Abstract
Two distinct modes of development in the common polychaete Scoloplos armiger (O. F. Müller, 1776) occur in the North Sea region: holobenthic development in egg cocoons and pelagic larvae hatching from suspended eggs. In the northern Wadden Sea near the island of Sylt, we observed that egg cocoons are produced intertidally while pelagic larvae originate from the adjacent subtidal zone. A previous genetic comparison between these subtidal and intertidal populations revealed distinct gene pools, suggesting that reproductive differences are not phenotypic but heritable. In this study, crossbreeding experiments show that intertidal and subtidal populations are reproductively isolated. Couples with males and females from different habitats had no offspring. Production of egg cocoons is determined by female origin from the intertidal zone. Pelagic larvae occurred only in couples with subtidal females and subtidal males. Intertidal males have spermatozoa with heads twice as long as those from subtidal males and a significantly shorter flagellum. We suspect that deviating sperm morphology may cause the reproductive breakdown at the fertilization stage. Juveniles hatching from cocoons have shorter anal cirri compared to juveniles that metamorphosed from pelagic larvae. We conclude there to be two sympatric sibling species in S. armiger: 'type I' in intertidal areas, which have egg cocoons, no pelagic larvae, elongated sperm heads, shortened sperm flagella and anal cirri; and a subtidal 'type S', lacking egg cocoons but with pelagic larvae, short sperm heads, long sperm flagella and anal cirri.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In marine invertebrates, species are usually diagnosed by externally visible recognition marks using pictorial keys for identification. Problems with this arise when: (1) individuals within a species vary considerably in phenotype due to environmental factors during development; (2) speciation is incomplete with some gene exchange occurring along a cline between semispecies; or (3) there are sibling species which are identical or very similar in their morphological traits yet have no viable hybrids in nature (Mayr 1963; Dobzhansky et al. 1977; Ridley 1996).
How common sibling species are in the marine environment is unknown. Knowlton (1993), however, states that widely distributed species are frequently found to consist of distinguishable subspecies or siblings when examined in sufficient detail. Morphological comparisons have therefore been extended from the outer appearance to inner anatomy and ultrastructure and to include characteristics of juveniles or sperm. In some cases, a combination of several characters achieves a good separation of different taxa (McDonald et al. 1991). Particularly powerful has been the direct measurement of genetic distance and divergence. Crucial for the biospecies concept (Mayr 1942; Dobzhansky et al. 1977), however, are field and experimental data on reproductive isolation. Isolation mechanisms may be prezygotic, such as separation by habitat, season, mating behavior, and by gametes failing to attract each other or being unable to fuse. After zygote formation, hybrid sterility or nonviability may cause a reproductive breakdown (postzygotic isolation).
The polychaete Scoloplos armiger (O. F. Müller, 1776) is purported to be a cosmopolitan species known from all zoogeographic regions (Hartmann-Schröder 1996). It ranges from low salinities in the Baltic Sea to fully marine conditions, from the intertidal zone down to depths of 200 m in Norwegian fjords (Holte and Gulliksen 1998) and 2,000 m off Greenland (Wesenberg-Lund 1950) or off Japan (Annenkova 1938), and from tropical sites (Frith et al. 1976) to polar regions (Sveshnikov 1960). Beesley et al. (2000), however, doubted that S. armiger found around Australia and elsewhere in the world all belong to the same species. In the eastern North Atlantic, two developmental types have been reported: (1) holobenthic development in egg cocoons in the intertidal zone (Gibbs 1968 and references therein); and (2) pelagic larvae in open water (Banse 1955; Sveshnikov 1960; Giere 1968; Bosselmann 1991; Plate and Husemann 1991). After comparing descriptions of both S. armiger larval types from the Atlantic (Anderson 1959) and the White Sea (Sveshnikov 1960) with those of Orbiniidae from California, Blake (1980) suspected sibling species or misidentification in S. armiger. He noted that holobenthic larvae of S. armiger more closely resemble those of Leitoscoloplos pugettensis than pelagic S. armiger larvae and that the latter are more similar to the larvae of S. acmeceps than to holobenthic S. armiger larvae.
