Abstract
The distribution, feeding and oxygen consumption of Calanus sinicus were studied in August 2001 on a transect across Yellow Sea Cold Bottom Waters (YSCBW) and two additional transects nearby. The distribution of C. sinicus adults and copepodites stage CV appeared to be well correlated with water temperature. They tended to concentrate in the YSCBW (>10,000 ind. m−2) to avoid high surface temperature. Gut pigment contents varied from 0.44 to 2.53 ng chlorophyll a equivalents (chl a equiv.) ind.−1 for adults, and from 0.24 to 2.24 ng chl a equiv. ind.−1 for CV copepodites. We found no relationship between gut pigment contents and the ambient chl a concentrations. Although the gut evacuation rate constants are consistent with those measured for other copepods, their low gut pigment contents meant an estimated daily herbivorous ingestion of <3% of body carbon in the YSCBW and <10% outside the YSCBW. However, based on estimates of clearance rates, C. sinicus feeds actively whether in the YSCBW or not, so the low ingestion rates probably reflect shortage of food. Oxygen consumption rates of C. sinicus ranged from 0.21 to 0.84 μl O2 ind.−1 h−1, with high rates often associated with high temperature. From the oxygen consumption rates, daily loss of body carbon was estimated to be 4.0–13.7%, which exceeds our estimates of their carbon ingestion rates. C. sinicus was probably not in diapause, either within or outside the YSCBW, but this cold-water layer provides C. sinicus with a refuge to live through the hot, low-food summer.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Calanus sinicus is distributed widely in the Northwest Pacific Ocean, from Japan to the South China Sea (Anon. 1977; Uye 2000). It generally dominates the mesozooplankton in the Inland Sea of Japan, the Bohai Sea, the Yellow Sea and the East China Sea. Being primarily a herbivore and an important food species for fish (e.g. sardine, anchovy, etc.), it occupies a key trophic position between the phytoplankton and higher levels, such as fish larvae and pelagic fish in this area (Meng 2003). Its population dynamics was one of the principal foci of the Chinese GLOBEC Yellow Sea and East China Sea Program.
This copepod occurs continuously throughout the year in the Inland Sea of Japan, the Bohai Sea, the Yellow Sea and the East China Sea, but both its abundance and distribution show a strong seasonal variation. Its numbers are highest in May–June, when it is found from the coast to the offshore Pacific. In summer, however, its population declines sharply and becomes limited to the shelf water, especially the near-shore area, where the population is almost absent (Anon. 1977; Uye 2000; Wang et al. 2003). Although some biological attributes of C. sinicus have been demonstrated in previous studies (Uye 2000, and references cited therein), the oversummering mechanism is still unclear.
C. sinicus has been considered a warm-temperate and coastal copepod (Chen and Zhang 1965; Hulsemann 1994), with a wide tolerance range of temperature and salinity (Huang and Zheng 1986; Huang et al. 1993; Uye 2000). However, temperature is conceivably one of the most important limitations to its population development in summer (Wang et al. 2003). Hatching of C. sinicus is possible between 5°C and 22.6°C, and ~23°C is probably the upper limit for its reproduction (Uye 1988; Huang et al. 1993). Further, an upper thermal limit for occurrence of this copepod is 26–27°C (Huang and Chen 1985; Huang et al. 1991; Lin and Chen 1992).
The Yellow Sea is one of the main population distribution centers of C. sinicus in the shelf waters of the Northwest Pacific. Although the surface water temperature in most parts of the Yellow Sea was above the upper thermal limit (>26°C) in mid-summer, the Yellow Sea Cold Bottom Waters (YSCBW) provide an optimal oversummering site for this copepod (Wang et al. 2003). Some behaviors exhibited by the C. sinicus population in YSCBW indicated that this copepod may habe been in diapause, with a near lack of diel vertical migration, much lower reproduction rates and with copepodite stage CV dominating the population (Sun et al. 2002). However, whether this can be considered true diapause is unclear. The clutch size of C. sinicus was still at a comparatively high level in summer within the YSCBW, and the low reproduction rate was mainly due to the low temperature, the low food concentration and the strong thermocline (Zhang et al. 2002; Wang et al. 2003). Thus, further work is needed to clarify the mechanism by which C. sinicus populations oversummer.
A cruise was designed to study the oversummering strategy of C. sinicus in August 2001. This paper presents data on the activities of herbivorous feeding and respiration of C. sinicus in and outside the YSCBW.
