Abstract
This study describes the density variation and phenology of Macrocystis integrifolia and M. pyrifera populations from northern and southern Chile, respectively. Samples of both species were taken in wave-exposed and wave-protected areas. In addition, spore production, germination and early growth rate of sporophytes of each population was studied at monthly intervals under three temperature and salinity regimes. Results indicate that M. integrifolia from northern Chile presents perennial plants with a mean density of three individuals per 0.25 m2 throughout the year and that it reproduces mainly during spring and winter. Although, M. pyrifera in exposed areas of southern Chile also have a perennial-type life strategy, they are able to reproduce all year round. In contrast, M. pyrifera populations in protected areas of southern Chile show a clear annual cycle, with high recruitment during late winter and fertile sporophytes in summer and autumn, although the populations become completely decimated thereafter. The effect of temperature and salinity on M. integrifolia shows that it is independent of water movement, but requires low temperatures and high salinities for the release of zoospores, germination and early sporophyte growth. This pattern differs from that of M. pyrifera in southern Chile, which has a broader tolerance range for salinity and temperature than does M. integrifolia. However, in southern Chile wave-protected populations showed higher spore release and germination at 15°C and 18°C, whereas sporophyte growth responded better at the lowest temperature tested (8°C). In general, these results are contrary to those expected, since a seasonal reproductive pattern was observed in M. integrifolia inhabiting a less seasonally variable environment. In exposed sites of southern Chile, plants showed greater tolerance and continuous reproduction throughout the year, despite the greater environmental variability. Finally, population dynamics of protected kelps in southern Chile shows an annual pattern, which is contrary to the expected perennial strategy shown by exposed populations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Two species of Macrocystis (Lessoniaceae) have been identified along the Chilean coast (Hoffmann and Santelices 1997). M. integrifolia is present along the Peruvian and northern Chilean coast (6–32°S), and inhabits bays at a depth of 0–15 m in wave-protected and semiprotected areas. Periodically, these northern Chilean populations suffer the effects of El Niño–Southern Oscillation (ENSO) phenomena (Vásquez and Buschmann 1997; Vásquez et al. 1998). On the other hand, M. pyrifera, the most common species, can be found from 37°S down to Patagonia (55°S). This latter species is found in bays open to the Pacific Ocean, as well as in channels and fjords that are protected from strong wave action south of 41°S (Dayton 1985). At lower latitudes, these populations are usually subjected to fewer and less-intensive seasonal variations in salinity, nutrients and temperature when compared to the southern region, where M. pyrifera is the dominant kelp and ENSO effects are normally less marked.
In general it has been indicated that M. integrifolia and M. pyrifera tolerate a broad range of temperatures, which can reach highs of 18–20°C (North et al. 1986). Nevertheless, summer nutrient depletion, together with high temperatures, is responsible for canopy die-off (e.g. North et al. 1986). Water motion certainly increases nutrient and carbon uptake (e.g. Wheeler 1980,1982; Gerard 1982), but may cause the dislodgment of plants (Seymour et al. 1989; Graham et al. 1997). This situation explains the general effects of El Niño: temperatures increase, nutrient concentrations decline and winter storms increase (e.g. Gerard 1984; Dayton and Tegner 1984; Harrold and Reed 1985). The effect of salinity has not been studied as closely as the above environmental parameters, but it has been suggested that kelp tolerate seawater dilution of almost one-third (North et al. 1986). In spite of El Niño events in the north, we postulate that the stronger seasonal variation in southern Chile is correlated with a higher tolerance to environmental changes, such as salinity, due to the higher precipitation in the south. Furthermore, effects of water movement on populations from northern Chile are not expected, but, in southern Chile, protected populations should be much more variable and show higher tolerance limits than exposed populations, because of the stronger environmental variability present in inland water habitats (Buschmann 1992).
This study shows the effects of water motion, temperature and salinity on the population dynamics of Macrocystis spp. in the southeastern Pacific coast. We describe annual variation in plant density in relation to water motion, and assess the effects of temperature and salinity on sporulation, spore germination and early sporophytic growth in wave-exposed and -protected populations. The results are discussed in relation to latitudinal distribution, local upwelling, El Niño events, and the temperature and salinity variability present at the study sites along the Chilean coast.
