Abstract
Vital fluorescent probes have routinely been used to distinguish viable from non-viable embryos in various veterinary and aquaculture studies. Here, we present new protocols to rapidly detect embryo viability in the copepod Calanus helgolandicus using three of these probes, fluorescein diacetate (FDA), SYTOX green and 7-aminoactinomycin D (7-AAD), and the confocal laser scanning microscope. The percentage of fluorescent-FDA embryos and non-fluorescent SYTOX green and 7-AAD embryos were compared with the percentage of hatched unstained embryos and with the percentage of embryos that had been stained, washed, and allowed to hatch. Results showed that all three dyes accurately predicted embryo viability and could be used to rapidly calculate C. helgolandicus egg-hatching success. We also tested the possible applications of SYTOX green in egg-production/egg-hatching assays in which the dinoflagellate Prorocentrum minimum or the diatom Skeletonema costatum are used to investigate for the possible negative impact of diatoms on embryo viability. Other possible applications for fluorescence methods in studies on the reproductive biology of zooplankton, and in particular of copepods, are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fluorescence-based cell viability assays have been widely used on a variety of cells, from bacteria (Breeuwer and Abee 2000; Joux and Lebaron 2000) to human cultured cells (Yang et al. 2002). Evaluation of embryo viability with fluorescent probes is one common practice in veterinary science (Huhtinen et al. 1996; Vanderwall 1996), or to discriminate the quality of cryopreserved embryos (Leveroni Calvi and Maisse 1998). In marine biological studies, this technique has mostly been used to determine phytoplankton cell viability (Veldhuis et al. 1997; Brussaard et al. 2001; Agusti and Sanchez 2002), except for a recent study by Buttino et al. (2003), who developed a new staining protocol that allows for the visualization of viable marine copepod embryos with the confocal laser scanning microscope. Copepods are dominant zooplanktonic grazers in shelf and coastal waters (Mauchline 1998). Until now, hatching success of these small crustaceans in most laboratory (e.g. Poulet et al. 1994; Turner et al. 2001) and field (Laabir et al. 1995; Miralto et al. 2003) studies have been calculated by allowing eggs to develop undisturbed to hatching, which usually requires about 24–72 h in most temperate and sub-temperate copepods (Ianora 1998). Here, we propose a rapid assay to determine copepod embryo viability, soon after egg spawning, by using three different vital fluorescent probes: fluorescein diacetate (FDA), SYTOX green and 7-aminoactinomycin D (7-AAD).
FDA is a cell-permeant dye that constitutes a substrate for active enzymes inside the cells. When FDA penetrates into viable cells, esterases produce free fluorescent fluorescein and cells appear fluorescent in green, whereas cells with an inactive metabolism are not fluorescent. SYTOX green is a non-permeant nucleic acid stain that enters only into cells with damaged plasma membranes, such as in dead cells, which then appear with green fluorescent nuclei. 7-AAD is a fluorescent DNA-intercalator that is excluded from live cells; dead cells appear with red fluorescent nuclei. These techniques are proposed as alternative methods to determine copepod embryo viability in laboratory experiments, such as in egg-production studies, or in field studies to evaluate copepod recruitment rates in situ.