Materials and methods
The cruise was conducted by the R.V. “Beidou” from 11 to 24 August 2001. The research area, transects and stations are shown in Fig. 1. Two stations (A7 and B3), where the ship was anchored for 27–30 h, were chosen to study the copepod diel feeding rhythms and ingestion rates.
Temperature and salinity were recorded with a CTD (Sea Bird Electronics, SBE-19). Seawater samples (500 ml) for the measurement of chlorophyll a (chl a) were collected from depths of 0, 10, 20, 30, 50 m and the bottom and filtered through GF/F glass-fiber filters. The filters were then extracted in 90% aqueous acetone for 24 h at 0°C, and the extracts were measured in a Turner Designs fluorometer in accordance with the method of Yentsch and Menzel (1963).
Zooplankton samples were collected at each station with an 80-cm-diameter closing net (mesh size: 300 μm). Vertical tows were made in different layers: 10–0 m, 20–10 m, 40–20 m, 60–40 m and the bottom to 60 m, to study the vertical distribution pattern. Samples were preserved in 5% neutralized formalin seawater solution. All Calanus sinicus were counted under a dissecting microscope.
Zooplankton samples for gut pigment analysis were collected with the same type of net, but with a sealed cod-end towed vertically from the bottom to the surface. Immediately after sampling, a portion of the cod-end contents was passed through a 500-μm screen and gently rinsed with filtered (GF/C) seawater to remove debris and phytoplankton cells that may have adhered to the animals. The contents were then filtered on the GF/C filters and kept frozen at −30°C in the dark until being transferred to the laboratory. This operation took ca. 3–5 min.
In the laboratory, samples were thawed in the dark and then sorted under a dissecting microscope; about 30 adults and 40 CV-stage C. sinicus were individually picked. Following brief grinding in a glass tube, copepods were rinsed into a 10-ml glass centrifuge tube, and gut pigment was extracted in 90% aqueous acetone for 24 h in the dark at −30°C. Then, the tubes were centrifuged for 10 min, and the fluorescence of the suspension from each tube was measured before and after acidification with 10% HCL using a Turner Designs model II fluorometer. Total gut pigment content (GPC) is expressed in chlorophyll a equivalent weight. A mean carbon/chlorophyll ratio of 50 (Zhang, unpublished data) was used to convert chl a into phytoplankton carbon.
Diurnal variations in GPC were investigated at the anchor stations to study diurnal feeding rhythms. At each station, samples were collected at 3-h intervals for a complete daily cycle, and the GPC was measured as above. Night was defined as the time from sunset to sun rise.
Within 3–5 min after capture, living samples for gut evacuation experiments were gently rinsed with filtered seawater to wash away phytoplankton cells and placed in several 2-l beakers containing 0.45-μm-filtered seawater. Beakers were kept in a dark incubator set to the temperature of the layer in which C. sinicus were most abundant. Initially, and after 10, 20, 30, 60, 90 and 120 min, animals were taken from the respective containers, filtered on the GF/C filters and frozen at −30°C in darkness. Subsequent treatments and measurements were the same as gut pigment analysis. The gut evacuation rate constant (k) was calculated by using a negative exponential equation (Mackas and Bohrer 1976). Assuming that the animals were feeding at a constant rate, ingestion rates (I) were calculated from the expression: I=GPC×kl(1−b′), where GPC was the average value over a daily cycle and b’′ was the pigment destruction rate. Pigment loss was assumed to be constant and equal to 33% (Dam and Peterson 1988).
Oxygen consumption rates of C. sinicus were determined by a water-bottle method. Zooplankton was collected with the same type of net as that for samples used in feeding measurements. Seawater for washing and incubation was collected by a large water sampler from the same layer, filtered through a cellulose acetate filter (0.45 μm), aerated to near saturation (lasting 2–3 h), and preserved at the same temperature as that of the sampling layer. Immediately after the collection, undamaged specimens were gently washed and transferred into the glass bottles (500 ml) filled with filtered seawater. Twenty individuals were incubated in each bottle. Specimens were transferred by pipettes during the experiments. The bottles were sealed, wrapped with aluminum foil, and kept at in situ temperature for 24 h. Control bottles without zooplankton were prepared concurrently.
At the end of incubation, two 100-ml water samples were siphoned out for duplicate determination of dissolved oxygen. The copepods were inspected under a dissecting microscope to determine whether they were still alive, filtered on a GF/C filter to remove seawater adhering to the body, and stored frozen at −30°C for later weighing and elemental analyses ashore.