Our own observations near the island of Sylt in the eastern North Sea revealed that holobenthic development in egg cocoons is confined to intertidal habitats, while pelagic larvae originate from adjacent subtidal areas. Adjacent intertidal and subtidal gene pools have a greater genetic distance than do gene pools from within these habitats over a wider area (Kruse et al. 2003). This suggests that there may be two species of Scoloplos in the North Sea, separated by habitat and by their mode of development.
The aim of this study is to test for reproductive isolation using crossbreeding experiments, coupling mates from adjacent intertidal and subtidal sediments and comparing results of couples from within or between the two habitats. Evidence of sufficient reproductive isolation to prevent all or most gene exchange would provide further evidence for the existence of two sibling species within S. armiger. A clarification of the species status is of some interest because not only is S. armiger widespread and one of the most dominant members of the macrofauna in soft-sediments of the eastern North Atlantic (e.g. Stripp 1969; Beukema 1976; Ziegelmeier 1978; Olenin and Schiedek 1996) but also this case could show that the lower tide line suffices as a divide for speciation.
Materials and methods
Study area
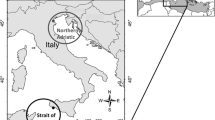
Scoloplos armiger was collected in the Sylt-Rømø Bight, a tidal basin in the North Sea (Fig. 1). The bight is part of a continuous tidal area extending over 500 km of coastline, called the Wadden Sea. Tides are semidiurnal with a range of about 2 m. The Sylt-Rømø Bight covers about 400 km2, of which 33% belong to the intertidal zone, 57% is shallow subtidal habitat (<5 m depth) and 10% consists of deeper tidal channels (max. depth ~ 40 m). Exchange of water between the Sylt-Rømø Bight and the open North Sea takes place through a 2.8-km-wide tidal channel. More information about the area was given in Gätje and Reise (1998).
S. armiger was collected at intertidal or subtidal sites at location "Odde" (Fig. 1) for crossbreeding experiments and for comparison of spermatozoa. The subtidal position was at 55° 1.47′ N, 8° 27.98′ E; the intertidal position was at 55° 1.09′ N, 8° 26.00′ E. The location "Odde" refers to earlier genetic studies on S. armiger (Kruse et al. 2003).
Crossbreeding experiments
To investigate interbreeding of intertidal and subtidal S. armiger, one male and one female from the same habitat or different habitat (subtidal or intertidal) were placed together in aquaria (21.5 cm×10.5 cm×23 cm). Reciprocal crosses between habitats and crosses within habitats were replicated seven times. Additionally, seven intertidal females were cultured in isolation. Worms were collected in the field prior to the spawning season in February, when gonads are ripe and males and females are easily distinguishable. Subtidal specimens were sampled from a research vessel at 5 m water depth and 3 km from where intertidal specimens were collected. These sampling sites at location "Odde" refer to earlier genetic studies on S. armiger (Kruse et al. 2003). The experiment was set up on 3 March 2000. All 35 aquaria contained 4.5 cm sediment from the subtidal habitat, which was sieved through 1-mm meshes and covered with 14 cm seawater, filtered through a 40-μm mesh. Aquaria were aerated and subject to artificial light in day-night rhythm (14 h light and 10 h darkness). Temperature was first adjusted to the current water temperature in the field (5°C) and then gradually raised to 14°C over a period of 7 days. Each day, deposition of egg cocoons was controlled. Occurrence of pelagic larvae and eggs was recorded by sampling the water column every 3rd day, using a tube to take a 3-l water sample from each aquarium and filtering it through an 80-μm mesh. Water was replaced by new 40-μm-filtered seawater. Three weeks after no more spawning could be observed, the occurrence of benthic larvae was examined. The sediment surface of the aquaria was sampled by sucking up the upper few millimeters using a tube of 6 mm width. Juvenile worms were extracted by shaking this sediment with added seawater within a beaker and decanting the supernatant water, with suspended sediment, through an 80-μm mesh. The residue in the sieve was examined for larvae using a dissecting microscope.