Materials and methods
Zooplankton was collected in the northern Adriatic Sea with a 200-µm mesh plankton net, from March to May 2003. Adult females of the copepod Calanus helgolandicus were sorted and incubated in 500-ml bottles filled with 0.22-µm-filtered seawater (FSW) and with an algal suspension of the dinoflagellate Prorocentrum mininum (PRO) (Pavillard) Sciller, isolated from the Gulf of Naples, at a concentration of about 7×103 cells ml−1. Bottles, containing about 20 females each, were transported to the Stazione Zoologica of Naples in a refrigerated box. In the laboratory, about 40 C. helgolandicus females were individually transferred into crystallizing dishes containing 100 ml FSW and either PRO, at a concentration of 3×103 cells ml−1, or the diatom Skeletonema costatum (SKE), isolated from the North Adriatic Sea, at a concentration of 3×104 cells ml−1, equivalent to a daily carbon input of about 50–60 µg C (Carotenuto et al. 2002). Algae were in the exponential growth phase and cultured as described in Carotenuto et al. (2002). Females were incubated at 20°C for about 10–15 days; each day, embryos were collected and females were transferred to new crystallizing dishes containing fresh algae. Embryos were divided into two groups: one batch of embryos was left undisturbed for 24 h in FSW until hatching, as a control; a second group of embryos was incubated in FSW containing 1 U ml−1 chitinase (EC 3.2.1.14; Sigma-Aldrich, Milan, Italy) for 50 min at 20°C. After incubation in chitinase, embryos were rinsed in FSW and further divided into two groups. One group was left to hatch, to determine the effect of the chitinase enzyme on egg-hatching success, and another group was incubated, at 20°C in the dark, in one of the following vital dyes: fluorescein diacetate (FDA) (Sigma-Aldrich), SYTOX green, and 7-aminoactinomycin D (7-AAD) (Molecular Probes, Leiden, The Netherlands). Stained embryos were observed in epifluorescent, confocal and transmitted-light modes, with an inverted confocal laser scanning microscope (LSM; Zeiss 410) equipped with a Plan-neofluar 25× water immersion objective (NA 0.80).
FDA was prepared by dissolving 7.3 mg FDA in 5 ml of the buffer dimethylsulphate (DMSO) (Sigma-Aldrich). After incubation of embryos for 50 min in 7.5 µM of this solution, stained embryos were rinsed three times with FSW and observed with the LSM using an Argon laser (488 nm wavelength, λ). To evaluate the toxicity of DMSO, a group of embryos was incubated for 50 min in 2.5 µl DMSO ml−1, without the dye. After chitinase incubation, another group of embryos was incubated for 50 min in 20 µM SYTOX green. After rinsing three times, embryos were observed with the LSM using the same setting as for FDA. A third group of embryos was incubated for 50 min in 20 µg 7-AAD ml−1. A stock solution of 7-AAD was prepared by dissolving 1 mg in 1 ml methanol. 7-AAD/DNA complexes were excited by the He-Neon laser (543 nm λ). To test the effect of methanol on egg-hatching success, a group of embryos was incubated in 20 µl methanol ml−1 alone.
Fluorescent embryos were optically z-sectioned and three-dimensional (3D) fluorescent images were acquired with the Zeiss software. Transmitted light images were acquired with a He-neon laser (633 nm λ) on a single focal plane. The percentages of FDA-fluorescent, and SYTOX green– and 7-AAD–non-fluorescent embryos were determined with the LSM in epifluorescence mode. After LSM observations, each group of embryos was left untouched at 20°C for 24 h, until they hatched, to verify the number of living nauplii. Percentage hatching success was also calculated for control unstained embryos, and for embryos incubated in chitinase, DMSO and methanol alone. Each data point represents a mean of 5–15 replicates of about 30 embryos each. Comparisons between groups of data points were performed using unpaired statistical t-test analyses (Graphpad software).
To determine the length of time that samples maintained their fluorescence, embryos were stained with each of the vital dyes, as described above, observed with the LSM, and then fixed in 4% paraformaldehyde in FSW. After 24 h, fixed embryos were rinsed in phosphate-buffered saline (PBS; pH 7.4) and left in the PBS solution containing 0.02% sodium azide at 4°C in the dark for different lengths of time. Percentage of fluorescent embryos was calculated each 24–48 h, up to 408 h after fixation, with the epifluorescent microscope.