Dissolved oxygen was determined by a Winkler titration method (Strickland and Parsons 1968). Frozen copepod specimens were dried at 60°C for 24 h and weighed to obtain dry weight. Carbon content was measured with a P-E 240C elemental analyzer using acetanilide as a standard.
Results
Hydrographic conditions and chl a
During the study period, the presence of YSCBW resulting from summer stratification was apparent. For transect A, through the YSCBW, the seasonal thermocline was well developed and was located between 10 and 35 m depth. Surface temperatures were in the range of ca. 25–28°C, and bottom temperatures were ca. 12–15°C inshore and ~8°C in the YSCWM (Fig. 2). On two additional transects outside the YSCBW, the thermoclines were less evident and the bottom temperatures were higher than 15°C (Fig. 2). According to the boundary determined by Weng and Wang (1982), seven stations (stations A3, A4, A5, A6, A7, A7′, A8) were located in the YSCBW.
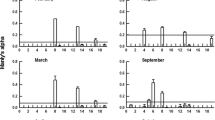
The chl a concentrations were at a comparatively low level (<0.5 mg m−3 on average at most stations) during the study period. Spatially, the chl a concentrations were lower in the YSCBW-occupied area than in that outside the YSCBW, and lower below the thermocline than above it (Fig. 3).
Distribution of Calanus sinicus
C. sinicus was found at all stations, but its abundance varied greatly from 120 to 25,220 ind. m−2 (Fig. 4). It resided mainly in the area of the YSCBW, with low numbers in the coastal and southern area. The vertical distribution of C. sinicus (Fig. 5) shows a concentration in the deep, cold layer occupied by the YSCBW. Even through the distribution center of the population moved up somewhat to the middle layer during darkness, very few C. sinicus were found above the thermocline. On transects B and C, the copepod also mainly resided in deeper waters, but abundances were much lower.
Feeding of C. sinicus
The gut pigment contents of C. sinicus during the study period are shown in Fig. 6. The minimum and maximum values were 0.44 and 2.53 ng ind.−1 for adults and 0.24 and 2.24 ng ind.−1 for CV copepodites, respectively. There was no apparent correlation between the gut pigment contents and the ambient chlorophyll concentrations. Chlorophyll was always less abundant than phaeopigment in the gut of C. sinicus, averaging 20.4% (range: 11.7–38.5%) of total pigment contents. The difference in the percentage compositions of chlorophyll was not related to differences in ambient chlorophyll levels.
Calanus sinicus. Gut pigment contents in relation to mean chlorophyll a concentrations. The sampling time at each station was the same as that in Fig. 5
Diel variation in the gut pigment contents of C. sinicus was observed at two anchor stations (Fig. 7). At station A7, located in the YSCBW, the diurnal amplitudes in gut pigment of adults and CV copepodites were only 0.72 and 0.14 ng ind.−1, respectively. But at station B3, outside the YSCBW, C. sinicus exhibited pronounced diel feeding rhythms and the diurnal amplitudes were 4.20 ng ind.−1 for adults and 1.20 ng ind.−1 for CVs.
Four experiments were carried out to estimate gut evacuation rate constants of C. sinicus at the two anchor stations (Fig. 8). Gut pigment contents decreased sharply during the initial 20–30 min, and slowed or stopped thereafter. Copepods that were starved for 60–120 min still contained some pigment in the gut. The gut evacuation rate constant was calculated using data obtained for the first 30 min to effects of food deprivation. At station A7, where the experimental temperature was 10°C, the estimated gut evacuation rate constant was 0.0320 and 0.0283 min−1 for adults and CVs, respectively. At station B3, the rates increased to 0.0373 min−1 for adults and 0.0388 min−1 for CVs at 18°C.
Daily individual pigment-specific ingestion rates are shown in Table 1. Individual ingestion rates at station A7 were lower than those measured at station B3 for both adults and CVs. Assuming a carbon-to-chl a ratio of 50 and a 70% assimilation efficiency (Conover 1978), Daily pigment-specific ingestion rates were converted to carbon units. Daily carbon ingestion derived from herbivorous feeding by C. sinicus accounted for ca. 2.7–2.8% and ca. 8.0–9.1% of their body carbon weight at stations A7 and B3, respectively (Table 1).
We have estimated the clearance rates of C. sinicus from the average concentrations of chl a in the water column in which >95% of C. sinicus lived (from the bottom to 20 m and 10 m for stations A7 and B3, respectively) and the estimated ingestion rates. The clearance rates of adults were higher than those of CVs at both stations (Table 2). For the same stage, however, no significant difference appeared between the two stations.