Sperm morphology
After the crossing experiment ended on 22 March 2002, male S. armiger were used for comparison of spermatozoa from intertidal and subtidal specimens. Three males with ripe gametes of each habitat were harvested from the experiments. Additional males with ripe spermatozoa were collected in the field at the sites already sampled for the crossbreeding experiment. The intertidal site was sampled on 24 March 2002, harvesting 10 unspawned worms; the subtidal site was sampled on 28 March 2002, when 6 unspawned males were collected.
Spermatozoa of all males were gained by tapping their body walls. Morphology of spermatozoa was examined by immersion light microscope and by scanning electron microscope (SEM). For the latter, spermatozoa were fixed with 2.5% glutaraldehyde in sea water and dehydrated with 30%, 50% and 70% ethanol in sea water. Using a vacuum pump they were put on membrane filters (Nucleopore), laid in an acidified dimethoxypropane (DMP) bath for 30 min (0.1 ml concentrated HCl per 100 ml DMP) and then transferred to neutral DMP for 2 days until the DMP had evaporated. Filters were then glued onto aluminium stubs and sputtered with gold-palladium using a BAL-TEC SCD 50. Spermatozoa were examined and pictures taken using a Zeiss DSM 940 SEM. Heads and flagella were measured from SEM photos of five sperm from each male from the intertidal and subtidal habitats.
Length of anal cirri
Juvenile S. armiger of early benthic stages raised in laboratory cultures were noted to differ in length of anal cirri depending on mode of development (i.e. holobenthic or pelagic). Samples of worms were collected on 16 May 1998 when strong easterly winds caused an extraordinary exposure of the subtidal habitat, allowing access. The subtidal sample site (55° 1.09′ N, 8° 26.06′ E) was 700 m northeast of the intertidal site (55° 1.85′ N, 8° 25.82′ E) and 0.8 m below spring low tide level. Anal cirri of the worms from the two habitats were systematically compared under a microscope (magnification 200–400).
Anal cirri of 50 intertidal and 45 subtidal juvenile S. armiger were measured after worms were anesthetized with magnesium chloride (8% in sea water) and placed on microscope slides. S. armiger has two anal cirri of equal length. Only specimens with both anal cirri of equal length were included in the analysis. Developmental stage of individuals was determined by counting setigers of the juveniles to ensure that equal size groups of intertidal and subtidal habitat were compared.
Results
Crossing experiments
Only couples where male and female came from the same habitat produced viable offspring (Fig. 2, where symbols are as follows:

egg cocoon;

pelagic larvae;

benthic juveniles). Egg cocoons were produced by intertidal parents in five out of seven replicates, giving rise to benthic juveniles in four replicates. The eggs in the cocoons turned from orange to the brown color of hatching juveniles. Pelagic larvae were recorded in four of seven replicates from subtidal parents, developing to benthic juveniles in three replicates. In mixed couples, with specimens from different habitats, no viable pelagic larvae could be raised. In crosses with intertidal females and subtidal males, egg cocoons were produced in six replicates, but several days after being spawned, the eggs decayed. Under a dissecting microscope the eggs appeared paler and inflated compared to those from intertidal couples. The eggs turned from orange to pale pink, presumably indicating bacterial infection. The eggs produced by the seven intertidal females kept on their own suffered the same fate.
Crossbreeding experiment with couples consisting of one male and one female of Scoloplos armiger from different habitats and the same habitat. Each combination was replicated seven times. In the third and fourth rows, results are indicated by symbols with numbers showing replicates with reproductive output. Int intertidal specimen, Sub subtidal specimen
The females from the intertidal habitat produced egg cocoons only. Two females from the subtidal site also spawned egg cocoons, where eggs decayed. In the experiment, egg cocoons were spawned from 11 March to 29 March; eggs were shed into the water from 27 March to 4 April.
The experimental set-up was maintained. In March of the following year a second spawning was observed. All spawning females maintained their egg spawning behavior. Seventeen females of the intertidal, which had spawned egg cocoons did so again and one female that had spawned pelagic larvae did so again.