The SYTOX green staining protocol was tested as an experimental protocol to evaluate egg-hatching success in classical copepod egg-production experiments: three females of C. helgolandicus were individually incubated in 100 ml FSW containing an algal suspension of the dinoflagellate PRO, and three other females were incubated with the diatom SKE at the final cell concentrations reported above. Each day, females were transferred to new crystallizing dishes containing fresh algal cultures; embryos were collected after 1, 4 and 8 days of feeding on these diets, and were further divided into two groups. One group of embryos (control) was left undisturbed for 24 h to develop normally to hatching, as described above, and another group of embryos was stained with SYTOX green. The percentage of (control) hatched embryos was compared with the percentage of SYTOX green–non-fluorescent embryos.
Results
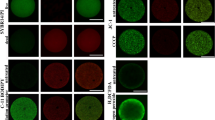
Viable embryos stained with FDA appeared with a green fluorescence. Figure 1A shows a 3D-reconstructed viable Calanus helgolandicus embryo at the 64-blastomere stage in which symmetrical blastomeres are clearly visible. Figure 1B shows a single focal plane of the same embryo observed in transmitted light. Figure 1C shows two 3D-reconstructed embryos, but only one, at the gastrula stage, is fluorescent; the other embryo, at the 32-blastomere stage, is not fluorescent even if it is slightly visible due to the high brightness value. Figure 1D shows the same embryos as in panel C observed in transmitted light and on a single focal plane. Figure 1E and F show a 3D-reconstructed viable embryo a few minutes prior to hatching and corresponding transmitted-light images, respectively.
Calanus helgolandicus. Viable embryos stained with fluorescein diacetate and observed with the confocal laser scanning microscope. A Fluorescent 3D image of a 64-cell-stage embryo. B Single focal plane of the same embryo in panel A observed in transmitted light. C 3D-reconstructed image of a fluorescent embryo at the gastrula stage (left), and a non-fluorescent 32-stage embryo (right). D The same embryos as in panel C observed in transmitted light on a single focal plane. E 3D reconstruction of a developed viable embryo before hatching. F The same embryo as in panel E observed in transmitted light on a single focal plane. Scale bar: 64.3 µm
SYTOX green marks only non-viable embryos with compromised membrane permeability. Figure 2A shows a 3D reconstruction of a SYTOX green–positive embryo. Nuclei are clearly visible even though the embryo does not reveal any apparent malformations. When observed in transmitted light (Fig. 2B), the same embryo appears darker and less transparent than the nearest viable embryos, which were not fluorescent (Fig. 2A). Figure 2C shows an abnormal non-viable embryo, in which the nuclei are asymmetrically distributed within the cytoplasm, and Fig. 2D shows the same embryo observed in transmitted light.
Calanus helgolandicus. Non-viable embryos stained with SYTOX green and observed with the confocal laser scanning microscope. A Fluorescent 3D image of non-viable morula embryo. B Transmitted light of the same field as in panel A; the darker embryo at the center of the field is positively stained with SYTOX green. C Fluorescent 3D image of an abnormal embryo with dispersed chromatin. D The same embryo as in panel C observed in transmitted light on a single focal plane. Scale bar: 58.5 µm
Figure 3 shows C. helgolandicus embryos stained with 7-AAD. This dye penetrates into dead cells and emits a red fluorescence when it does so. The embryo in Fig. 3A appears as a normally developed morula stage, with small, symmetrical nuclei in the cytoplasm. However, the red fluorescence indicates that this embryo is not viable. Figure 3B shows the same embryo as in Fig. 3A, observed in transmitted light. Figure 3C shows an embryo with red nuclei that has undergone asymmetric development. When observed in transmitted light (Fig. 3D), the same embryo appears dark and with incomplete cytokinesis. Sometimes, unstained embryos show a slight auto-fluorescence after chitinase incubation, when images are acquired with the same configuration as for 7-AAD (Fig. 3E, G). However, in auto-fluorescent embryos, nuclei are not well distinguished and images appear out of focus (Fig. 3E, G) compared to 3D images of non-viable embryos stained with 7-AAD (Fig. 3A, C). Figure 3F and H show the same embryos as in Fig. 3E and G, observed in transmitted light on a single focal plane.
