Abstract
Hector's dolphin (Cephalorhynchus hectori) is a small New Zealand delphinid with a coastal distribution. Within a strip of 1 km from shore, the present study quantified the habitat used by the dolphins (n=461 groups) over a 19-month period (216 field days with 966 survey hours) by recording the abiotic factors sea surface temperature (SST), water depth and water clarity. Resource selection functions were used to distinguish the properties of 461 "used" sites (dolphins present) from 425 "unused" sites (no dolphins present) in six different study areas. Most dolphins were encountered in waters <39 m depth, with <4 m Secchi disk visibility and >14°C temperature. The preference of Hector's dolphins for warm and turbid waters was tested using eight models. Water depth, water clarity, SST and the study area explained dolphin presence to a very significant degree (p<0.001), and the model allowed the creation of probability plots for a variety of combinations of the variables. Habitat selection by dolphins differed between study areas, particularly between east and west coasts, in summer (December–February) and winter (June–August). Dolphin abundance appeared to change seasonally in some study areas, possibly due to a more offshore distribution of their prey in the winter, with its lower SSTs. This was so especially in summer (the main reproductive season), when dolphins (frequently with calves) occupied shallow and turbid waters, whereas in winter less use was made of this habitat.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cetaceans live in a complex, three-dimensional habitat with a dynamic regime of physical and chemical properties. Habitat selection by delphinids generally has been studied by relating their distribution to environmental factors. Such selected factors often describe the habitat used either as some measure of space (e.g. water depth, bottom topography, distance to shore, or thermocline depth) or according to physical and/or chemical properties of the water (e.g. temperature, current velocity, water clarity, salinity). These abiotic factors may be direct determinants of cetacean distribution, or may act indirectly by influencing prey distribution (Jaquet and Whitehead 1996; Fiedler et al. 1998).
One factor correlating with the distribution of a number of different cetacean species is sea surface temperature (SST). It has been shown to correlate with seasonal and interannual variation in harbour porpoise (Phocoena phocoena) distribution (Watts and Gaskin 1985) as well as short-finned pilot whale (Globicephala macrorhynchus) occurrence (Montero and Arechavaleta 1996). The seasonal distribution of four pelagic dolphin species off New Zealand correlated with SST (Gaskin 1968). Similarly, Atlantic white-sided dolphins (Lagenorhynchus acutus) in the Northwest Atlantic occurred in areas where SSTs in combination with different salinities were low, whereas common dolphins (Delphinus delphis) were found in warmer, more saline waters (Selzer and Payne 1988). In some seas, cetacean abundance correlated with SSTs indicative of nutrient-rich upwelling (Croll et al. 1998), or the confluence of cyclone–anticyclone eddy pairs (Davis et al. 2002), both providing good feeding opportunities for cetaceans.
Seasonal changes of cetacean distribution are well documented. For example, several populations are either observed only in warmer waters (Goodall et al. 1995), or are known to leave the studied area when the water becomes colder in winter (Maze and Würsig 1999). Generally, SSTs display a high annual variation in shallow, near-shore waters, which is where frequently a strong correlation between SSTs and seasonal dolphin distribution is found (Heithaus and Dill 2002). A second abiotic factor widely studied is water depth.
The effect of water clarity on habitat use by dolphins is more difficult to measure and no studies have detected a clear preference for turbid waters (Maze and Würsig 1999; Karczmarski et al. 2000). This suggests that an apparent preference for turbid waters by humpback dolphins (Sousa chinensis) in some areas may simply reflect a preference for inshore waters where prey is found.
A weakness of many studies is that they examined only a single factor when attempting to describe habitat selection. Furthermore, measurements were obtained only from sites where dolphins were actually observed, but not from neighbouring unused sites. Only the comparison of measurements from used and unused sites within the available habitat allows testing and prediction of the animal's choice using a resource selection function (Manly et al. 1993).
Hector's dolphin (Cephalorhynchus hectori), endemic to New Zealand, is one of the smallest cetacean species. Both anecdotal reports (Baker 1978; Cawthorn 1988) and systematic boat surveys (Dawson and Slooten 1988; Bräger and Schneider 1998) show that the species inhabits shallow, turbid, coastal waters. However, although the studies confirmed the presence of dolphins in such waters, they were not able to confirm a preference for the conditions found there. Furthermore, not all authors agree on the relative importance of the various habitat parameters, and the apparent influence of different physical factors on distribution has never been formally studied.
The abundance of Hector's dolphins in coastal waters that display a range in abiotic factors makes this species a very good candidate for the application of a resource selection function. This study examines the importance of water depth, water clarity and SST for the habitat selection of this species. Six different study areas were chosen to incorporate a wide variety of habitat selection behaviours and to reduce the constraints of local hydrographical conditions. This study thus represents the first comprehensive assessment of habitat selection in dolphins and, in addition, is the first use of modelling by the habitat selection function to address distribution in any odontocete species.