Materials and methods
Study sites
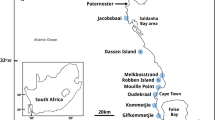
This study was carried out in 12 locations, 6 in northern (~30°S) and 6 in southern Chile (~41°S) (Fig. 1). In each location, three exposed and three protected study sites were defined by using calcium carbonate blocks, as described by Doty (1971). Five blocks per site were installed along a depth gradient, for a period of 3 days, a procedure that was repeated on six occasions in different seasons. This methodology permitted us to identify study sites with different degrees of water movement by determining the dissolution rate of the carbonate blocks. Water temperature was determined daily (at hourly intervals) by using submerged digital thermographs (StowAway Tidbit), whereas salinity was measured at bi-weekly intervals on three samples per station with an Atago refractometer (±0.5‰). Temperature and salinity were measured at different depths (1–10 m) to obtain an average value for each location.
Map of the study areas of Macrocystis integrifolia in northern and M. pyrifera in southern Chile. Playa Blanca (ESN1 and PSN1) 28°11′S; 71°09′W; Punta Choros (ESN2 and PSN2) 29°14′S; 71°31′W; San Lorenzo (ESN3 and PSN3) 30°20′S; 71°49′W; Pucatrihue (ESS1) 40°33′S; 73°43′W; Bahía Mansa (ESS2 and ESS3) 40°34′S; 73°46′W; and Metri (PSS1, PSS2 and PSS3) 41°36′S; 72°42′W
In northern Chile, wave-exposed sites (ESN1, ESN2 and ESN3) are characterized by southern-facing areas with a sea bottom dominated by consolidated rocks from the intertidal zone up to a depth of 12 m. Patches of sand and boulders can be found between these rocky areas. At depths >12 m, the sand and boulder areas outnumber the areas of rocky substrate. Wave-protected sites (PSN1, PSN2 and PSN3) face towards the north and present a similar substratum structure as described for exposed sites. Macrocystis integrifolia in these locations reaches a height of 5 m, with an average holdfast diameter of 30 cm and with up to 20 stipes and 50 sporophylls per plant. No published information on the population dynamics of M. integrifolia exists at present (see Vásquez and Buschmann 1997). Under the M. integrifolia canopy a second kelp (Lessonia) is present. An understory assemblage of algae, composed principally by coralline crustose algae and turfs of Gelidiales and Ceramiales, can also be found. Other algae present in these areas are Halopteris spp., Glossophora kunthii, Asparagopsis armata and Rhodymenia sp. (Vásquez 1992). At depths >10 m, Lessonia dominates over Macrocystis. The gastropod snail Tegula atra, the black sea urchin Tetrapygus niger and the fish Aplodactylus punctatus are the main herbivorous species controlling kelp abundance (Vásquez 1993).
In southern Chile, the open coast is generally exposed (sites ESS1, ESS2 and ESS3), with a rocky sea bottom surrounded by boulder fields. Algal populations can be found in the shallow intertidal zone up to depths of 10–12 m (Buschmann 1992). M. pyrifera present at these locations can be 6 m long, with a holdfast diameter of 25 cm and with only 3–4 stipes and 30 sporophylles per plant. Data related to the population dynamics of M. pyrifera in southern Chile indicate that it has a perennial-type of life history (Santelices and Ojeda 1984; Westermeier and Möller 1990). In addition to M. pyrifera stands, exposed sites in this area present an understory of flora dominated by Ulva rigida, but which also includes Desmarestia, Sarcothalia crispata and Trematocarpus, along with other less abundant red seaweed species. In the inner seas of southern Chile, wave-protected areas (PSS1, PSS2 and PSS3) can be found, with a conspicuous Macrocystis belt surrounding the coastline. The substratum is mostly granitic rock or boulder fields, and the kelp population can be found up to a depth of 12 m. The most common algal species are U. rigida and S. crispata. Two sessile mollusks are very abundant in this area, Aulacomya ater and Crepidula fornicata fecunda. In both exposed and protected sites of southern Chile, the black snail Tegula atra is the most abundant grazer in terms of number and biomass.