Calanus helgolandicus. Non-viable embryos stained with 7-aminoactinomicycin D (A–D) and auto-fluorescent unstained embryos (E–H), observed with the confocal laser scanning microscope. A 3D reconstruction of a fluorescent embryo at the morula stage. B The same embryo as in panel A observed in transmitted light on a single focal plane. C 3D reconstruction of a fluorescent embryo; nuclei are asymmetrically distributed in the cytoplasm. D The same embryo as in panel C observed in transmitted light on a single focal plane. E, G 3D reconstructions of viable embryos emitting red auto-fluorescent. F, H The same embryos as in panels E and G observed in transmitted light. Scale bar: 54.3 µm
The percentage of fluorescent-FDA embryos (FDA positive) and non-fluorescent SYTOX green and 7-AAD embryos (referred to as stained embryos) were determined and compared with the percentage of hatched unstained (control) embryos, and embryos that had been stained, washed, and allowed to hatch to test the effect of the various dyes on hatching viability (for simplicity, these embryos are referred to as 24 h after staining) (Fig. 4). The percentage of FDA-positive embryos (N=15) was 70.4±28.3 (mean±SD) compared to 25.9±26.2, 24 h after staining. The percentage of control unstained embryos was 76.1±24.5, similar to the percentage of FDA-positive embryos. Unpaired t-test indicates that the difference between the FDA-positive and control unstained embryos is not statistically significant (P=0.66), whereas the percentage egg viability 24 h after FDA staining was significantly lower than that of FDA-fluorescent and control hatched nauplii (P<0.0001).
Calanus helgolandicus. Comparison between the percentage of egg viability for unstained embryos (controls), FDA-fluorescent embryos, for SYTOX green– and 7-AAD–non-fluorescent embryos (stained embryos), and for embryos stained with the three probes and then allowed to hatch (24 h after staining) (mean±SD)
In the case of SYTOX green, the percentage of non-fluorescent embryos (76.0±31.6; N=11) was similar to the percentage of hatched embryos 24 h after staining (88.3±11.2; N=11) and control hatched nauplii (90.5±7.6; N=15) (Fig. 4). Also, in the case of 7-AAD, the percentage of non-fluorescent embryos (58.3±21.8; N=5) was very similar to hatched embryos 24 h after staining (53.5±20.9; N=5) and controls (56.4±26.4; N=8). Differences were not significantly different (unpaired t-test).
To verify if DMSO was responsible for the low hatching success of FDA-stained embryos, another group of embryos was incubated in this blank solvent alone. Figure 5 shows that the percentage hatching success of embryos incubated in DMSO is similar to that recorded for controls (63.9±10.8 and 70.3±12.7, respectively); the difference was not statistically significant. The percentage of hatching success for FDA-stained embryos is lower with respect to both controls and embryos incubated in DMSO alone (20.4±35.2), even if the difference is not statistically significant (unpaired t-test). Methanol incubation did not interfere with percentage egg-hatching success (data not shown).
Figure 6 reports the percentage of fluorescent embryos over time, for each of the dyes. Fluorescence due to FDA rapidly bleaches after 24 h, with a loss of fluorescence in 50% of the embryos (Fig. 6A). After 48 h, only a mean value of 23.3% of the embryos was positively stained with FDA, and after 72 h none of the embryos were fluorescent. 7-AAD was a somewhat better stain than FDA, with about 20% of the embryos losing their fluorescence after 24 h, and 45.3%, after 48 h. After 72 h, only 17% of the embryos were still fluorescent, whereas after 96 h none of the embryos were fluorescent (Fig. 6C). SYTOX green was the best of the three dyes (Fig. 6B); after 144 h a mean value of 92% of the embryos was still positively stained, and after 240 h fluorescence still occurred in 54% of the embryos, subsequently dropping to 28% and 0% after 312 and 408 h, respectively.