Materials and methods
Fieldwork
Inshore surveys were carried out between October 1995 and April 1997 and simultaneously recorded three abiotic factors: SST, water depth and water clarity. Six study areas were chosen, spread along both the west and the east coast of the South Island of New Zealand, covering a wide range of geographical and environmental variability. On the east coast, "Kaikoura", "Banks Peninsula" and "Moeraki" covered coastal waters between the Clarence River mouth (42°9′S; 174°0′E) and Karitane (45°39′S; 170°41′E). On the west coast, "Westport", "Greymouth" and "Jackson Bay" covered coastal waters as far apart as Karamea (41°16′S; 172°5′E) and Big Bay (44°19′S; 167°59′E) (Bräger and Schneider 1998). Each area was visited on at least three different occasions, for several days to weeks at a time (Table 1). The actual level of sampling, however, relied on favourable weather, as these coasts are very exposed at times. The locations of a total of 461 dolphin groups out of 1,060 observed were sampled during 966 survey hours over 216 field days (Table 1).
A 4.5 m aluminium vessel was used to conduct surveys at a speed of 10–15 knots under good weather conditions, i.e. sea states of 3 Beaufort (Bft.) or less. This speed prevented dolphins from following the vessel over extended periods and prevented repeated sightings of the same individuals. Care was taken to ensure that sighting conditions at sampling did not consistently deteriorate with distance from shore. Average (±SD) sea state was 1.8±1.22 Bft. (n=886) and did not differ (z=0.299, p=NS) between winter (mean=1.7±1.11 Bft., n=137) and summer (mean=1.7±1.23 Bft., n=474). Depth was measured with a depth sounder up to 183 m. One case where depth was greater was recorded conservatively as 183 m. Clarity was measured with a 30 cm diameter Secchi disk to the nearest 0.1 m, and SST was measured with a digital thermometer to the nearest 0.1°C.
The following sampling protocol was used. Two observers surveyed a strip of 4–500 m on either side of the vessel, aiming to cover completely a strip of up to 1 km off the shore. Dolphins were always first sighted above the water and were identified by their unique round dorsal fin as the animals surfaced to breathe. The turbidity of the water, therefore, did not influence the probability of detection. The magnitude of the overall probability of detection remained unknown, but a similar survey in 1984/1985 estimated it to be 0.78 (Dawson and Slooten 1988). Only one set of measurements was taken per group of dolphins, usually in close proximity to the animals (<15 m). Within the survey strip, the sighting distance of the dolphins was not measured (as, e.g., in line-transect sampling) because of complete coverage of the strip. To avoid observer bias, surveys were restricted to good sighting conditions.
Conditions at sites used and not used by Hector's dolphins were compared with a resource selection function (Manly et al. 1993: p 70), to assess habitat selection by dolphins. For this purpose, about half of all measurements were taken whenever Hector's dolphins were encountered and as close as possible to the location where they were first sighted. These measurements are assumed to be a random sample of used sites. The other half of the measurements were taken at frequent intervals to cover a wide range of potential dolphin habitat (in near-shore waters often between sites with dolphins) at sites where no dolphins were present at the time. These measurements are assumed to be equivalent to a random sample of unused sites. This approach allows the comparison between conditions at used sites and those at available sites, in order to quantify the extent to which the habitat utilisation of dolphins may be related to the three abiotic factors.
All sampling sites with and without dolphins were at least 5 min and, in most cases, 10–30 min of boating time apart. This translates into varying distances (minimum of 1,000 m) depending on the speed of the vessel, but provided sufficient physical distance to ensure that repeated measurements of the same group were avoided. A site was defined as a geographic location within 15 m, or about ten body lengths, from the nearest dolphin. The population for all sites is defined as all such points along the track within 500 m of each side of the vessel. There is clearly a very large number of these, but only a few used by dolphins at any one time.
It is unknown how quickly and over what distances dolphins react to environmental changes in their habitat. Around Banks Peninsula, however, individual Hector's dolphins usually range (±SE) over a stretch of 31±2.4 km of coastline (n=32; Bräger et al. 2002), and short-term movements in the west coast study areas extended along and up to a maximum of 61.4 km of coastline (Bräger 1998).
Data analysis procedures
The data were analysed using a logistic regression model (Harraway 1995) of the form:
to compare the characteristics of "used" sites, at which dolphins were present, and "unused" sites, at which dolphins were not present when the site data were collected. The "response" variable was binary, taking the value of 1 if a site was "used" and 0 if a site was "unused". The probability Π i of a site i being "used" depended on variables x 1, x 2, ..., x p , which consist of one or more of the abiotic variables water depth, water clarity and SST, measured at each of the 886 sites sampled in six areas over 19 months, plus variables made up of the powers and products of these variables. The probability of site i being "unused" was 1−Π i .