Population and laboratory experiments
Plants were counted at each study site at monthly intervals for a year from July 2000 to 2001, with the use of nine 0.25-m2 random quadrats along a transect, starting at 3–12 m. All plants (juveniles and adults) were counted in each quadrat. Following the counting of sporophytes, sporophylls were collected (n=60 from at least 30 random plants), packed in plastic bags and transported in ice to the laboratory. When no fertile tissues or successful sporulation was observed, the period was described as non-reproductive. Otherwise, irrespective of whether the fertile sporophylls produced few or numerous spores, we qualified the period as a reproductive one. Every month, 45 randomly selected sporophylls from all study areas of M. integrifolia, as well as from all study areas of M. pyrifera, were brought to the laboratory. The sporophylls were gently brushed and rinsed with filtered (0.2 µm), sterile seawater and packed with filter paper and aluminum foil for 12 h at a temperature of 8°C. After this mild desiccation period, 1-cm2 discs (one per sporophyll) were cut off each fertile sorus and placed in Petri dishes (5 cm diameter) with Provasoli culture medium (McLachlan 1973) to induce sporulation, germination and growth of new sporophytes. Five Petri dishes with tissues originating from different plants were considered for each of the following treatments: three temperatures (8°C, 15°C and 18 °C) and three salinities (27‰, 31‰ and 34‰). The ranges of temperature and salinity chosen were in accordance with those found in the natural environment. Incubation was carried out in culture chambers under constant conditions, considering a neutral photoperiod (12 h light:12 h dark) and a photon flux of 40 µmol m−2 s−1 provided by 40 W fluorescent tubes (Phillips). The photon flux rate was measured with a Li-Cor LI-189 photometer. The culture medium was changed on a weekly basis. The number of spores produced by each 1-cm2 sorus disc was counted after 48 h of incubation, whereas the number of spores with a germination tube was determined after 144 h. Counting was carried out using an inverted Nikon microscope attached to a digital camera and an image analyzer (Image-Pro version 4.0). Three random areas of each dish were photographed, from which the total numbers of settled spores and the spores with the presence of germination tubes were counted. With this data we estimated the number of spores produced by a 1-cm2 piece of sorus, and the germination percentage was calculated as the number of spores with a germination tube divided by the total number of initially settled spores (×100). After 30 days of incubation, the length of all the young sporophytes in each ocular field was determined following the same procedure as described above, to estimate the growth rate (mm month−1) of the sporophytes under different temperature and salinity conditions.
After a year of repeated monthly experiments, the different data for protected and exposed northern and southern localities were pooled by season (winter, spring, summer and autumn). Means and standard errors were calculated for each case, and the data were analyzed by three-way ANOVA using SYSTAT 5.0, after verifying normality and homocedasticity of the data. The factors analyzed for every season were temperature, salinity and location of plant collection (exposed or protected sites). If significant differences were detected for salinity and/or temperature, an a posteriori Tukey test was performed using SYSTAT 5.0.
Results
Abiotic conditions
In both northern and southern Chile, calcium carbonate blocks permitted discrimination between protected and exposed areas. The northern area presents a significant (P<0.01) reduction in the dissolution rate when exposed sites are compared to protected sites (mean diffusion rate: 0.60 and 0.32 g h−1, respectively). In the south, the dissolution rate was three to four times higher (P<0.001) in the exposed sites compared to the protected sites (mean diffusion rate: 0.68 and 0.17 g h−1, respectively). Temperature and salinity variation during the year is presented in Fig. 2. Data show that in northern Chile there is almost no seasonal variation in salinity, with a mean value of 33‰, and that temperature varied between 13°C and 18°C (5°C), with no differences between protected and exposed sites. On the other hand, in southern Chile salinity variation was greater than in northern Chile; salinity varied between 28‰ and 33‰ in exposed locations, whereas, in protected locations, values fluctuated between 22‰ and 31‰ (Fig. 2). Temperature in the exposed areas of the southern region varied from 8°C to 16°C (8°C) and in the protected areas from 8°C to 15°C (7°C).
Salinity (‰) versus temperature (°C) variations over a 1-year period in protected and exposed Macrocystis spp. study locations (see Fig. 1 for abbreviations) in northern and southern Chile
Abundance and phenology
The plant density of Macrocystis integrifolia in all three study locations from northern Chile showed a very conservative annual variation (Fig. 3). In the exposed sites, the plant density varied from 2 to 4 plants per 0.25 m2. Numbers were very similar in the protected sites, although one study site (PSN3) registered a density of over 5 plants per 0.25 m2 in summer. Nevertheless, no significant differences were found between localities and seasons (Fig. 3).
Macrocystis spp. Annual variations in plant density (individuals per 0.25 m2) of M. integrifolia in northern and M. pyrifera in southern Chile, also with respect to wave exposure (site abbreviations, see Fig. 1)
The case of M. pyrifera in southern Chile is quite different (Fig. 3). In the exposed area, two of the most exposed sites (EES1 and EES3) showed a similar pattern to that found in northern Chile, with almost no temporal variation in abundance and values varying between 2 and 4 plants per 25 m2; in one exposed site (ESS2), a massive recruitment event was detected in early spring (Fig. 3). However, the most striking difference was seen in the protected areas of southern Chile, where M. pyrifera populations showed an annual cycle, with recruitment starting during winter (July) and reaching densities of 15 or more plants per 0.25 m2. Density at the protected sites in the south begins to decline during spring and summer, but the largest specimens are found in early summer (Fig. 3). All the plants disappear in autumn, when it is possible to observe necrotized holdfasts. Regrowth of these holdfasts was never observed, even after several years (5) of observation.