Oxygen consumption rates of C. sinicus
Oxygen consumption rates of C. sinicus were determined at six stations (Table 3). At stations A2, A7, A9 and B3, animals for experiments were collected below the thermocline, where C. sinicus tended to concentrate. At stations A7′ and A8, animals were collected from the thermocline. Oxygen consumption rates varied from 0.21 to 0.84 μl O2 ind.−1 h−1; they generally increased with increasing water temperature. Results from the stations in the YSCBW area indicated that the animals collected from the thermocline (water temperature: ca. 15–28°C) showed higher oxygen consumption rates than those collected below the thermocline (water temperature: <11°C). However, for the animals living at the same temperature, oxygen consumption was lower in the YSCBW (station A7′) compared with outside the YSCBW (station B3).
In order to calculate daily metabolic loss as a percentage of body carbon, oxygen consumption rates were converted to CO2 evolution rates, using a respiratory quotient (RQ) value of 0.97 (protein metabolism, cf. Gnaiger 1983), i.e. oxygen consumption rate×0.97×12/22.4, where 12/22.4 is carbon mass (g) in 1 mol of CO2 (22.4 l). The daily metabolic loss of body carbon for C. sinicus thus estimated ranged from 4.0% to 13.7%, which was higher than the daily carbon ingestion derived from herbivorous feeding during the study period (Table 3).
Discussion
This paper reported on the distribution, feeding and respiration of the oversummering population of Calanus sinicus in the Yellow Sea. Together, our data on vertical distribution and ingestion, clearance and respiration rates show that the copepods had reduced their metabolic losses by entering the YSCBW. Below we discuss the lines of evidence suggesting that they were active there and not in a state of diapause.
Distribution
Population density of C. sinicus decreased to a very low level in most parts of the Yellow Sea, but a high abundance of adults and CVs resided in the YSCBW during the study period. This distribution pattern is in agreement with previous studies in the Yellow Sea during the same season (Anon. 1977; Wang et al. 2003). Although C. sinicus performs pronounced diel vertical migration (Uye et al. 1990; Huang et al. 1992), the vertical distribution pattern indicated that few animals appeared above the thermocline, whether in the daytime or at night, especially in the area occupied by the YSBCW, during the study period. A possible explanation for these observations is that C. sinicus was avoiding the high temperature above the thermocline (Huang and Chen 1985; Lin and Chen 1992; Huang et al. 1993). Furthermore, the presence of a pronounced thermocline possibly affected the vertical migration of copepods (Williams 1985).
Feeding
The gut fluorescence method is one approach to measure in situ phytoplankton ingestion rates of zooplankton, although it is not free of methodological problems. One inaccuracy of this method is that pigment destruction indeed occurs during gut passage and that the degree of pigment destruction varies greatly (e.g. Conover et al. 1986; Head and Harris 1992; McLeroy-Etheridge and McManus 1999). We did not measure the pigment loss level during this study and chose to correct for pigment destruction using an estimated average value of 33% (Dam and Peterson 1988).
Compared with previous reports, the gut pigment contents of C. sinicus during this study period were at relatively lower levels, ranging from 0.24 to 2.53 ng ind.−1. The maximum value observed under laboratory and field conditions was 21.6 and 28.2 ng ind.−1, respectively (Uye and Yamamoto 1995). Typical pigment levels for C. sinicus in the field are 1–10 ng ind.−1 (Wang et al. 1998; Li and Wang 2000; Li et al. 2002a, 2003).
As reported previously by investigators (Uye and Yamamoto 1995; Li et al. 2002a, 2003), C. sinicus exhibited diel feeding rhythms, with a peak in ingestion during the period of darkness at station B3, outside the YSCBW. However, at station A7, within the YSCBW, diel feeding rhythms did not become apparent, with the diurnal amplitude in gut pigment only 0.74 and 0.12 ng ind.−1 for adults and CVs, respectively. Except for the endogenous physiological rhythms, external factors such as change in light intensity, food availability and risk of predation have been considered as potential triggering components of copepod diel feeding variation (Huntley and Brooks 1982; Stearns 1986; Bollens and Stearns 1992; Dam and Peterson 1993; Bollens et al. 1994). As discussed before, C. sinicus mainly lived below the thermocline, where the diurnal change in light intensity was insignificant in the present study. The main predators of C. sinicus in the Yellow Sea are the planktivorous fish anchovy and sierra, but they exerted negligible impact on the population dynamics of this copepod (Meng 2003). For food availability, the vertical profiles of chl a concentrations showed that the maximum value occurred on the underside of the thermocline at station B3. However, for station A7, the maximum value was above the thermocline and with much lower concentrations (<0.1 mg chl a m−3) below it (Fig. 9). This indicated that C. sinicus lived in a continually food-limited environment at station A7. When food is limited, copepods usually feed continuously to obtain the minimum food requirements (Calbet et al. 1999). Thus, the reasons for the difference in feeding behaviors between the two stations may be related to the concentrations and vertical distributions of food.