The logistic regression function with separate sampling of used and unused sites is a resource selection probability function, except that the constant term β 0 is modified in an unknown way (Manly et al. 1993: p 128). It must be recognised, therefore, that the estimated logistic function does not give a true probability of use for different sites. Indeed, the true probability of inhabiting a given site at any moment in time is very low. The function that is estimated does, however, serve as an index of selection in the sense that, if sites are placed in order on the basis of the probabilities from the logistic function, then this would be the same order as for probabilities of use (Manly et al. 1993: p 129).
The logistic regression routine of the statistical package by SPSS (1999) was used to obtain maximum-likelihood estimates of the parameters β 0 to β p in the equation for Π i . Besides providing estimates of the β parameters, their standard errors and the significance level of each parameter, the program produced values for an analysis of deviance table (Harraway 1995: p 157) and values of Akaike's information criterion (AIC) for each model. The analysis of deviance and the AIC values were used to test how successful the models, which included different combinations of the abiotic variables water depth, water clarity (underwater visibility) and SST, were at explaining the observed pattern in site usage. The assessed models included further X variables: namely area (six categories: Kaikoura, Banks Peninsula, Moeraki, Westport, Greymouth and Jackson Bay), season (spring, summer, autumn and winter), and coast (east coast and west coast). To include "study area" in these models, five dummy X variables were set up as follows:
-
X kk=1, if site at Kaikoura, 0 otherwise
-
X bp=1, if site at Banks Peninsula, 0 otherwise
-
X mo=1, if site at Moeraki, 0 otherwise
-
X wp=1, if site at Westport, 0 otherwise
-
X gm=1, if site at Greymouth, 0 otherwise.
Here, Jackson Bay is the reference area, and the parameters associated with the variables X kk to X gm give a comparison of each area with Jackson Bay.
To include "season" in the models, three further dummy X variables were set up as follows:
-
X sp=1, if site sampled in spring, 0 otherwise
-
X su=1, if site sampled in summer, 0 otherwise
-
X au=1, if site sampled in autumn, 0 otherwise.
Here, winter is the reference season, and the parameters associated with the variables X sp to X au give a comparison of each season with winter.
The introduction of these eight dummy variables allowed pooling of the data from the six areas around the South Island in the different seasons. The null model in the subsequent analysis included the area and season effects and the area by season interaction, thus taking account of these effects on the estimated probabilities. The alternative was to estimate the logistic function separately for each area, using the same basic function of depth, clarity and SST as the model in each area.
Three approaches were adopted to address the problem of selection of the best model to fit to the observed proportions of sites used. The first two were based on l i , the maximised log-likelihood for model i (calculated automatically by SPSS), and the deviance, D i =−2l i , for model i, while the third calculated the percentage of all 886 sites with usage correctly classified by a model as either a "dolphin present" site or a "dolphin absent" site (based on an estimated probability from the logistic regression greater than, or less than 0.5).
The first approach used the deviance difference between model i and model i+1, D i −D i+1, where model i+1 included all the parameters contained in model i plus some additional parameters; that is, model i was nested in model i+1. If D i −D i+1 is significantly large in comparison with a chi-squared distribution with degrees of freedom equal to the number of additional parameters included in model i+1 but not in model i, then the extra parameters in model i+1 are considered to be necessary to describe the data (Harraway 1995: p 156). This procedure was used to test for improvement in model build-up when adding additional parameters. The best model is the one for which there is no significant improvement by fitting more parameters.
The second approach, which produced the same best model as the analysis of deviance, involved the use of the deviance to calculate AIC, which, for model i, is defined to be AIC i =D i +2(i+1), where the second term on the right-hand side is twice the number of estimated parameters in model i (Manly et al. 1996). The model with the smallest AIC value is preferred; the penalty for an increased number of parameters has to be compensated for by a smaller deviance, D i , or, equivalently, an increase in the log-likelihood, l i , before a model is preferred over an alternative with fewer parameters.
Preliminary assessment of variables for inclusion in the model
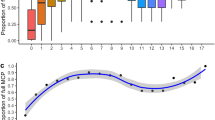
An initial inspection of the observed proportions of used sites as a function of the three variables, water depth (see Fig. 2), water clarity (see Fig. 3) and SST (see Fig. 4) indicated that powers up to at least the cube of these variables should be investigated for inclusion in any model (Table 2). These powers should give curves that fit the shape of the observed proportions.
Secondly, the data show that water depth, water clarity and SST vary with each area (see Fig. 1). The interactions measured by the products of study area dummy variables with each of the abiotic variables allow for the effects of depth, clarity and temperature on the probabilities to differ in each area. These interactions, along with interactions between clarity with season and temperature with season, were therefore included in the range of models assessed by AIC (Table 2). Any interaction between season and clarity and between season and temperature were not needed in the models chosen, as inclusion of these effects always increased AIC when "SST" was in a model.
Results
In the course of the study a range of different combinations of the selected environmental factors was encountered, depending in part on weather, study area and season (Table 1). Each study area had its environmental peculiarities, which were reflected in habitat selection by the dolphins. For example, water depths >84 m were only encountered near the southern termination of the Hikurangi Trench, south of Kaikoura, where the sea is >1,000 m deep in some areas. For comparison, all measurements of the three factors (with or without dolphins) are summarised for each of the six study areas in Fig. 1.