Spermatozoa
Intertidal and subtidal Scoloplos armiger show remarkable differences in spermatozoa morphology. Sperms of subtidal worms have significantly shorter heads (sperm head length 3.6±0.1 μm) than sperms of intertidal specimens (8.2±0.3 μm), where sperm heads have elongated nuclei (Fig. 3) (n=5, t-test, P<0.01). Additionally, sperm flagella of subtidal S. armiger were longer (61.5±1.2 μm) than those of intertidal origin (52.9±4.7 μm) (n=5, t-test, P<0.01). Sperm of all the examined males, 13 from the intertidal and 9 from the subtidal site, looked the same within each habitat.
Anal cirri length of subtidal and intertidal benthic juveniles
Subtidal benthic juveniles of S. armiger had longer anal cirri (25.2±10.8 μm) than juveniles from the intertidal habitat (9.8±3.1 μm) (Figs. 4 and 5a, b). At 95% confidence limits, regression lines showed no overlap (the equation for intertidal specimens was y=1.759+0.34x+eps; for subtidal specimens, y=8.025+0.69x+eps). Compared juveniles had the same setiger number and there was no significant difference between intertidal (24±4 setigers) and subtidal S. armiger (25±7 setigers) (t-test, P>0.1). Worm size differences and length of anal cirri between intertidal and subtidal habitats were analysed using STATISTICA 5.1. Heterogeneous variances were log-transformed.
Discussion
Proof of reproductive isolation
The crossbreeding experiment between intertidal and subtidal Scoloplos armiger suggests complete reproductive isolation because between-habitat couples lacked offspring while within-habitat couples produced benthic juveniles and pelagic larvae, respectively. The experiment simulated subtidal conditions. Nevertheless, intertidal females produced egg cocoons. This rejects the possibility of a phenotypic switch in reproductive mode induced by tidal exposure.
On the other hand, two females of subtidal origin produced egg cocoons, one in the presence of an intertidal male. If this female were of intertidal origin, the eggs should have been fertilized and should have developed into viable larvae. No explanation can be provided for the observed failure. Similarly, there was a failure of larval development in one of the egg cocoons in an intertidal-intertidal crossing. Possibly laboratory conditions were unfavorable for further development or we misclassified some individuals. We assume that our operational categories of intertidal and subtidal worms defined by their occurrence at two respective sites has its limits. Although egg cocoons are anchored in the sediment and juveniles hatch through the vertical stalks directly into subsurface sediment, which may help to keep worms within the narrow tidal zone (Gibbs 1968; Reise 1979), resuspension and subsequent drift of benthic juveniles with tidal currents has been recorded during phases of strong wave action (Armonies 1994, 1998). With respect to the propagules of the subtidal S. armiger, no mechanism is known by which pelagic larvae could avoid settlement in the intertidal zone. Thus we expect the lower tide line to be a permeable borderline allowing for some overlap in occurrence between intertidal and subtidal S. armiger. Consequently, there was no guarantee that all experimental worms belonged to the designated categories. However, the complete lack of offspring out of the six egg cocoons in the cross between intertidal female and subtidal male compared with the emergence of viable larvae in four replicates in the intertidal-intertidal cross allows us to conclude that reproductive isolation between intertidal and subtidal S. armiger exists. Infertility in couples of mates from different habitats was shown in spite of a potential mix-up of intertidal and subtidal population, which is conservative in respect to the hypothesis being tested.
Intertidal females also produced egg cocoons when kept alone and in the presence of subtidal males. Apparently these eggs remained unfertilized. Thus, there is no indication of parthenogenesis, and ripe females may simply have to get rid of the egg load in absence of males by spawning in an ordinary way. Worms surviving the spawning season spawned again in the next year and stuck to the original mode of reproduction. This corroborates respective inferences from field data (Gibbs 1968) that individuals may spawn in more than one season, and provides additional support for the view that the mode of reproduction is inherited and not triggered by environmental conditions prevalent during gonad development.
How is reproductive isolation achieved?