We also tested the possible applications of these fluorescent probes in egg-production/egg-hatching assays in which different algal diets are tested for their possible negative effects on embryo viability (e.g. Turner et al. 2001). Figure 7 compares the number of hatched C. helgolandicus nauplii 24 h after spawning and the percentage of embryos negatively fluorescent with SYTOX green after 1, 4 and 8 days of feeding on either PRO or SKE. In both cases, the number of hatched nauplii was similar to the number of non-fluorescent embryos stained with SYTOX green (N=56 for PRO and N=47 for SKE). Figure 7B shows that the percentage egg viability decreases after 8 days of maternal feeding with SKE, passing from about 80% on the first day to about 30% after 8 days, similar to other laboratory findings for this copepod and diatom species (Ianora et al. 2003).
Calanus helgolandicus. Percentage egg viability for females fed Prorocentrum minimum (PRO) and Skeletonema costatum (SKE) for 8 days. Comparison between percentage egg viability calculated for unstained embryos allowed to develop to hatching (control), and for SYTOX green–non-fluorescent embryos stained immediately after spawning
Discussion
In this paper we describe, for the first time, new methods for the rapid detection of copepod embryo viability soon after egg laying. Using the three different fluorescent probes FDA, SYTOX green and 7-AAD, we show how it is possible to distinguish between viable and dead embryos, depending on the biochemical properties of the probes used. FDA activity is based on intracellular enzyme characteristics, and selection is for viable embryos that appear fluorescent. On the other hand, the activity of SYTOX green and 7-AAD is based on membrane integrity, and selection is for non-viable embryos that appear fluorescent. All three dyes gave good results in terms of predicting egg-hatching success, compared with control untreated embryos that were allowed to develop undisturbed to hatching (Fig. 4). These dyes penetrated well into copepod embryos, but only after chitinase treatment. In fact, even though SYTOX green and 7-AAD generally penetrate into cells that have lost membrane integrity (Breeuwer and Abee 2000; Waters et al. 2002), in the case of copepod embryos the chitinous wall represents an impermeable barrier to the dyes also in dead embryos (Buttino, unpublished data). FDA is incapable of penetrating into viable copepod embryos, even if it easily penetrates into other viable cell types, such as fungus (Brul et al. 1997), plant (Windholm 1972) and animal cells (see Johnson 1998, for a review). In our experiments, treatment with chitinase enzyme did not affect egg-hatching success at the concentrations tested or for the time of incubation used in this protocol. Hence, copepod eggs can be pre-treated with this enzyme without compromising egg-hatching viability.
Both “dead or live cell” stains can be used to assess reproductive success. To date, most copepod egg-production/egg-hatching experiments (Poulet et al. 1994; Ianora et al. 1996, 2003; Turner et al. 2001; Ceballos and Ianora 2003) have determined embryo viability by allowing eggs to develop undisturbed to hatching, which usually requires 24–72 h. Here, we show that embryo viability can also be rapidly assessed using FDA, whereas SYTOX green and 7-AAD stains can be used to determine the percentage embryo mortality.
Of the three dyes, FDA appears to be the least appropriate. Most of the viable embryos stained with FDA were not viable 24 h later (Figs. 4, 5), probably due to the toxicity of this dye. Inhibition of egg hatching was not due to DMSO, the solvent in which FDA was dissolved, at the concentrations tested (Fig. 5). The toxic effect of FDA on cell viability has never been reported before, but our results indicate that this dye may underestimate hatching viability if embryos are not examined immediately after staining. The two other dyes gave good results soon after staining and 24 h later, indicating that they were not toxic. However, embryos treated with chitinase and excited for 7-AAD at times appeared slightly auto-fluorescent (Fig. 3E, G), indicating possible overestimation of dead embryos. SYTOX green was therefore the best of the three dyes tested, since embryos stained with this dye gave good results immediately after staining and 24 h later, and there were no problems due to auto-fluorescence. SYTOX green was also the better of the three dyes because it lost its fluorescence much more slowly than the other two and, once fixed, the percentage embryo viability could be determined with accuracy up to 120 h after staining. This dye could, therefore, potentially be a good probe to allow for the determination of hatching success in fixed samples collected in the field and estimated later in the laboratory.