The phenology of M. integrifolia indicates that, in both protected and exposed locations of northern Chile, this kelp contains sporophylls all year round (Fig. 4). However, during summer the number of spores produced is very low. On the other hand, M. pyrifera is reproductive all year round in exposed areas. The reproductive season of the protected populations in southern Chile is limited to summer and early autumn (Fig. 4). For this reason the protected population of M. pyrifera follows an annual cycle, in contrast to the other kelp populations studied.
Temperature and salinity experiments
In the north of Chile, the spores produced by a standardized area of sorus occur mainly in winter and spring, while the number of spores that can be obtained in summer seems minimal (<30 spores mm−2), both in protected and exposed sites (Fig. 5). In autumn, these northern populations did not produce any spores. Statistical analysis indicates that the number of spores released by M. integrifolia was significantly (F=5.830; 2, 792 df; P<0.003) greater in spring and autumn at 8°C than at the higher temperatures. Salinity had a significant effect on spore release in winter (F=7.367; 2, 792 df; P<0.001) and summer (F=4.901; 2, 792 df; P<0.008), showing a higher success at 34‰ than at the two lower salinities studied (Fig. 5). Finally, spore production between the protected and exposed plants differed significantly in winter (F=6.789; 2, 792 df; P<0.009), spring (F=17.908; 2, 792 df; P<0.001) and summer (F=4.245; 2, 792 df; P<0.040), with a higher spore release observed in the exposed plants (Fig. 5). It is important to indicate that temperature interacted significantly with locality (exposed and protected sites) both in spring (F=7.671; 2, 792 df; P<0.001) and in summer (F=4.248; 2, 792 df; P<0.015), and that the effect of temperature was stronger for the exposed populations (Fig. 5).
Spore release by M. pyrifera in southern Chile presented a different pattern (Fig. 6). In this case, temperature only has a significant (F=33.57; 2, 792 df; P<<0.001) effect in autumn, when spore release is higher at 15°C than at 8°C. Salinity only had a significant effect in autumn (F=4.727; 2, 792 df; P<0.009), indicating that spore production was higher at 27‰ than at 34‰ (Fig. 6). However, in all four seasons significant (winter: F=400.76; 2, 792 df; P<<0.001; spring: F=111.37; 2, 792 df; P<<0.001; summer: F=77.89; 2, 792 df; P<<0.001; F=16.454; 2, 792 df; P<0.001) differences were found between the sporulation of protected and exposed sites, showing that in winter and autumn the spore production was higher in the exposed sites, but in summer and autumn the plants collected in protected areas had a higher spore production capacity (Fig. 6). Furthermore, in autumn the temperature interacted significantly (F=23.296; 792 df; P<<0.001) with locality, indicating that at 15°C spore production was higher at 27‰ than at the two higher salinities tested in this study (Fig. 6). In summer, salinity interacted significantly (F=6376; 2, 792 df; P<0.002) with the collection locality, indicating that its effect was stronger for protected populations (Fig. 6). Another significant (F=3.086; 4, 792 df; P<0.016) interaction was detected between temperature and salinity in summer, showing that spore release at low salinities and increased temperature enhances sporulation, whereas at higher salinities and higher temperatures sporulation is reduced (Fig. 6). Finally, a significant (F=4.143; 4, 792 df; P<0.003) interaction was found in autumn, indicating that a temperature of 15°C produced a stronger effect on sporulation for the protected kelps, especially at lower salinities.
Spore germination success determined for M. integrifolia indicated that, in the best situation, the germination percentage never exceeded 25%, whereas in autumn no response was observed for the few spores obtained (Fig. 7). In winter and summer temperature had a significant (winter: F=3.400; 2, 792 df; P<0.034; summer: F=6.570; 2, 792 df; P<0.001) effect on germination percentage, indicating that the success was higher at 15°C than at 8°C or 18°C. In contrast, germination showed higher success in spring at 31‰ and in summer at 31‰ and 34‰ than at 27‰ (Fig. 7). A significant (F=22.241; 2, 792 df; P<0.001) difference between exposed and protected locations was only found in spring, when the germination response was higher (Fig. 7). The effect of temperature in winter and spring also showed a significant (spring: F=7.363; 2, 792 df; P<0.001; winter: F=4.310; 2, 792 df; P<0.014) interaction with the location of the algal stands. Furthermore, salinity did not interact with locality, but interacted significantly (F=3.485; 4, 792 df; P<0.008) with temperature in winter, and, finally, interaction of the three factors was also significant (F=4.349; 4, 792 df; P<0.002; spring: F=2.505; 4, 792 df; P<0.041) in winter and spring (Fig. 7).