If the feeding activity of copepods were a function of food concentration, then one would expect to see gut pigment level positively correlated to ambient chlorophyll levels, because food was limited in the Yellow Sea in summer. Results from this study did not support this expectation. There was no apparent correlation between the concentration of chl a and the gut pigment content of C. sinicus. The same results have been reported previously for C. sinicus (Uye and Yamamoto 1995) and other copepods (Dagg and Wyman 1983; Dam and Peterson 1993). Absence of correlation may be due to individual variance, diel feeding rhythms, food availability and/or feeding synchronization (Uye and Yamamoto 1995, and references cited therein).
Although gut evacuation rate constants were estimated only at the two anchor stations, our results showed that the gut evacuation rate constant increased with temperature, as reported by other investigators (Kiørboe et al. 1982; Dam and Peterson 1988; Uye and Yamamoto 1995). Dam and Peterson (1988) described the gut evacuation rate constant of copepods (E, min−1) in relation to temperature (T, °C) such that:
Based on the data from the Inland Sea of Japan, Uye and Yamamoto (1995) also proposed a linear regression equation for C. sinicus such that:
Results of their study (ca. 0.0283–0.0320 and 0.0373–0.0388 min−1 at 10°C and 18°C, respectively) were lower than the values calculated from Eq. 2 (0.0500 and 0.0722 min−1, respectively), but close to those from Eq. 1 (0.0296 and 0.0443 min−1, respectively). The reasons for the difference between our results and those of Uye and Yamamoto (1995) may be related to both the gut pigment contents and phytoplankton concentrations being at low levels in the present study (Tirelli and Mayzaud 1999, and references cited therein).
Although the daily individual pigment-specific ingestion rates at station A7, in the YSCBW, were less than one-third of those at station B3, outside it, there was no significant difference in the clearance rates (i.e. feeding effort) of C. sinicus between the two stations. Calculated from the data reported by Uye and Yamamoto (1995), the clearance rates of C. sinicus ranged from 202 to 1,066 ml ind.−1 day−1 in the Inland Sea of Japan in June. Previous studies on the herbivorous ingestion rates of mesozooplankton in the Yellow Sea indicated that the clearance rates of large copepods (dominated by C. sinicus) were about 213–318 ml ind.−1 day−1 in spring and 146–371 ml ind.−1 day−1 in autumn (Li et al. 2002b). Clearance rates estimated in the present study, whether within the YSCBW or not, fell within the data range cited above. This suggests that C. sinicus still feeds actively in summer, even in the YSCBW. Thus, the low ingestion rate may be mainly due to the lack of food.
Respiration
Ikeda et al. (2001) demonstrated that 93–96% of the variance in oxygen consumption rates could be attributed to body mass and temperature. Comparing our results for all stations to the rates calculated from the empirical model of Ikeda et al. (2001), there is no significant difference between our data and the model (a covariance test: F=1.14; df=5,5; P>0.05). However, oxygen consumption rates did not always increase with the temperature (Table 3). For example, the rate at station B3 was twice as high as that at station A7′, at the same temperature. Possibly C. sinicus for the station A7′ experiments only ascends into the warmer thermocline occasionally and lives in the cold, deep water most of the time (>95% of C. sinicus occurred below the themocline). As a result of being in a cold environment for a long time, C. sinicus living in the YSCBW reduce their metabolic rates to a very low level. Although these individuals possibly enter the upper warm water occasionally, they are unlikely to adjust their metabolic rates to a comparable level as quickly as the permanent inhabitants. Such an influence of prior environmental temperature on metabolic rates has also been reported for Calanus finmarchicus (Halcrow 1963).