Intertidal and adjacent subtidal S. armiger may often get into contact with each other, and there is no sign of a temporal mismatch in spawning time. A behavioral mismatch may be likely. Weber (1992) observed intertidal males and females in the same burrow tube when egg cocoons are produced, and Chapman (1965) assumed that male and female S. armiger come together in a burrow so that the eggs are fertilized as they leave the nephridia of the female. Further, in the cocoon-spawning polychaete Anaitides mucosa, sperms and eggs are shed right after cocoon jelly has been secreted (Sach 1975), representing the only detailed observation on mating behavior, to our knowledge, of polychaetes that spawn egg cocoons. Subtidal males and females of S. armiger may not associate that closely, releasing sperms and eggs into the water column. However, this aspect needs further study.
The striking difference in sperm morphology originating from intertidal and subtidal males strongly suggests gametic isolation. Sperm morphology has been widely regarded as a valid means to separate sibling species from another (Franzén 1956; Rice and Simon 1980; Olive 1983; Pfannenstiel and Gruenig 1990). Among nine Capitella, Capitomastus and Capitellides species, significant differences were found in nucleus lengths (Eckelbarger and Grassle 1987). Representing a case similar to S. armiger, all these species differ strikingly in reproductive modes, while they are hard to distinguish morphologically. Variations in sperm dimensions were quite low within the species but overlap within the genera. According to Jamieson and Rouse (1989), elongation of the nucleus is a common evolutionary trend in polychaete sperm related to a close association of male and female during sperm transfer. Intertidal S. armiger support this model. Correspondingly, the short nucleus and long flagella in the sperm of subtidal S. armiger suggests fertilization in the water column. A similar correspondence between sperm morphology and mode of reproduction is known from the siblings Platynereis dumerilii , which has short-headed spermatozoa and releases gametes into the water, and P. massiliensis, which has long-headed spermatozoa and spawns within a brooding tube (Pfannenstiel et al. 1987).
Two sibling species in S. armiger in the North Sea
Based on habitat separation and distinct gene pools (Kruse et al. 2003), contrasting mode of reproduction, lack of viable hybrids in crossbreeding experiments, differences in sperm shape and in anal cirri length of juveniles, though adult morphology reveals no other differential characters, we suggest the existence of two sympatric sibling species termed S. armiger 'type I' dwelling in the intertidal and 'type S' in the adjacent subtidal zone of the North Sea.
The geographic ranges of these siblings still need to be investigated. Reports of egg cocoon occurrence of S. armiger in the literature are restricted mainly to the soft bottom intertidal of the North Sea region (e.g. Schultze 1855; De Groot 1907; Thamdrup 1935; Gibbs 1968), and adjacent waters [for the English Channel, see Prenant in Cabioch et al. (1968); for the Irish Sea see Hornell (1891); and for the Kattegat, which is the transition zone between the North Sea and the Baltic Sea and lacks an extended intertidal zone, see Rasmussen (1973)]. From the Baltic, Mau (1882) reports short sperm heads (3.9 μm) and Banse (1955) mentions pelagic larvae. From the Skagerrak, Franzén (1956) depicts S. armiger sperm with a short, spherical head. Presumably all these may belong to our 'type S'. Outside the North Sea, pelagic larvae of S. armiger have been reported from the White Sea (Sveshnikov 1960).
Further investigation is needed to decide if S. armiger 'type I' and 'type S' have separated within the North Sea region and if they speciated in allopatry or in sympatry, a distributional situation which is present in the Wadden Sea. If sympatric speciation has occurred, the question is whether the lower tide line suffices as a dividing barrier for a speciation process. However, in face of the cosmopolitan distribution of S. armiger, the many siblings in Capitella (Grassle and Grassle 1976) may serve as a warning against the assumption that all S. armiger without egg cocoons belong to a single species.
