gambi et al. 1998). This species, together with Platynereis dumerilii, is also very abundant in different Australian seagrass systems (Hutchings 1982). Conversely, this family has no relevance in the Canarian assemblage, while the Questidae, represented by the interstitial species Periquesta canariensis (which is absent in the Mediterranean) is very important within the C. nodosa meadows.
The Canarian assemblage is characterized by a high abundance of larvae and juveniles from a variety of species, belonging either to the macrofauna or to the meiofauna. Again, the larvae of Streptosyllis spp. must be pointed out; these larvae are present in all monthly samples, which indicates the high reproductive potential of the species of this genus in this particular habitat throughout the year. Many other larval and juvenile stages belonging to numerous families have also been found. Among them (ordered according to their abundance) we should mention the larvae of Paraonidae and Ophelidae, the juveniles of Parapionosyllis (Syllidae), the larvae of Chaetopteridae, Maldanidae and Spionidae and the juveniles of Sabellidae and Syllides (Syllidae). Certainly, the use of fine sieves in the present study may contribute to explain the remarkably high presence of larvae, stressing the relevance of studying the different size ranges of infauna to obtain a more complete picture of the temporal trends.
The multivariate analyses point out the existence of temporal differences in the polychaete assemblage from C. nodosa beds, with two main periods being recognized throughout the year: a longer cold period (i.e. January, February, March, April, May, October, November and December) and a shorter warm period (July, August and September). On the other hand, the assemblage found in June differs from both the assemblages found in summer and in winter. As a consequence, it has been considered a transition assemblage, which is characterized by a high abundance of Schroederella laubieri, the absence of C. minimus and the presence of Spiochaetopterus costarum. This pattern, with only two clearly distinguishable seasonal periods, does not agree with the results obtained in some earlier studies of both Mediterranean (Formentera, Balearic Islands) and Atlantic (Ria de Ferrol, Galicia) polychaete communities, where four seasonal periods were identified (Besteiro et al. 1990; Soler et al. 1997). However, when considering whole assemblages (including non-vegetated systems, but also C. nodosa and Ruppia cirrhosa meadows), a common seasonal pattern has been proposed for the NW Mediterranean mediolittoral macroinfauna, which is generally characterized a high-abundance (mid-autumn to late spring) and a low-abundance (early summer to early autumn) season (Sardá et al. 1995). Conversely, sublittoral macroinfaunal assemblages differ in having fairly constant annual densities, with maxima from early spring to early summer (Sardá et al. 1995). In both cases, however, the seasonal patterns seem to be connected mainly to the reproductive activities of the respective species (Sardá et al. 1995).
The major faunistic descriptors (i.e. density, species richness and diversity) generally maintain considerable uniformity throughout the year. The month with the highest density, richness and diversity is September, while the lowest density values occur in May and June and those of richness and diversity in July. Accordingly, the existence of low richness and diversity values during July was also registered in the Mediterranean polychaete assemblages associated with C. nodosa (Gambi et al. 1998).
Most of the species inhabiting rhizomes are also typical of the soft bottoms that occur in the nearby sand (Lanera and Gambi 1993). Nevertheless, the presence of a meadow increases the fine particles and organic matter in the sediment, thus allowing it to maintain a quite homogenous and stable assemblage throughout the year. Furthermore, density, species richness and diversity are usually much higher in the meadows than in the adjacent sandy zones. Some species inhabiting the assemblage (e.g. C. perdidoensis, Parapionosyllis labronica, S. laubieri and S. costarum) tend to show a patchy distribution, as indicated by the high variability and standard deviation between replicates.