Vital fluorescent probes could also find useful applications in other studies on copepods. For example, in copepod-rearing experiments such as those of Carotenuto (1999) and Carotenuto et al. (2002), SYTOX green or 7-AAD could be used to eliminate dead embryos from the culture. The same probes could also be used to test for negative effects of pollutants and chemicals on embryo viability in in vitro assays. Fluorescent probes are currently being used in ecotoxicological studies to assess the metabolic activity of microalgae in water samples (e.g. Gilbert et al. 1992) or to test pathogen-derived compounds inducing cell death (Chand et al. 1994; Clarke et al. 2001). However, they have rarely been used in copepod studies, except for the vital fluorescent probe Hoeschst 33342, used to identify early anomalies in copepod embryogenesis (e.g. Poulet et al. 1995) and for fluorescent probes specific for apoptosis (programmed cell death), which have recently been used to differentiate necrotic or apoptotic processes in copepod embryos and nauplii (Ianora et al. 2003; Poulet et al. 2003; Romano et al. 2003).
The techniques proposed in our study indicate that fluorescent probes such as FDA, SYTOX green and 7-AAD can also rapidly assess copepod embryo viability. In recent years, the use of fluorescent probes to study various physiological processes in cells has grown at a rapid rate (see Johnson 1998, for a review) and the possibility of applying fluorescent techniques to copepod embryos opens new perspectives in studies on the reproductive physiology of copepods and zooplankton, in fields that have not been investigated until now.
References
Agusti S, Sanchez MC (2002) Cell viability in natural phytoplankton communities quantified by a membrane permeability probe. Limnol Oceanogr 47:818–828
Breeuwer P, Abee T (2000) Assessment of viability of microorganisms employing fluorescence techniques. Int J Food Microbiol 55:193–200
Brul S, Nussbaum J, Dielbandhoesing SK (1997) Fluorescent probes for wall porosity and membrane integrity in filamentous fungi. J Microbiol Meth 28:169–178
Brussaard CPD, Marie D, Thyrhaug R, Bratbak G (2001) Flow cytometric analysis of phytoplankton viability following viral infection. Aquat Microb Ecol 26:157–166
Buttino I, Ianora A, Carotenuto Y, Zupo V, Miralto A (2003) Use of the confocal laser scanning microscope in studies on the developmental biology of marine crustaceans. Microsc Res Tech 60:458–464
Carotenuto Y (1999) Morphological analysis of larval stages of Temora stylifera (Copepoda, Calanoida) from the Mediterranean Sea. J Plankton Res 21:1613–1632
Carotenuto Y, Ianora A, Buttino I, Romano G, Miralto A (2002) Is post-embryonic development in the copepod Temora stylifera negatively affected by diatom diets? J Exp Mar Biol Ecol 276:49–66
Ceballos S, Ianora A (2003) Different diatoms induce contrasting effects on the reproductive success of the copepod Temora stylifera. J Exp Mar Biol Ecol 294:189–202
Chand S, Lusuzi I, Veal DA, Williams LR, Karuso P (1994) Rapid screening of the antimicrobial activity of extracts and natural products. J Antibiot (Tokyo) 47:1295–1304
Clarke MJ, Gillings MR, Altavilla N, Beattie AJ (2001) Potential problems with fluorescein diacetate assays of cell viability when testing natural products for antimicrobial activity. J Microbiol Meth 46:261–267
Gilbert F, Galgani F, Cadiou Y (1992) Rapid assessment of metabolic activity in marine microalgae: application in ecotoxicological tests and evaluation of water quality. Mar Biol 112:199–205