References
Anderson DT (1959) The embryology of the polychaete Scoloplos armiger. Q J Microsc Sci 100: 69–166
Annenkova NP (1938) Polikheti severnoi chasti iaponskogo moria i ikh fatsial'noe i vertikal'noe raspredelenie—Polychaeta of the North Japan Sea and their horizontal and vertical distribution. Gidrobiol eksped 1934 g. Iaponskoje More. Trudy 1: 81–230
Armonies W (1994) Drifting meio- and macrobenthic invertebrates on tidal flats in Königshafen: a review. Helgol Meeresunters 48: 299–320
Armonies W (1998) Driftendes Benthos im Wattenmeer: Spielball der Gezeitenströmungen? In: Gätje C, Reise K (eds) Ökosystem Wattenmeer: Austausch-, Transport- und Stoffumwandungsprozesse. Springer, Berlin Heidelberg New York, pp 473–498
Banse K (1955) Über das Verhalten von meroplanktischen Larven in geschichtetem Wasser. Kiel Meeresforsch 11: 188–200
Beesley PL, Ross GJB, Glasby CJ (2000) Polychaetes and allies: the southern synthesis. Fauna of Australia, vol 4A. Polychaeta, Myzostomida, Pogonophora, Echiura, Sipuncula. CSIRO, Melbourne
Beukema JJ (1976) Biomass and species richness of the macro-benthic animals living on the tidal flats of the Dutch Wadden Sea. Neth J Sea Res 10: 236–261
Blake JA (1980) The larval development of Polychaeta from the northern California coast IV. Leitoscoloplos pugettensis and Scoloplos acmeceps (Familiy Orbiniidae). Ophelia 19: 1–18
Bosselmann A (1991) Recruitment and postlarval growth of some macrozoobenthos species in the German Bight. Meeresforsch 33: 141–158
Cabioch L, L'Hardy JP, Rullier F (1968) Inventaire de la Faune marine de Roscoff. Annélides. Trav Stat Biol Roscoff 17: 1–95
Chapman G (1965) The egg cocoons of Scoloplos armiger O. F. Müller. Biol Bull 128: 189–197
De Groot GJ (1907) Aaanteekenigen over de ontwikkelung van Scoloplos armiger. Dissertation, University of Leiden
Dobzhansky T, Ayala F, Stebbins GL, Valentine JW (1977) Evolution. Freeman, San Francisco
Eckelbarger KJ, Grassle JP (1987) Spermatogenesis, sperm storage and comparative sperm morphology in nine species of Capitella, Capitomastus and Capitellides (Polychaeta: Capitellidae). Mar Biol 95: 415–429
Franzén Å (1956) On spermiogenesis, morphology of the spermatozoon, and biology of fertilization among invertebrates. Zool Bidr Från Uppsala 31: 355–482
Frith DW, Tantanasiriwong R, Bhatia O (1976) Zonation of macrofauna on a mangrove shore, Phuket Island. Res Bull Phuket Mar Biol Cent 10: 1–37
Gätje C, Reise K (1998) Ökosystem Wattenmeer, Austausch-, Transport- und Stoffumwandlungsprozesse. Springer, Berlin Heidelberg New York
Gibbs PE (1968) Observations on the population of Scoloplos armiger at Whitstable. J Mar Biol Assoc UK 48: 225–254
Giere O (1968) Die Fluktuationen des marinen Zooplanktons im Elbe-Aestuar. Arch Hydrobiol [Suppl] 3/4: 379–546
Grassle JP, Grassle JF (1976) Sibling species in the marine pollution indicator Capitella (Polychaeta). Science 192: 567–569
Hartmann-Schröder G (1996) Polychaeta. In: Dahl F, Schumann H (eds) Die Tierwelt Deutschlands und angrenzender Meeresteile nach ihren Merkmalen und ihrer Lebensweise, 2nd edn. Fischer, Stuttgart
Holte B, Gulliksen B (1998) Common macrofauna dominant species in the sediments of some north Norwegian and Svalbard glacial fjords. Polar Biol 19: 375–382
Hornell J (1891) Report on the polychaetous annelids of the L.M.B.C. district. Trans Liverpool Biol Soc 5: 223
Jamieson BGM, Rouse GW (1989) The spermatozoa of the Polychaeta (Annelida): an ultrastructural review. Biol Rev 64: 93–157
Knowlton N (1993) Sibling species in the sea. Annu Rev Ecol Syst 24: 89–216
Kruse I, Reusch TBH, Schneider MV (2003) Sibling species or poecilogony in the polychaete Scoloplos armiger? Mar Biol 142: 937–947