Carbon budget
A comparison of the daily herbivorous carbon rations of C. sinicus and the cost of respiration indicated that C. sinicus could not meet their metabolic requirements on a herbivorous diet alone, which is consistent with some previous results for copepods (Dagg et al. 1980; Dam et al. 1993; Li et al. 2003). Copepods are known to be omnivorous, and predation on microzooplankton has been commonly observed (e.g. Gifford 1993; Ohman and Runge 1994; Atkinson 1996). Gut content analysis of C. sinicus indicated that 16% of gut items, by weight, were ciliates and flagellates in the Bohai Sea in summer (Yang 1997). Unfortunately, grazing on microheterotrophs by C. sinicus was not measured in the present study. According to Sun et al. (2002), CV copepodites dominated the C. sinicus population, and egg production of females declined to its lowest seasonal level in the YSCBW during the study period. Furthermore, if the specimens collected from the YSCBW were cultured in a food-enriched environment at the same temperature, CV copepodites could develop to adults and egg production could return to normal level (Pu et al. 2002). This suggests that lack of food is one of the important factors limiting the development of the C. sinicus population.
In general, copepod growth increases with temperature (Hirst and Bunker 2003). However, when food is limited, the animals will be subjected to greater stress at high than at low temperatures, because they need more energy to meet the high metabolic requirements. In this case, low temperature may be more propitious to maintain the population. The results from the present study also showed that, though the herbivorous ingestion rates of C. sinicus were lower in the YSCBW than outside it, the deficit of energy between feeding and metabolism was smaller inside the YSCBW than outside it (1.3% and 4.1–5.2% of body carbon per day, respectively). This may be one of the reasons why C. sinicus can keep a steady population in the YSCBW in summer.
Although some biological attributes, such as the low amplitude of migration, much lower reproduction rates, and copepodite stage CV dominating the population, implied that C. sinicus may be inactive in the YSCBW in summer (Sun et al. 2002), the results from this paper indicated that both feeding and metabolism were at normal levels, which suggests that the population was not in diapause, whether inside or outside the YSCBW. As discussed above, the low ingestion rates and respiration rates in the YSCBW may be mainly due to the cold, low-food concentration environment. Uye (2000) suggested that shelf waters provide a suitable habitat for C. sinicus, because temperature, phytoplankton food supply and depth are ideal. In the YSCBW, though the phytoplankton was at a lower level, the cold temperature caused the energy consumption of C. sinicus to decline to a much lower level. So, in view of the energy budget, the YSCBW may provide C. sinicus with a compatible refuge to live through the hot, low-food summer. The subsequent development of the C. sinicus population in the Yellow Sea also supports this suggestion (Anon. 1977).
In conclusion, the oversummering C. sinicus mainly reside in the central, cold (<12°C), bottom waters of the Yellow Sea. Their physiological condition, examined by feeding and respiration rates, indicates that the animals were hiding in the YSCBW to “cut their losses”, but were not in diapause.
References
Anon. (1977) Study on plankton in China Seas (in Chinese). In: Anon. (ed) Scientific reports of ‘Comprehensive Expedition in China Seas’, vol 8. Ocean Research Office, Tianjun, pp 1–159
Atkinson A (1996) Subantarctic copepods in an oceanic low chlorophyll environment: ciliate predation, food selectivity and impact on prey populations. Mar Ecol Prog Ser 130:85–96
Bollens SM, Stearns DE (1992) Predator-induced changes in the diel feeding cycle of a planktonic copepod. J Exp Mar Biol Ecol 156:179–186
Bollens SM, Frost BW, Cordell J (1994) Chemical, mechanical and visual cues in the vertical migration behavior of the planktonic copepod Acartia hudsonica. J Plankton Res 16:555–564
Calbet A, Saiz E, Irigoien X, Alcaraz M, Trepat I (1999) Food availability and diel feeding rhythms in the marine copepods Acartia grani and Centropages typicus. J Plankton Res 21:1009–1015
Chen Q, Zhang S (1965) The planktonic copepods of the Yellow Sea and East China Sea. I. Calanoida (in Chinese with English abstract). Stud Mar Sin 7:20–131