Parameters of the final model
In addition to the categorical variables in the null model reflecting the different effects in each study area in each season, the best habitat selection models should include the continuous variables water depth, water clarity and SST, as well as their quadratic and cubic effects and the interactions study area with depth, study area with water clarity and study area with SST. The following eight nested models were assessed by deviance differences (Table 3). In addition, at each step, the values of AIC (Table 2) were calculated for models involving deletion or addition of other variables.
- Model 1: :
-
(null model): the observed probability of use varies by study area and season, which is allowed for by including terms for the study area, season and study area by season interaction.
- Model 2: :
-
habitat selection based on all variables in the null model plus linear terms of depth, clarity and SST.
- Model 3: :
-
habitat selection based on all variables in model 2 plus interactions of depth by study area and clarity by study area.
- Model 4: :
-
habitat selection based on all variables in model 3 plus quadratic terms of depth, clarity and SST.
- Model 5: :
-
habitat selection based on all variables in model 4 plus interactions of study area with the quadratic terms of depth and clarity.
- Model 6: :
-
habitat selection based on all variables in model 5 plus cubic terms of depth, clarity and SST.
- Model 7: :
-
habitat selection based on all variables in model 6 plus interaction of study area with the cubic term of depth.
- Model 8: :
-
habitat selection based on all variables in model 7 plus interaction of study area with the cubic term of clarity.
The analysis of deviance table (Table 3) lists models from the simplest ("no selection") and null models to the most complicated (model 8, with all cubic powers of depth, clarity, SST, and their two- and three-way interactions, different parameters estimated for each study area taking one study area, Jackson Bay, as reference, and different parameters estimated for each study area interacting with depth, clarity and SST). At no stage was the interaction of study area with SST significant.
The analysis of deviance table shows that the null model, model 1, improves on the "no selection" model (p=0.0000 for deviance difference 110.756), and model 2 improves on the null model (p=0.0000 for 34.511). Models 3, 4, 5, 6 and 7 improve on models 2, 3, 4, 5 and 6, respectively, but the more complicated model 8 is no better than model 7 (p=0.1043 for deviance difference 9.122). The values of AIC (Table 2) confirm the best model to be model 7. This model increased the predictive power of 64.79% for the null model to 72.57% by introducing 34 additional parameters (Table 4).
The significance levels listed show that depth, SST, clarity, and the higher powers (square and cube) of SST and clarity affect the probabilities of habitat usage after allowing for depth and clarity to vary with the study area. The effects of depth on the probability of habitat selection by dolphins at Kaikoura, Banks Peninsula and, to a lesser extent, Westport differ significantly from the effect at the reference study area, Jackson Bay. In addition, the effect of water clarity on the probability of finding dolphins at Westport differs significantly from the effect at Jackson Bay. Although parameters involving the cube of depth are not significant individually, the removal of these terms resulted in substantially higher values of AIC, and for this reason these terms were retained in the final model. Inclusion of the SST by site interaction increased the values of AIC and these interactions were omitted. The estimated parameters, the standard errors and the significance levels are presented in Table 4.
Fit of the model
The general fit of the resource selection probability function was checked by comparing the predicted proportions of usage for the three parameters, water depth (Fig. 2), water clarity (Fig. 3) and SST (Fig. 4) with the observed proportions of used sites (i.e. dolphins present).
To test the two distributions against each other the following approximate chi-squared statistic was used:
where N is the number of groups used in the plot for which frequencies are five or more, \( \hat p_i \) is the probability of "used" sites predicted by the model in Table 4, \(\bar p_i \) is the observed proportion of used sites and n i is the number of sites.
Both the observed and predicted proportions of sites used by Hector's dolphins decreased with water clarity. The distributions showed a major peak at 2–4 m Secchi disk visibility. There were three used sites at 8–10 m visibility, but site usage was zero just before and beyond this range. The usage predicted by the model matched the observed usage closely (\( \chi _6^2 = 11.46 \) with p=0.1770; Fig. 3). The observed and predicted proportions of sites used also decreased with water depth almost continuously from 3 to 45 m, but the fit was not good over the range of depths from 12 to 21 m (\( \chi _{10}^2 = 18.04 \) with p=0.0050; Fig. 2). For SST, both the observed and predicted proportions were relatively small for the lower winter temperatures, but increased with the warmer summer temperatures, the distributions both showing a minimum at 12.1–13.0°C and a maximum at 16.1–17.0°C. In other respects these proportions agreed only for 8 of the 13 temperature intervals (Fig. 4), although the agreement was good for intervals with the largest frequencies.
Habitat utilisation probabilities for different study areas
With the fitted resource selection function it was possible to estimate habitat usage probabilities for various combinations of water depth, water clarity and SST in the six study areas after allowing for different functional relationships within each study area involving depth and clarity (SST did not interact significantly with study area). Contour plots of constant probabilities at different water depth and water clarity values are presented for five of the six study areas (with n>100 sites sampled) for summer and for winter (Figs. 5, 6, 7, 8, 9). In the other study area (Moeraki), no data were collected in deep water.