Mau W (1882) Über Scoloplos armiger O. F. Müller. Z Wiss Zool (Leipz) 36: 389–432
Mayr E (1942) Systematics and the origin of species. Columbia University Press, New York
Mayr E (1963) Animal species and evolution. Harvard University Press, Cambridge
McDonald JH, Seed R, Koehn RK (1991) Allozyme and morphometric characters of three species of Mytilus in the Northern and Southern hemispheres. Mar Biol 111: 323–335
Olive PJ (1983) Annelida—Polychaeta. In: Adiyodi KG, Adiyodi RG (eds) Reproductive biology of invertebrates, vol 2. Spermatogenesis and sperm function. Wiley, Chichester
Olenin S, Schiedek D (1996) Is the polychaete Scoloplos armiger a biological marker of saline water inflows into subhalocline areas of the Baltic proper? In: Abstracts of the Baltic marine science conference, Rønne, Bornholm, p 3
Pfannenstiel H-D, Grünig C (1990) Spermatogenesis and sperm ultrastructure in the polychaete genus Ophryotrocha (Dorvilleidae). Helgol Meeresunters 44: 159–171
Pfannenstiel H-D, Grünig C, Lücht J (1987) Gametogenesis and reproduction in nereidid sibling species (Platynereis dumerilii and P. massiliensis). Biol Soc Wash Bull 7: 272–279
Plate S, Husemann E (1991) An alternative mode of larval development in Scoloplos armiger (O. F. Müller, 1776) (Polychaeta, Orbiniidae). Helgol Meeresunters 45: 487–492
Rasmussen E (1973) Systematics and ecology of the Isefjord marine fauna (Denmark). Ophelia 11: 1–507
Reise K (1979) Spatial configurations generated by motile benthic polychaetes. Helgol Meeresunters 32: 55–72
Rice S, Simon J (1980) Intraspecific variation in the pollution indicator polychaete Polydora ligni (Spionidae). Ophelia 19: 79–115
Ridley M (1996) Evolution. Blackwell Scientific, Boston
Sach G (1975) Zur Fortpflanzung des Polychaeten Anaitides mucosa. Mar Biol 31: 157–160
Schultze MS (1855) Über die Entwicklung von Arenicola piscatorum nebst Bemerkungen über die Entwicklung anderer Kiemenwürmer. Abh Naturf Ges Halle 3: 211
Stripp K (1969) Die Assoziationen des Benthos in der Helgoländer Bucht. Veröff Inst Meeresforsch Bremerhaven 12: 95–141
Sveshnikov VA (1960) Pelagic larvae of some polychaeta in the White Sea. Zool Zh 39: 343–355
Thamdrup HM (1935) Beiträge zur Ökologie der Wattenfauna auf experimenteller Grundlage. Medd Danm Fisk Havunders (Ser Fiskeri) 10: 1–125
Weber K (1992) Fortpflanzung, Populationsdynamik und Lebensweise von Scoloplos armiger. Diploma thesis, Christian-Albrechts-Universität zu Kiel, Kiel
Wesenberg-Lund E (1950) The polychaeta of West Greenland. Medd Grönland 151: 1–171
Ziegelmeier E (1978) Macrobenthos investigations in the eastern part of the German bight from 1950 to 1974. Rapp P-v Réun Cons int Explor Mer 172: 432–444
Acknowledgements
We wish to thank everyone from the Wadden Sea Station Sylt involved in this study, especially the crew of the ship "Mya", Niels Kruse, Peter Elvert and Timo Wieck, for excellent team work during the sampling of the subtidal habitat. Hanne Halliger ran the SEM and prepared the samples for it. This study was financially supported by the German Federal Ministry of Education and Research (BMBF) under grant 03F0179A and by the Alfred-Wegener-Institut fuer Polar- und Meeresforschung (AWI). All experiments were conducted in agreement with German law.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by O. Kinne, Oldendorf/Luhe
Rights and permissions
About this article
Cite this article
Kruse, I., Reise, K. Reproductive isolation between intertidal and subtidal Scoloplos armiger (Polychaeta, Orbiniidae) indicates sibling species in the North Sea. Marine Biology 143, 511–517 (2003). https://doi.org/10.1007/s00227-003-1112-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1112-x