The results of the study of vertical distribution indicate that the polychaete assemblage of the C. nodosa meadows in the Ensenada de los Abades inhabits the sediments to depths of at least 30 cm, although nearly 90% of the assemblage occurs within the upper 5 cm. In the Bay of Els Alfacs, the polychaetes reached depths of 15 cm, both in the C. nodosa and R. cirrhosa meadows. However, they were more uniformly distributed in the former (39% in the upper 5 cm, 41% between 5 and 10 cm and 20% between 10 and 15 cm) than in the latter (>90% within the upper 5 cm). In the Canarian C. nodosa assemblage, the most frequent species inhabiting deep layers of the sediment were: C. perdidoensis, A. assimilis, C. minimus, Cirrophorus armatus, P. canariensis and S. bidentata, while, in the Mediterranean, they were Malacoceros fuliginosus, Mastobranchus trinchesi and Cirroforus furcatus. Conversely, the infauna from the R. cirrhosa meadows was dominated by capitellids (i.e. Capitella spp.) and, particularly, by spionids (i.e. Streblospio shrubsolii). In the Canarian assemblage, no remarkable seasonal differences in abundance of polychaetes at the different depth layers have been observed, although an increase in the number of specimens in the deep layers during some sporadic periods with high hydrodynamics has been observed.
In environments characterized by silty-sand sediments, the fauna tends to inhabit mainly the upper 2 cm of sediment (Cruz and Vargas 1987), largely because of the lack of oxygen and the degree of compaction. Similar results have been obtained for the Mediterranean polychaete fauna, where 84% inhabited the upper 5 cm of the sediment in C. nodosa meadows (Gambi et al. 1998), and the upper 4 cm, in Posidonia oceanica meadows (Willsie 1983; Somaschini et al. 1994). The results obtained in the R. cirrhosa meadow of the Bay of Els Alfacs confirmed this trend. As the sediments are highly compacted, have high organic matter and fine sediment contents, and low Redox potential, the associated infauna is mainly composed of small, numerous opportunistic species such as Capitella spp. and S. shrubsolii (Palacín et al. 1991; Martin et al. 1993, 2000). Conversely, the C. nodosa meadows from the Bay of Els Alfacs clearly differ, as the polychaetes were more-or-less uniformly distributed along the upper 15 cm of sediment. This was likely connected to the low fine sediment contents, the high oxygenation and the low compactness of the sediments and, thus, to a polychaete assemblage dominated by species characteristic of biologically controlled assemblages (sensu Sanders 1969), e.g. those of the genera Mastobranchus, Phylo, or Neanthes (Palacín et al. 1991; Martin et al. 1993, 2000).
C. nodosa and R. cirrhosa do not show a high degree of architectural complexity within the sediment, in contrast, for instance, with Posidonia oceanica, which usually modifies the structure of the bottom so that the type of sediment has a less important influence on the associated assemblages than the plant itself. In contrast, our results support that, besides the biogeographical variability in the infaunal polychaete species pool inhabiting a given small-sized seagrass meadow, the structural characteristics of the assemblages appeared to be more strongly controlled by the characteristics of the sediment than by the seagrass species building the meadow. It should be taken into account, however, that, in addition to the structure of the rhizomes, other factors such as seagrass density or the way in which each seagrass species modifies the water flow inside the meadow can affect sediment characteristics, and this seems certainly be the case in C. nodosa and R. cirrhosa meadows.
References
Afonso-Carrillo J, Gil-Rodríguez MC (1980) Cymodocea nodosa (Ucria) Ascherson (Zannichelliaceae) y las praderas submarinas o sebadales de las Islas Canarias. Vieraea 8:365–376
Allison LE, Moodie CD (1965) Carbonate. In: Black CA (ed) Methods of soil analysis. American Society of Agronomy, Madison, Wisc., pp 1389–1392