Germination success was much higher in southern Chile than in the north, as it reached values as high as 100% during several seasons and under different culture conditions (Fig. 8). Most importantly, only kelps collected in the protected areas of southern Chile provided viable spores in summer and autumn. Temperature had a significant (winter: F=22.07; 2, 792 df; P<<0.001; summer: F=4.284; 2, 792 df; P<0.014; autumn: F=19.47; 2, 792 df; P<<0.001) effect on the germination percentage in winter, summer and autumn. In winter germination was more successful at 18°C than at 8°C or 15°C; in the summer and autumn germination performance was better at 15°C and 18°C than at 8°C (Fig. 8). Salinity only had a significant effect in winter (F=4.577; 2, 793 df; P<0.011) and summer (F=15.043; 2, 792 df; P<0.001). These data indicate that, in winter, germination performance was higher at 31‰ and 27‰ than at 34‰, but, in summer, the improved performance only occurred at 31‰ (Fig. 8). Significant differences between exposed and protected locations were found in winter (F=6903.8; 1, 792 df; P<<0.001), spring (F=234.6; 1, 792 df; P<<0.001) and also in summer (F=19.075; 1, 792 df; P<0.001). In summer, a significant interaction indicated that at 18°C the exposed population had a higher germination percentage, while the protected sites had a better germination performance at 8°C (Fig. 8). Furthermore, a significant interaction between temperature and salinity was found during the winter (F=4.889; 4, 792 df; P<0.001) and summer (F=3.734; 4, 792 df; P<0.005), when the impact of increasing the temperature was more profound at higher salinities (Fig. 8).
After maturation of gametophytes, growth rate (length month−1) of the sporophytic phase of M. integrifolia under culture conditions was determined (Fig. 9). Temperature only had a significant (F=3.892; 2, 792 df; P<0.021) effect on the growth in summer, when the highest growth rate was found at 15°C. However, overall, the highest growth rate occurred in the plants collected in spring (Fig. 9). Salinity, on the other hand, significantly affected the growth rate in winter (F=4.169; 2, 792 df; P<0.016), spring (F=4.653; 2, 792 df; P<0.010) and summer (F=3.892; 2, 792 df; P<0.021), indicating that better growth rates were found at 31‰ and 34‰ (Fig. 9). Significant (F=7.425; 1, 792 df; P<0.007) differences between exposed and protected locations were only found during spring, when sporophytes produced in protected areas showed a higher growth rate than those from exposed plants. Furthermore, a significant interaction (F=4.403; 2, 792 df; P<0.013) between locality and temperature was found in spring, as the effect of temperature had a synergic effect on the growth of protected populations of this kelp at 8°C and 15°C (Fig. 9).
Once again, the growth rate of M. pyrifera from southern Chile varied under the different studied factors (Fig. 10). The exposed population produced viable spores all year round, but sporophytes were mostly produced during winter and spring, and strongly reduced in autumn, whereas no growth rate was found for the sporophytes collected in summer. On the other hand, the protected population produced sporophytes only in summer and autumn. Temperature significantly affected the growth rate of sporophytes in winter (F=3.623; 2, 792 df; P<0.027), summer (F=5.260; 2, 792 df; P<0.005) and autumn (F=75.88; 2, 792 df; P<<0.001), showing higher growth rates at 8°C and 15°C than at 18°C (Fig. 10). A significant interaction was found between the origin of the spores (exposed or protected) and temperature, indicating that in summer (F=3.339; 2, 792 df; P<0.036) and particularly in autumn (F=72.26; 2, 792 df; P<<0.001) the protected plants grew better at low temperatures, which was not the case for the exposed kelps (Fig. 10). Salinity did not have a significant effect on the growth rate of M. pyrifera in any season.