Huhtinen M, Reilas T, Katila T (1996) Recovery rate and quality of embryos from mares inseminated at the first post-partum oestrum. Acta Vet Scand 37:343–350
Ianora A (1998) Copepod life history traits in subtemperate regions. J Mar Syst 15:337–349
Ianora A, Poulet SA, Miralto A, Grottoli R (1996) The diatom Thalassiosira rotula affects reproductive success in the copepod Acartia clausi. Mar Biol 125:533–539
Ianora A, Poulet SA, Miralto A (2003) The effects of diatoms on copepod reproduction: a review. Phycologia 42:351–363
Johnson I (1998) Fluorescent probes for living cells. Histochem J 30:123–140
Joux F, Lebaron P (2000) Use of fluorescent probes to assess physiological functions of bacteria at single-cell level. Microb Infect 2:1523–1535
Laabir M, Poulet SA, Ianora A (1995) Measuring production and viability of eggs in Calanus helgolandicus. J Plankton Res 17:1125–1142
Leveroni Calvi S, Maisse G (1998) Cryopreservation of rainbow trout (Oncorhynchus mykiss) blastomeres: influence of embryo stage on postthaw survival rate. Cryobiology 36:255–262
Mauchline J (1998) The biology of calanoid copepods. Academic , San Diego,
Miralto A, Guglielmo L, Zagami G, Buttino I, Granata A, Ianora A (2003) Inhibition of population growth in the copepods Acartia clausi and Calanus helgolandicus during diatom blooms. Mar Ecol Prog Ser 254:253–268
Poulet SA, Ianora A, Miralto A, Meijer L (1994) Do diatoms arrest embryonic development in copepods? Mar Ecol Prog Ser 111:79–86
Poulet SA, Laabir M, Ianora A, Miralto A (1995) Reproductive response of Calanus helgolandicus. I. Abnormal embryonic and naupliar development. Mar Ecol Prog Ser 129:85–95
Poulet SA, Richer de Forges M, Cueff A, Lennon JF (2003) Double-labelling methods used to diagnose apoptotic and necrotic cell degradations in copepod nauplii. Mar Biol 143:889–895
Romano G, Russo GL, Buttino I, Ianora A, Miralto A (2003) A marine diatom-derived aldehyde induces apoptosis in copepod and sea urchin embryos. J Exp Biol 206:3487–3494
Turner TJ, Ianora A, Miralto A, Laabir M, Esposito F (2001) Decoupling of copepod grazing rates, fecundity and egg-hatching succession mixed and alternating diatom and dinoflagellate diets. Mar Ecol Prog Ser 220:187–199
Vanderwall DK (1996) Early embryonic development and evaluation of equine embryo viability. Vet Clin 12:61–83
Veldhuis MJW, Cucci TL, Sieracki ME (1997) Cellular DNA content of marine phytoplankton using two new fluorochromes: taxonomic and ecological implications. J Phycol 33:527–541
Waters WR, Harkins KR, Wannemuehler MJ (2002) Five-color flow cytometric analysis of swine lymphocytes for detection of proliferation, apoptosis, viability, and phenotype. Cytometry 48:146–152
Widholm JM (1972) The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol 47:189–194
Yang A, Cardona DL, Barile FA (2002) In vitro cytotoxicity testing with fluorescence-based assays in cultured human lung and dermal cells. Cell Biol Toxicol 18:97–108
Acknowledgements
We thank F. Esposito for algal culturing. We declare that the experiments comply with the current laws of the country in which experiments were performed.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
Buttino, I., do Espirito Santo, M., Ianora, A. et al. Rapid assessment of copepod (Calanus helgolandicus) embryo viability using fluorescent probes. Marine Biology 145, 393–399 (2004). https://doi.org/10.1007/s00227-004-1317-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1317-7