The single variable graphs in Figs. 2, 3 and 4 display the abiotic variables individually, whereas the contour plots (Figs. 5, 6, 7, 8, 9) allow for the effects of the three abiotic variables together by producing a convenient graphical representation of the final model (Table 4). These plots demonstrate the impact that water depth and water clarity have on the probability of encountering dolphins and how the occurrence of the dolphins changes between summer and winter. To investigate the effect of temperature, values of SST were fixed to the summer and winter seasonal mean values: 13.9°C and 8.5°C for Kaikoura, 14.9°C and 8.5°C for Banks Peninsula, 15.9°C and 11.2°C for Westport, 17.0°C and 10.6°C for Greymouth, and 16.5°C and 9.8°C for Jackson Bay. Furthermore, they give probability estimates for certain combinations of factors. These estimates must be treated with caution, however, if they are for values of the abiotic variables which have not been encountered at a particular study area, or which are far from values associated with sampled sites.
The habitat selection function surfaces for different depth-clarity combinations (Figs. 5, 6, 7, 8, 9) show distinct differences in usage probabilities between the areas and, to a lesser degree, between seasons within areas. At the two east coast areas, the model predicts that Hector's dolphins prefer deeper, more-or-less turbid waters (Figs. 5, 6), whereas two different patterns emerge on the west coast. In Westport, dolphins prefer very turbid and mostly shallow waters (Fig. 7). Further south, in Greymouth and Jackson Bay, the model produces a bimodal pattern with greater predicted use of both very shallow and deep waters at low to medium clarities in summer as well as in winter (Figs. 8, 9). An overall bimodal pattern of depth use agrees with the field observations (Fig. 2).
Usage probabilities for Banks Peninsula, Westport and Greymouth show differences between summer and winter (Figs. 6, 7, 8). There is a general trend towards higher predicted usage probabilities over any observed range of depth–clarity combinations in summer compared to winter. In summer, the dolphins are also likely to use a wider depth–clarity range than in winter. This pattern does not hold for all areas, however. At Kaikoura (Fig. 5), the reverse is predicted, i.e. in winter there is a higher probability of occurrence within the range of depth–clarity combinations used in summer. Furthermore, at Jackson Bay (Fig. 9), there is no difference detectable between seasons.
In all cases the plots show that for both summer and winter the dolphins appear to concentrate in the turbid waters across the range of depths that occur in each area. In the three west coast areas, the dolphins used habitats with shallow, turbid water when available: no areas had clarities <1 m. At Kaikoura and Banks Peninsula, the probability surfaces confirm the different influences of depth on the probability of finding dolphins (compared with the reference area Jackson Bay); deeper water is used at Kaikoura and Banks Peninsula, whereas at Jackson Bay and the other west coast areas deeper turbid water is not encountered.
Discussion
Output of the habitat selection model
Singly, as well as in combination, all three parameters water depth, water clarity, and SST significantly influence the habitat selection of Hector's dolphins (Cephalorhynchus hectori). Most dolphins were encountered in waters <39 m depth, with <4 m Secchi disk visibility and >14°C surface temperature (Figs. 2, 3, 4). Habitat selection by dolphins varied between study areas, particularly between east and west coasts, and between summer (December–February; panel A in Figs. 5, 6, 7, 8, 9) and winter (June–August; panel B in Figs. 5, 6, 7, 8, 9). As confirmed by the model, the probabilities of observing dolphins in shallow and turbid waters tended to be greater in summer than in winter.
We provide an inclusive analysis of dolphin habitat selection based on a number of environmental parameters both in used and unused sites. This approach using a habitat selection function has not been tried previously for any species of cetacean, but see Manly et al. (1993) for successful examples of use on terrestrial species. Such modelling of habitat selection allows a more holistic approach, by incorporation of any measured variable as well as interactions and powers of variables, though there is also a danger of over-parameterisation. Furthermore, employing a habitat selection function with dummy variables to identify different areas allows the use of data from different populations and can be used to highlight differences between them.
The three variables we used here (i.e. depth, clarity and SST) are not likely to be the only factors determining habitat selection in Hector's dolphin. Other factors such as spatial differences in environmental structure, e.g. river mouths, underwater reefs and prominent headlands, appear to contribute to small-scale variability in site usage and distribution unrelated to water depth, water clarity, or SST (e.g. Cawthorn 1988; Bräger and Schneider 1998). The inclusion of additional relevant variables into the habitat selection model will strengthen its predictive power still more.
Factors affecting habitat selection in dolphins
Environmental factors such as the three investigated in this study are omnipresent and, thus, play a fundamental role in determining the distribution of any cetacean species. They may influence the animals directly, e.g. thermoregulatory and energetic demands, or indirectly, by acting upon other biotic factors such as prey availability, predator avoidance, or the facilitation of social interactions (e.g. Wells et al. 1980; Scott et al. 1990). For example, water depth and certain features of bottom topography (e.g. underwater rocks) are known to correlate with patch dynamics in fish and dolphin distributions (Würsig and Würsig 1979; Gelwick et al. 1997).