Discussion
The reproduction patterns of both Macrocystis species presented striking differences. The northern populations of M. integrifolia showed a more intense reproductive period in winter and spring, which was independent of exposure. In contrast, continuous reproduction in M. pyrifera was observed in the southern populations located at exposed sites, while in protected sites the reproductive period was restricted to summer and early autumn. Exposed site patterns are similar to M. pyrifera in the northern hemisphere, which is continuously fertile (Reed et al. 1996), but contrast with M. integrifolia, which also show continuous recruitment throughout the year (Druehl and Wheeler 1986). Populations and species of southern Chile differ, not only in their reproductive cycle, but also in their abundance patterns. In northern Chile, M. integrifolia populations present a very stable value of 2–4 plants per 0.25 m2, which is similar to that found in exposed locations of M. pyrifera in southern Chile. However, the protected kelp populations show a clear annual cycle (see also Buschmann 1992). In summary, we present three distinct patterns: (1) in northern Chile, a stable population abundance is observed, but with a clearly seasonal reproductive pattern (in winter); (2) in southern Chile, the exposed population shows stable population dynamics, with continuous reproduction; and (3) also in the south, we observed an annual population with a reproductive season restricted to summer (Fig. 4). Therefore, the adaptive capacity of the abundance and reproductive patterns shown by these kelps to different environmental conditions is a very important characteristic.
Seasonal fluctuations in the environment typically induce growth and reproduction in perennial organisms that live long enough to experience repeated seasonal changes. Furthermore, it is typical that plants living outside the tropics, i.e. in more fluctuating environments, do not reproduce all year round (e.g. Battey and Lyndon 1990). However, M. pyrifera is reproductive throughout the year in the northern hemisphere (e.g. Reed et al. 1996). Seasonal environmental changes provide external signals that trigger internal processes in plants. Only when habitat conditions are constant and benign, will the growth and reproductive patterns arise solely from the plant itself (Lyndon 1992). Population patterns found in this study are not correlated with the fairly constant environmental conditions found in northern Chile, where the population is characterized by more seasonal reproductive dynamics than their southern counterparts in exposed areas. The explanation seems to be that, in the northern environment, M. integrifolia requires winter conditions (lower temperatures) to reproduce, even though they can grow all year round. In southern Chile, the appropriate reproduction conditions are present throughout the year, and, in this case, M. pyrifera has developed a reproductive strategy that enables plants to produce spores continuously. For this reason the populations in the north appear to have developed a different strategy: their populations are more perennial than those in southern Chile and their holdfast regeneration capacity is greater, as they are subjected to less intense winter storms than populations in southern Chile (Vásquez and Buschmann 1997). On the other hand, these results show that the exposed M. pyrifera reproductive strategy is to produce spores independent of water conditions, in contrast to the M. integrifolia populations in northern Chile that produce spores during a restricted time period that coincides with the sporophyte growth period.
Seaweed populations can be affected by the existence of upwelling regimes and ENSO effects (e.g. Dayton and Tegner 1984; Ormond and Banaimoon 1994). The three study locations in northern Chile show stable or discontinuous upwelling regimes (Vásquez et al. 1998). Thus, if M. integrifolia requires low temperatures in the spring (8°C) to enhance growth of the sporophytes (Fig. 9), we suggest that upwelling could be an important oceanographic requirement for the maintenance of these populations in northern Chile. Nevertheless, the different wave regimes do not alter the population density and reproduction patterns for unknown reasons. Furthermore, effects of El Niño events have been decimating populations of the kelp Lessonia nigrescens from Chile and other Laminariales in the northern hemisphere for decades (Dayton and Tegner 1984; Tegner and Dayton 1987; Castilla and Camus 1992; Camus 1994; Martínez et al. 2003). The link between these periodical events on the northern Chilean coast and the recovery of macroalgal populations requires close attention in the near future.
Salinity remains almost constant along southern California and Baja California, and has no apparent effect on kelp distribution except in the inner portions of estuaries (North et al. 1986). The same authors mention that, in water bodies in northern and central California, salinity may affect the population biology of giant kelp. Druehl (1979) observed that M. integrifolia beds from Vancouver Island are present in habitats where salinity varied between 23‰ and 30‰. In contrast, M. integrifolia in northern Chile shows a significantly better response at higher (>30‰) salinities. On the other hand, in the salinity-variable environments of southern Chile, M. pyrifera shows a tendency to perform better at 27‰ and 30‰. In some cases, the interaction with locality indicates that the better responses at lower salinities generally occur in protected annual kelp populations. This result suggests that giant kelp may have differentiated responses in relation to salinity, allowing this species to inhabit estuarine environments like those in southern Chile.