Conover RC (1978) Transformation of organic matter. Mar Ecol 4:221–499
Conover R, Durvasula R, Roy S, Wang R (1986) Probable loss of chlorophyll-derived pigments during the passage through the gut of zooplankton and some of the consequences. Limnol Oceanogr 31:878–886
Dagg MJ, Wyman KD (1983) Natural ingestion rates of copepods Neocalanus plumchrus and N. cristatus calculated from gut contents. Mar Ecol Prog Ser 13:37–46
Dagg MJ, Cowles T, Whitledge T, Smith S, Howe S, Judkins D (1980) Grazing and excretion by zooplankton in the Peru upwelling system during April 1977. Deep-Sea Res 27:43–59
Dam HG, Peterson WT (1988) The effect of temperature on the gut clearance rate constant of planktonic copepods. J Exp Mar Biol Ecol 123:1–14
Dam HG, Peterson WT (1993) Seasonal contrasts in the diel vertical distribution, feeding behavior, and grazing impact of the copepod Temora longicornis in Long Island Sound. J Mar Res 51:561–594
Dam HG, Miller CA, Jonasdottir SH (1993) The trophic role of mesozooplankton at 47°N, 20°W during the North Atlantic Bloom Experiment. Deep-Sea Res II 40:197–212
Gifford DJ (1993) Protozoa in the diets of Neocalanus spp. in the oceanic subarctic Pacific Ocean. Prog Oceanogr 32:223–237
Gnaiger E (1983) Calculation of energetic and biochemical equivalents of respiratory oxygen consumption. In: Gnaiger E, Horstner H (eds) Polarographic oxygen sensors. Springer, Berlin Heidelberg New York, pp 337–345
Halcrow K (1963) Acclimation to temperature in the marine copepod, Calanus finmarchicus (Gunner). Limnol Oceanogr 8:1–8
Head EJH, Harris LR (1992) Chlorophyll and carotenoid transformation and destruction by Calanus spp. grazing on diatoms. Mar Ecol Prog Ser 186:229–238
Hirst AG, Bunker AJ (2003) Growth of marine planktonic copepods: global rates and patterns in relation to chlorophyll a, temperature, and body weight. Limnol Oceanogr 48:1988–2010
Huang C, Uye S, Onbe T (1992) Ontogenetic diel vertical migration of the planktonic copepod Calanus sinicus in the Inland Sea of Japan. II. Late fall and early spring. Mar Biol 113:391–400
Huang C, Uye S, Onbe T (1993) Geographical distribution, seasonal life cycle, biomass and production of a planktonic copepod Calanus sinicus in the Inland Sea of Japan and its neighboring Pacific Ocean. J Plankton Res 15:1229–1246
Huang J, Chen B (1985) Species composition and distribution of planktonic copepods in the Jiulongjiang estuary (in Chinese with English abstract). Taiwan Strait 4:79–88
Huang J, Zheng Z (1986) The effects of salinity on the distribution of some copepods in the Jiulongjiang estuary (in Chinese with English abstract). Acta Oceanol Sin 8:83–91
Huang J, Li S, Chen Y (1991) Distribution of pelagic zooplankton in Luoyuan Bay, Fujian (in Chinese). J Oceanogr 10:46–51
Hulsemann K (1994) Calanis sinicus Brodsky and C. jashnovi, nom. nov. (Copepoda: Calanoida) of the North-West Pacific Ocean: a comparison, with notes on the integumental pore pattern in Calanus s. str. Invertebr Taxon 8:1461–1482
Huntley M, Brooks ER (1982) Effects of age and food availability on diel vertical migration of Calanus pacificus. Mar Biol 71:23–31
Ikeda T, Kanno Y, Ozaki K, Shinada A (2001) Metabolic rates of epipelagic marine copepods as a function of body mass and temperature. Mar Biol 139:587–596
Kiørboe T, Mohlenberg F, Nicolajsen H (1982) Ingestion rate and gut clearance in the planktonic copepod Centropages hamatus (Lilljeborg) in relation to food concentration and temperature. Ophelia 21:181–194
Li C, Wang R (2000) Copepod feeding activities in the Laizhou Bay, Bohai Sea, in summer (in Chinese with English abstract). Oceanol Limnol Sin 31:15–22
Li C, Zuo T, Wang R (2002a) A study on grazing of planktonic copepods in the Yellow Sea and East China Sea. I. Population abundance and gut pigment contents (in Chinese with English abstract). Oceanol Limnol Sin 33[Suppl]:100–110
Li C, Wang R, Zhang F, Wang X (2002b) A study on grazing of planktonic copepods in the Yellow Sea and East China Sea. II. Population abundance and gut pigment contents (in Chinese with English abstract). Oceanol Limnol Sin 33[Suppl]:111–119
Li C, Wang R, Sun S (2003) Horizontal distribution and feeding activities of Calanus sinicus in the anchovy spawning ground in the southern Yellow Sea. J Fish China 27[Suppl]:55–63
Lin J, Chen R (1992) Distribution of planktonic copepods in Dongshan Bay, Fujian Province (in Chinese with English abstract). Mar Sci Bull 11:41–46