The geographical distribution of dolphins reflects a choice for specific habitats with transit places in between them. Contrary to some expectations, individuals do not regularly migrate along the coast on a semi-annual basis beyond their home range (Bräger et al. 2002). There are, however, strong indications that Hector's dolphins shift their home range from inshore to offshore areas on a seasonal basis (Dawson and Slooten 1988; Bräger and Schneider 1998). Since the areas of this study included only nearshore waters, the sighting probabilities for colder (winter) SSTs were reduced (Fig. 2). However, it is doubtful whether winter offshore SSTs are any higher than inshore ones.
The seasonal shift in distribution is almost certainly related to changes in food supply. Most potential prey species (fish and squid: Slooten and Dawson 1988, 1994) show some inshore migration in spring and summer (Ayling and Cox 1982; McDowall 1990). Furthermore, inshore waters and surf environments provide a wide variety of fish species that peak in abundance and diversity in spring and summer (Lasiak 1984; Jellyman et al. 1997). Consequently, seasonal movements of harbour porpoise (Watts and Gaskin 1985), killer whale (Orcinus orca; Condy et al. 1978; Nichol and Shackleton 1996), bottlenose dolphin (Tursiops truncatus; Wells et al. 1990; Bräger 1993; Maze and Würsig 1999), Atlantic white-sided dolphin (Selzer and Payne 1988; Gowans and Whitehead 1995), Peale's dolphin (Lagenorhynchus australis; Lescrauwaet 1997), common dolphin (Cockcroft and Peddemors 1990; Bräger and Schneider 1998), and mixed groups of three Stenella species (Reilly 1990) are frequently explained as following the availability of prey. Prey movements, however, have seldom been studied concurrently with cetacean distribution (for examples see Nichol and Shackleton 1996; Heithaus and Dill 2002).
Shark predation has been suggested to be a major factor for the selection of resting habitats (Corkeron 1990; Wells and Norris 1994; Heithaus and Dill 2002) and calving habitats (Wells et al. 1980; Mann et al. 2000) in various dolphin species. Some dolphins might avoid shark predation by moving into shallow waters, e.g. Hawaiian spinner dolphins (Wells and Norris 1994), dusky dolphins (Würsig and Würsig 1980), bottlenose dolphins in Florida (Wells et al. 1980) and Australia (Mann et al. 2000). South African and Australian bottlenose dolphins also appear to rest in deeper waters when sharks invade shallow waters (Cockcroft et al. 1989; Heithaus and Dill 2002). It is unknown to what degree inshore waters provide shelter for dolphins from predators such as seven-gill sharks (Notorhynchus cepidianus), a species known to prey on Hector's dolphins (Cawthorn 1988) and other coastal dolphin species (Heithaus 2001a). Dusky dolphins, for example, seek shelter from killer whales by hiding in the surf zone (Constantine et al. 1998). There are, however, no records of killer whale predation on Hector's dolphins (Jefferson et al. 1991). In various bottlenose dolphin populations in Australia and Florida, 31–74% of the individuals bear bite scars indicating high predation pressures by sharks (Corkeron et al. 1987; Urian et al. 1998; Heithaus 2001b). However, only very few living Hector's dolphins (<1%) bear obvious scars, from shark attacks (authors' personal observations). Prey availability is hence likely to be the major factor in habitat selection.
For prey with an optical primary sense, high turbidity will render most anti-predator behaviour ineffective (Abrahams and Kattenfeld 1997). Being predominantly acoustically oriented, low underwater visibility may not reduce the ability of dolphins to detect prey. Therefore, low visibility may increase the hunting efficiency of dolphin in turbid inshore waters, especially since the abundance of some fish species is also positively correlated with turbidity and temperature (Cyrus and Blaber 1987; Levin et al. 1997). Prey availability and hunting success may also determine dolphin group size, which again is a critical variable as it influences most aspects of social organisation. Most social interactions take place with more-or-less closely associated members of the social group (Bräger 1999). Group size can thus be understood as an equilibrium state of attracting and dispersing forces, i.e. the maximum available food supply and minimum functional group size, e.g. for vigilance or co-operative foraging (Würsig 1986; Acevedo-Gutierrez 1997).
Application of the model for conservation
This habitat selection model is an important tool for use in conservation as it allows the identification of critical habitat. The Hector's dolphin is an uncommon endemic to New Zealand waters, where at least three genetically distinct populations have been identified (Pichler et al. 1998). All populations are either potentially or critically endangered due to unsustainable gillnet mortality (Martien et al. 1999; Pichler and Baker 2000). The species' survival is likely to depend on gillnet-free sanctuaries covering the areas of critical habitat (Bräger et al. 2002). Habitat requirements can vary among populations, however, as our results have shown. Therefore, it is critical to carry out detailed analysis of habitat requirements for individual populations and areas, incorporating biotic parameters such as prey and predator distributions whenever possible.