Reproductive plasticity may be an adaptive character if the cost of producing propagules is low, as seems to be the case for seaweeds (Pfister 1992). In this context, the strategy of M. integrifolia in northern Chile and M. pyrifera in developing annual population dynamics with adjustment of their reproductive output to the prevailing environmental conditions is particularly noteworthy. In this last case, we have kelp populations growing and producing sporophylls earlier and producing more spores per area fertile tissue than the perennial population. Nevertheless, the spore production and germination capabilities are very similar in exposed and protected M. pyrifera populations. However, the early growth responses of the sporophytes showed very different patterns. Data show that growth of the sporophytes is mainly restricted to winter and spring for the exposed populations and that the protected kelps grow mainly in autumn, at low water temperatures similar to those present in southern Chile in winter. This evidence indicates that whenever the plants produce spores throughout the year they will become fertile, but will not produce viable sporophytes all year round. For this reason continuous production of new recruits cannot be extrapolated from continuous spore production. This result was only visible by repeating the experiments every month.
The main prerequisite for the annual M. pyrifera populations in southern Chile involves the coupling of spore production and the long survival period that is required. For seaweeds, the existence of seed banks has been indicated (Hoffmann and Santelices 1991; Santelices et al. 1995; Edwards 2003) and is invoked to explain the massive disappearance and subsequent recovery of decimated populations of M. pyrifera in the northern hemisphere (Ladah et al. 1999). In this case, we found that sporophyte growth is enhanced under low temperature conditions. This suggests that the plants produce propagules that are very successful in summer, but the sporophyte growth conditions are essential to survive the winter period. Light conditions have been demonstrated to be important in the field for M. pyrifera (Graham 1996). For this reason we suggest that the low radiation and low temperatures present in southern Chile are responsible for the dormancy of microscopic stages of M. pyrifera during the winter period. Recently it has been demonstrated that limiting resources can delay recruitment of embryonic giant kelp sporophytes for at least 1 month (Kinlan et al. 2003). These results suggest that in variable environmental locations, such as those in southern Chile, dormant stages appear to be a requirement, but further studies need to be undertaken on these annual populations.
References
Battey NH, Lyndon RF (1990) Reversion of flowering. Bot Rev 56:162–189
Buschmann AH (1992) Algal communities of a wave-protected intertidal rocky shore in southern Chile. In: Seeliger U (ed) Coastal plant communities of Latin America. Academic, Orlando, Fla., pp 91–104
Camus PA (1994) Dinámica geográfica en poblaciones de Lessonia nigrescens Bory (Phaeophyta) en el norte de Chile: importancia de la extinción local durante eventos de El Niño de gran intensidad. Invest Cienc Tecnol Ser Cienc Mar 3:58–70
Castilla JC, Camus PA (1992) The Humboldt–El Niño scenario: coastal benthic resources and anthropogenic influences, with particular references to the 1982–83 ENSO. S Afr J Mar Sci 12:703–712
Dayton PK (1985) The structure and regulation of some South American kelp communities. Ecol Monogr 55:447–468
Dayton PK, Tegner MJ (1984) Catastrophic storms and patch stability in a southern California kelp community. Science 224:283–285
Doty M (1971) Measurements in water movement in reference to benthic algal growth. Bot Mar 14:32–35
Druehl LD (1979) The distribution of Macrocystis integrifolia in British Columbia as related to environmental parameters. Can J Bot 56:59–69
Druehl LD, Wheeler WN (1986) Population biology of Macrocystis integrifolia from British Columbia, Canada. Mar Biol 90:173–179
Edwards MS (2003) The role of alternate life-history stages of a marine macroalga: a seed bank analogue? Ecology 81:2404–2415
Gerard VA (1982) In situ rates of nitrate uptake by giant kelp, Macrocystis pyrifera. Mar Biol 69:51–54
Gerard VA (1984) Physiological effects of El Niño on giant kelp in southern California. Mar Biol Lett 5:317–322
Graham MH (1996) Effect of high irradiance on recruitment of the giant kelp Macrocystis (Phaeophyta) in shallow waters. J Phycol 32:903–906
Graham MH, Harrold C, Lisin S, Light K, Watanabe JM, Foster MS (1997) Population dynamics of giant kelp Macrocystis pyrifera along a wave exposure gradient. Mar Ecol Prog Ser 148:269–279