Mackas D, Bohrer R (1976) Fluorescence analysis of zooplankton gut contents and an investigation of diel feeding patterns. J Exp Mar Biol Ecol 25:77–85
McLeroy-Etheridge SL, McManus GB (1999) Food type and concentration affect chlorophyll and carotenoid destruction during copepod feeding. Limnol Oceanogr 44:2005–2011
Meng TX (2003) Studies on the feeding of anchovy (Engraulis japonicus) at different life stages on zooplankton in the middle and southern waters of the Yellow Sea (in Chinese with English abstract). Mar Fish Res 24:1–9
Ohman MD, Runge JA (1994) Sustained fecundity when phytoplankton resources are in short supply: omnivory by Calanus finmarchicus in the Gulf of St. Lawrence. Limnol Oceanogr 39:21–36
Pu X, Sun S, Wang X, Wu Y (2002) Effects of temperature and food supply on CV copepodites of Calanus sinicus in the South Yellow Sea in summer (in Chinese with English abstract). Oceanol Limnol Sin 33[Suppl]:61–70
Stearns DE (1986) Copepod grazing behavior in simulated natural light and its relation to nocturnal feeding. Mar Ecol Prog Ser 30:65–76
Strickland JDH, Parsons TR (1968) A practical handbook of sea water analysis. Bull Fish Res Board Can 167:1–310
Sun S, Wang R, Zhang G, Yang B, Ji P, Zhang F (2002) A preliminary study on the oversummering strategy of Calanus sinicus in the Yellow Sea (in Chinese with English abstract). Oceanol Limnol Sin 33[Suppl]:92–99
Tirelli V, Mayzaud P (1999) Gut evacuation rates of Antarctic copepods during austral spring. Polar Biol 21:197–200
Uye S (1988) Temperature-dependent development and growth of Calanus sinicus (Copepoda: Calanoida) in the laboratory. Hydrobiologia 167/168:285–293
Uye S (2000) Why does Calanus sinicus prosper in the shelf ecosystem of the Northwest Pacific Ocean? ICES J Mar Sci 57:1850–1855
Uye S, Yamamoto F (1995) In situ feeding of the planktonic copepod Calanus sinicus in the Inland Sea of Japan, examined by the gut fluorescence method. Bull Plankton Soc Jpn 42:123–139
Uye S, Huang C, Onbe T (1990) Ontogenetic diel vertical migration of the planktonic copepod Calanus sinicus in the Inland Sea of Japan. Mar Biol 104:389–396
Wang R, Li C, Wang K, Zhang W (1998) Feeding activities of zooplankton in the Bohai Sea. Fish Oceanogr 7:265–271
Wang R, Zuo T, Wang K (2003) The Yellow Sea Cold Bottom Water—an oversummering site for Calanus sinicus (Copepoda, Crustacea). J Plankton Res 25:169–183
Weng X, Wang C (1982) Determination of the boundary and T–S bounds of the Huanghai Sea Cold Water Mass (in Chinese with English abstract). In: Anon. (ed) Hydrometeology. Chinese Society of Oceanology and Limnology Science, Beijing, pp 61–70
Williams R (1985) Vertical distribution of Calanus finmarchicus and C. helgolandicus in relation to development of the seasonal thermocline in the Celtic Sea. Mar Biol 86:145–149
Yang J (1997) Primary study on Calanus sinicus feeding in the Bohai Sea (in Chinese with English abstract). Oceanol Limnol Sin 28:376–382
Yentsch CS, Menzel DW (1963) A method for the determination of phytoplankton chlorophyll and phaeophytin by fluorescence. Deep-Sea Res 10:221–231
Zhang G, Sun S, Zhang F, Liu G (2002) Deleterious effects of temperature on the reproduction of Calanus sinicus in the southern Yellow Sea in summer (in Chinese with English abstract). Oceanol Limnol Sin 33[Suppl]:85–91
Acknowledgements
We thank the captain and crews of the R.V. “Beidou” for invaluable assistance in collecting zooplankton samples and in dong experiments on board ship. We are also grateful to Prof. R. Lu for providing the data on primary production and chlorophyll a and to Dr D. Huang for providing the CTD data. Dr A. Atkinson, one anonymous referee, and the contributing editor T. Ikeda substantially improved the final version of the manuscript. This research was supported by special funds from National Key Basic Research Program of China (G19990437) and the National Natural Science Foundation of China (40106016).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by T. Ikeda, Hakodate
Rights and permissions
About this article
Cite this article
Li, C., Sun, S., Wang, R. et al. Feeding and respiration rates of a planktonic copepod (Calanus sinicus) oversummering in Yellow Sea Cold Bottom Waters. Marine Biology 145, 149–157 (2004). https://doi.org/10.1007/s00227-004-1306-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-004-1306-x