References
Abrahams M, Kattenfeld M (1997) The role of turbidity as a constraint on predator–prey interactions in aquatic environments. Behav Ecol Sociobiol 40:169–174
Acevedo-Gutierrez A (1997) Group feeding in bottlenose dolphins at Isla cel Coco, Costa Rica: interspecific interactions with prey and other hunters. PhD thesis, Texas A&M University, College Station
Ayling T, Cox GJ (1982) Collins' guide to the sea fishes of New Zealand. Collins, Auckland
Baker AN (1978) The status of Hector's dolphin, Cephalorhynchus hectori (Van Beneden), in New Zealand waters. Rep Int Whal Comm 28:331–334
Bräger S (1993) Diurnal and seasonal behavior patterns of bottlenose dolphins (Tursiops truncatus). Mar Mamm Sci 9:434–438
Bräger S (1998) Behavioural ecology and population structure of Hector's dolphin (Cephalorhynchus hectori). PhD thesis, University of Otago, Dunedin, New Zealand
Bräger S (1999) Association patterns in three populations of Hector's dolphin, Cephalorhynchus hectori. Can J Zool 77:13–18
Bräger S, Schneider K (1998) Near-shore distribution and abundance of dolphins along the West Coast of the South Island, New Zealand. NZ J Mar Freshw Res 32:105–112
Bräger S, Dawson SM, Slooten E, Smith S, Stone GS, Yoshinaga A (2002) Site fidelity and along-shore range in Hector's dolphin, an endangered marine dolphin from New Zealand. Biol Conserv 108:281–287
Cawthorn MW (1988) Recent observations of Hector's dolphin, Cephalorhynchus hectori, in New Zealand. Rep Int Whal Comm Spec Issue 9:303–314
Cockcroft VG, Peddemors VM (1990) Seasonal distribution and density of common dolphins Delphinus delphis off the south-east coast of southern Africa. S Afr J Mar Sci 9:371–377
Cockcroft VG, Cliff G, Ross GJB (1989) Shark predation on Indian Ocean bottlenose dolphins Tursiops truncatus off Natal, South Africa. S Afr J Zool 24:305–310
Condy PR, van Aarde RJ, Bester MN (1978) The seasonal occurrence and behaviour of killer whales, Orcinus orca, at Marion Island. J Zool (Lond) 184:449–464
Constantine R, Visser I, Buurman D, Buurman R, McFadden B (1998) Killer whale (Orcinus orca) predation on dusky dolphins (Lagenorhynchus obscurus) in Kaikoura, New Zealand. Mar Mamm Sci 14:324–330
Corkeron PJ (1990) Aspects of the behavioural ecology of inshore dolphins Tursiops truncatus and Sousa chinensis in Moreton Bay, Australia. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic, San Diego, pp 285–293
Corkeron PJ, Morris RJ, Bryden MM (1987) Interactions between bottlenose dolphins and sharks in Moreton Bay, Queensland. Aquat Mamm 13:109–113
Croll DA, Tershy BR, Hewitt RP, Demer DA, Fiedler PC, Smith SE, Armstrong W, Popp JM, Kiekhefer T, Lopez VR, Urban J, Gendron D (1998) An integrated approach to the foraging ecology of marine birds and mammals. Deep-Sea Res II 45:1353–1371
Cyrus DP, Blaber SJM (1987) The influence of turbidity on juvenile marine fishes in estuaries, part 1. Field studies at Lake St Lucia on the southeastern coast of Africa. J Exp Mar Biol Ecol 109:53–70
Davis RW, Ortega-Ortiz JG, Ribic CA, Evans WE, Biggs DC, Ressler PH, Cady RB, Leben RR, Mullin KD, Würsig B (2002) Cetacean habitat in the northern oceanic Gulf of Mexico. Deep-Sea Res I 49:121–142
Dawson SM, Slooten E (1988) Hector's dolphin, Cephalorhynchus hectori: distribution and abundance. Rep Int Whal Comm Spec Issue 9:315–324
Fiedler PC, Barlow J, Gerrodette T (1998) Dolphin prey abundance determined from acoustic backscatter data in eastern Pacific surveys. Fish Bull (Wash DC) 96:237–247
Gaskin DE (1968) Distribution of Delphinidae (Cetacea) in relation to sea surface temperatures off eastern and southern New Zealand. NZ J Mar Freshw Res 2:527–534
Gelwick FP, Stock MS, Matthews WJ (1997) Effects of fish, water depth, and predation risk on patch dynamics in a north-temperate river ecosystem. Oikos 80:382–398
Goodall RNP, Würsig B, Würsig M, Harris G, Norris KS (1995) Sightings of Burmeister's porpoise, Phocoena spinipinnis, off southern South America. Rep Int Whal Comm Spec Issue 16:297–316
Gowans S, Whitehead H (1995) Distribution and habitat partitioning by small odontocetes in the Gully, a submarine canyon on the Scotian Shelf. Can J Zool 73:1599–1608
Harraway J (1995) Regression methods applied. University of Otago Press, Dunedin, New Zealand