Alós C (1988) Anélidos poliquetos de Cabo de Creus (Alt Empordà). Doctoral thesis, Universitat de Barcelona, Barcelona
Besteiro C, Urgorri V, Parapar J (1990) Estratificación vertical y variación temporal de la fauna mesopsámmica de arenas de Amphioxus en la ría de Ferrol (Galicia). Thalassas 8:107–115
Bilyard GR (1987) The value of benthic infauna in marine pollution studies. Mar Pollut Bull 18:581–585
Bloom SA, Simon JL, Hunter VD (1972) Animal–sediment relations and community analysis of a Florida estuary. Mar Biol 15:43–56
Bray JR, Curtis CT (1957) An ordination of the upland forest communities of southern Wisconsin. Ecol Monogr 27:325–349
Brito MC, Núñez J (2002) A new genus and species of Questidae (Annelida: Polychaeta) from the Central Macaronesia Region and a cladistic analysis of the family. Sarsia 87:281–289
Brito MC, Núñez J, San Martín G (2000a) The genus Streptosyllis Webster and Benedict, 1884 (Polychaeta: Syllidae: Eusyllinae) from the Canary Islands, with description of a new species. Bull Mar Sci 67:603–615
Brito MC, Núñez J, San Martín G (2000b) Parapionosyllis macaronesiensis, a new species of Exogoninae (Polychaeta: Syllidae) from the Macaronesian Region. Proc Biol Soc Wash 113:1147–1150
Brito MC, Núñez J, San Martín G (2001) Sílidos (Annelida: Polychaeta) intersticiales asociados a praderas de Cymodocea nodosa de las Islas Canarias. Avicennia 14:85–100
Buchanan JB (1984) Sediment analysis. In: Holme NA, McIntyre AD (eds) Methods for the study of marine benthos. Blackwell, Oxford, pp 41–65
Buchanan JB, Kain JM (1971) Measurement of the physical and chemical environment. In: Holme NA, McIntyre AD (eds) Methods for the study of marine benthos. Blackwell, Oxford, pp 30–52
Buia MC, Russo GF, Mazzella L (1985) Interrelazioni tra Cymodocea nodosa (Ucria) Aschers. e Zostera noltii Hornem in un prato misto superficiale dell’isola d’Ischia. Nova Thalassia 7[Suppl]3:406–408
Camp J, Romero J, Pérez M, Vidal M, Delgado M, Martínez A (1991) Production–consumption budget in an estuarine bay: How anoxia is prevented in a forced system? Oecol Aquat 10:145–152
Clarke KR (1993) Non-parametric multivariate analyses of changes in assemblage structure. Aust J Ecol 18:117–143
Clarke KR, Warwick RM (1994) Change in marine community: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Colognola R, Gambi MC, Chessa LA (1984) Polychaetes of Posidonia oceanica (L.) Delile foliar stratum: comparative observation. In: Boudouresque CF, Jeudy de Grissac A, Olivier J (eds) Proceedings of the first international workshop on Posidonia oceanica beds, vol 1. GIS Posidonia, pp 101–108
Cruz E, Vargas JA (1987) Abundancia y distribución vertical de la meiofauna en la playa fangosa de Punta Morales, Golfo de Nicoya, Costa Rica. Rev Biol Trop 35:363–367
Gambi MC, Giangrande A, Martilelli M, Chessa LA (1995) Polychaetes of a Posidonia oceanica bed off Sardinia (Italy): spatial and seasonal distribution and feeding guild analysis. Sci Mar 59:129–141
Gambi MC, Conti G, Bremec CS (1998) Polychaete distribution, diversity and seasonality related to seagrass cover in shallow soft bottoms of the Tyrrhenian Sea (Italy). Sci Mar 62:1–17
Giangrande A, Gambi MC (1986) Polychètes d’une pelouse a Cymodocea nodosa (Ucria) Aschers. du Golfe de Salerno (Mer Tyrrhénienne). Vie Milieu 36:185–190
Howard RK, Edgar GJ, Hutchings PA (1989) Faunal assemblages of seagrass beds. In: Larkum AWD, McComb AJ, Shepherd SA (eds) Biology of seagrasses: a treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, Amsterdam, pp 536–564
Hutchings PA (1982) The fauna of Australian seagrass beds. Proc Linn Soc NSW 106:181–200
Jackson ML (1960) Soil chemical analysis. Prentice Hall, New York
Kikuchi T (1980) Faunal relationships in temperate seagrass beds. In: Phillips RC, McRoy CP (eds) Handbook of seagrass biology: an ecosystem perspective. Garland STPM Press, New York, pp 153–172
Lanera P, Gambi MC (1993) Polychaete distribution in some Cymodocea nodosa meadows around the Island of Ischia (Gulf of Naples Italy). Oebalia 19:89–103