Harrold C, Reed DC (1985) Food availability, sea urchin grazing, and kelp forest community structure. Ecology 66:1160–1169
Hoffmann A, Santelices B (1991) Banks of algal microscopic forms: hypotheses and their function and comparison with seed banks. Mar Ecol Prog Ser 79:185–194
Hoffmann AJ, Santelices B (1997) Marine Flora of Central Chile. Ediciones Universidad Católica de Chile, Santiago
Kinlan BP, Graham MH, Sala E, Dayton PK (2003) Arrested development of giant kelp (Macrocystis pyrifera, Phaeophyceae) embryonic sporophytes: a mechanism for delayed recruitment in perennial kelp? J Phycol 39:47–57
Ladah LB, Zertuche-González JA, Hernández-Carmona G (1999) Giant kelp (Macrocystis pyrifera, Phaeophyceae) recruitment near its southern limit in Baja California, alter mass disappearance during ENSO 1997–1998. J Phycol 35:1006–1012
Lyndon RF (1992) The environmental control of reproductive development. In: Marshall C, Grace J (eds) Fruit and seed production. Cambridge University Press, Cambridge, pp 9–32
Martínez EA, Cárdenas L, Pinto R (2003) Recovery and genetic diversity of the intertidal kelp Lessonia nigrescens 20 years after El Niño 1982/83. J Phycol 39:504–508
McLachlan J (1973) Growth media—marine. In: Stein J (ed) Handbook of phycological methods. Culture methods and growth measurements. Cambridge University Press, Cambridge, pp 25–51
North WJ, Jackson GA, Manley SL (1986) Macrocystis and its environment, knowns and unknowns. Aquat Bot 26:9–26
Ormond RFG, Banaimoon SA (1994) Ecology of intertidal macroalgal assemblages on the Hadramout coast of southern Yemen, an area of seasonal upwelling. Mar Ecol Prog Ser 105:105–120
Pfister CA (1992) Costs reproduction in a intertidal kelp: patterns of allocation and life history consequences. Ecology 73:1589–1597
Reed DC, Ebeling AW, Anderson TW, Anghera M (1996) Differential reproductive responses to fluctuating resources in two seaweeds with different reproductive strategies. Ecology 77:300–316
Santelices B, Ojeda FP (1984) Population dynamics of coastal forests of Macrocystis pyrifera in Puerto Toro, Isal Navarino, southern Chile. Mar Ecol Prog Ser 14:175–183
Santelices B, Hoffmann AJ, Aedo D, Bobadilla M, Otaíza R (1995) A bank of microscopic forms on disturbed boulders and stones in tide pools. Mar Ecol Prog Ser 129:215–228
Seymour RJ, Tegner MJ, Dayton PK, Parnell PE (1989) Storm wave induced mortality of giant kelp, Macrocystis pyrifera, in southern California. Estuar Coast Shelf Sci 28:277–292
Tegner MJ, Dayton PK (1987) El Niño effects on Southern California kelp forest communities. Adv Ecol Res 17:243–279
Vásquez JA (1992) Lessonia trabeculata, a subtidal bottom kelp in northern Chile: a case of study for structural and geographical comparison. In: Seeliger U (ed) Coastal plant communities of Latin America. Academic, Orlando, Fla., pp 77–89
Vásquez JA (1993) Abundance, distributional patterns and diets of main herbivorous and carnivorous species associated to Lessonia trabeculata kelp beds in northern Chile. Ser Ocas Fac Cienc Mar Univ Cat Norte 2:213–229
Vásquez JA, Buschmann AH (1997) Herbivore–kelp interactions in Chilean subtidal communities: a review. Rev Chil Hist Nat 70:41–52
Vásquez JA, Camus PA, Ojeda FP (1998) Diversity, structure and functioning of rocky coastal ecosystems in northern Chile. Rev Chil Hist Nat 71:479–499
Westermeier R, Möller P (1990) Population dynamics of Macrocystis pyrifera (L.) C. Agardh in the rocky intertidal of southern Chile. Bot Mar 33:363–367
Wheeler WN (1980) Effect of the boundary layer transport on the fixation of carbon by the giant kelp Macrocystis pyrifera. Mar Biol 56:103–110
Wheeler WN (1982) Nitrogen nutrition of Macrocystis. In: Srivastava LM (ed) Synthetic and degradative processes in marine macrophytes. Gruyter, Berlin, pp 121–135
Acknowledgements
This paper was funded by FONDECYT (Chile) 1000044 and 1010706. The authors acknowledge the help, in the field and in the laboratory, of R. Espinoza, V. Muñoz, G. Aroca and C. Moreno. We also wish to recognize the advice and constructive criticism of D. Varela and R. Stead on various aspects of this manuscript and the English review of S. Angus. Paper No. 5 of i-mar.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P.W. Sammarco, Chauvin
Rights and permissions
About this article
Cite this article
Buschmann, A.H., Vásquez, J.A., Osorio, P. et al. The effect of water movement, temperature and salinity on abundance and reproductive patterns of Macrocystis spp. (Phaeophyta) at different latitudes in Chile. Marine Biology 145, 849–862 (2004). https://doi.org/10.1007/s00227-004-1393-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1393-8