Marr IL, Cresser MS, Gómez Ariza JL (1990) Química analítica del medio ambiente. Servicio de Publicaciones Universidad de Sevilla, Sevilla
Martin D, Ballesteros E, Gili JM, Palacín C (1993) Small-scale structure of infaunal polychaete communities in an estuarine environment: methodological approach. Estuar Coast Shelf Sci 36:47–58
Martin D, Pinedo S, Sardá R (2000) Distribution patterns and trophic structure of soft-bottom polychaete assemblages in a north-western Mediterranean shallow-water Bay. Ophelia 53:1–17
Molinier R, Picard J (1952) Recherches sur les herbiers de phanérogames marines du littoral Méditerranéen français. Ann Inst Oceanogr Paris NS 27:157–234
Palacín C, Martin D, Gili JM (1991) Features of spatial distribution of benthic infauna in a Mediterranean shallow-water bay. Mar Biol 110:315–321
Pielou EC (1969) An introduction to mathematical ecology. Wiley, New York
Reyes J (1993) Estudio de las praderas marinas de Cymodocea nodosa (Cymodoceaceae, Magnoliophyta) y su comunidad de epífitos, en el Médano (Tenerife, Islas Canarias). Doctoral thesis, Universidad de La Laguna, La Laguna, Tenerife, Canary Islands
Rizzo AE, Amaral ACZ (2000) Temporal variation of annelids in the intertidal zone of beaches of the São Sebastião Channel, southern Brazil. J Mar Biol Assoc UK 80:1007–1017
Sanders HL (1969) Benthic marine diversity and the stability-time hypothesis. Brookhaven Symp Biol 22:71–80
Sardá R, Martin D, Pinedo S, Dueso A, Cardell MJ (1995) Seasonal dynamics of shallow-water benthic communities in the western Mediterranean. In: Nicolaidou A (ed) The biology and ecology of shallow coastal waters. Olssen and Olssen, Fredensborg, pp 191–198
Shannon CE, Weaver W (1949) The mathematical theory of communication. Illinois University Press, Urbana
Soler A, Ballesteros M, Turón X (1997) Poliquetos el Estany des Peix (Formentera, Baleares). Aproximación al estudo faunístico y ecológico. Hist Anim 3:9–23
Somaschini A, Gravina MF, Ardizzone GD (1994) Polychete depth distribution in a Posidonia oceanica bed (rhizome and matte strata) and neighbouring soft and hard bottoms. Mar Ecol 15:133–151
Soyer J (1970) Bionomie benthique du plateau continental de la côte catalane française. III. Les peuplements de Copépodes Harpacticoides (Crustacea). Vie Milieu 21:337–511
Terlizzi A, Russo GF (1998) The molluscan taxocoene of different-exposed Cymodocea nodosa beds: year-long structural patterns and sampling methods. Boll Malacol 33:77–82
True-Schlenz R (1965) Données sur les peuplements des sédiments a petites phanérogames marines (Zoostera nana Roth et Cymodocea nodosa Ascherson) comparés a ceux des habitats voisins dépourvus de végétation. Rec Trav Stn Mar Endoume Fac Sci Mars 39:96–125
Walkey A (1947) A critical examination of a rapid method for determining organic carbon in soil. Soil Sci 63:251–263
Whitlatch RB (1977) Seasonal changes in the community structure of the macrobenthos inhabiting the intertidal sand and mud flats of Barnstable Harbor, Massachusetts. Biol Bull (Woods Hole) 152:275–294
Willsie A (1983) Zonation de la macrofaune endogée de la matte d’herbiers de Posidonia oceanica (L.) Delile. Rapp P-V Reun Comm Int Explor Sci Mer Mediterr 28:165–168
Young DK, Young MW (1977) Assemblage structure of the macrobenthos associated with seagrasses of the Indian River Estuary, Florida. In: Coull BC (ed) Ecology of Marine Benthos. Belle W. Baruch Institute for Marine Biology, University of South Carolina Press, Columbia, pp 339–381
Zar JH (1984) Biostatistical analysis. Prentice Hall, Engelwood Cliffs, N.J., USA
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Brito, M.C., Martin, D. & Núñez, J. Polychaetes associated to a Cymodocea nodosa meadow in the Canary Islands: assemblage structure, temporal variability and vertical distribution compared to other Mediterranean seagrass meadows. Marine Biology 146, 467–481 (2005). https://doi.org/10.1007/s00227-004-1460-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1460-1