Heithaus MR (2001a) Predator–prey and competitive interactions between sharks (order Selachii) and dolphins (suborder Odontoceti): a review. J Zool (Lond) 253:53–68
Heithaus MR (2001b) Shark attacks on bottlenose dolphins (Tursiops aduncus) in Shark Bay, Western Australia: attack rate, bite scar frequencies, and attack seasonality. Mar Mamm Sci 17:526–539
Heithaus MR, Dill LM (2002) Food availability and tiger shark predation risk influence bottlenose dolphin habitat use. Ecology 83:480–491
Jaquet N, Whitehead H (1996) Scale-dependent correlation of sperm whale distribution with environmental features and productivity in the South Pacific. Mar Ecol Prog Ser 135:1–9
Jefferson TA, Stacey PJ, Baird RW (1991) A review of killer whale interactions with other marine mammals: predation to co-existence. Mamm Rev 21:151–180
Jellyman DJ, Glova GJ, Sagar PM, Sykes JRE (1997) Spatio-temporal distribution of fish in the Kakanui River estuary, South Island, New Zealand. NZ J Mar Freshw Res 31:103–118
Karczmarski L, Cockcroft VG, McLachlan A (2000) Habitat use and preferences of Indo-Pacific humpback dolphins Sousa chinensis in Algoa Bay, South Africa. Mar Mamm Sci 16:65–79
Lasiak TA (1984) Structural aspects of the surf-zone fish assemblage at King's Beach, Algoa Bay, South Africa: long-term fluctuations. Estuar Coast Shelf Sci 18:459–483
Lescrauwaet A-K (1997) Notes on the behaviour and ecology of the Peale's dolphin, Lagenorhynchus australis, in the Strait of Magellan, Chile. Rep Int Whal Comm 47:747–755
Levin P, Petrik R, Malone J (1997) Interactive effects of habitat selection, food supply and predation on recruitment of an estuarine fish. Oecologia 112:55–63
Manly BFJ, McDonald LL, Thomas DL (1993) Resource selection by animals. Chapman and Hall, London
Manly BFJ, McDonald LL, Garner GW (1996) Maximum likelihood estimation for the double-count method with independent observers. J Agric Biol Environ Stats 1:170–189
Mann J, Connor RC, Barre LM, Heithaus MR (2000) Female reproductive success in bottlenose dolphins (Tursiops sp.): life history, habitat, provisioning, and group-size effects. Behav Ecol 11:210–219
Martien KK, Taylor BL, Slooten E, Dawson S (1999) A sensitivity analysis to guide research and management for Hector's dolphin. Biol Conserv 90:183–191
Maze KS, Würsig B (1999) Bottlenose dolphins of San Luis Pass, Texas: occurrence patterns, site-fidelity, and habitat use. Aquat Mamm 25:91–103
McDowall RM (1990) New Zealand freshwater fishes. Heinemann Reed, Auckland
Montero R, Arechavaleta M (1996) Distribution patterns, relationships between depth, sea surface temperature, and habitat use of short-finned pilot whales south-west of Tenerife. Eur Res Cetaceans 10:193–197
Nichol LM, Shackleton DM (1996) Seasonal movements and foraging behaviour of northern resident killer whales (Orcinus orca) in relation to the inshore distribution of salmon (Oncorhynchus Pages) in British Columbia. Can J Zool 74:983–991
Pichler FB, Baker CS (2000) Loss of genetic diversity in the endemic Hector's dolphin due to fisheries-related mortality. Proc R Soc Lond B Biol Sci 267:97–102
Pichler FB, Dawson SM, Slooten E, Baker CS (1998) Geographic isolation of Hector's dolphin populations described by mitochondrial DNA sequences. Conserv Biol 12:676–682
Reilly SB (1990) Seasonal changes in distribution and habitat differences among dolphins in the eastern tropical Pacific. Mar Ecol Prog Ser 66:1–11
Scott MD, Wells RS, Irvine AB (1990) A long-term study of bottlenose dolphins on the west coast of Florida. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic, San Diego, pp 235–244
Selzer LA, Payne PM (1988) The distribution of white-sided (Lagenorhynchus acutus) and common dolphins (Delphinus delphis) vs environmental features of the continental shelf of the northeastern United States. Mar Mamm Sci 4:141–153
Slooten E, Dawson SM (1988) Studies on Hector's dolphin, Cephalorhynchus hectori: a progress report. Rep Int Whal Comm Spec Issue 9:325–338
Slooten E, Dawson SM (1994) Hector's dolphin Cephalorhynchus hectori (van Beneden, 1881). In: Ridgway SH, Harrison R (eds) Handbook of marine mammals, vol 5. Academic, San Diego, pp 311–333
SPSS (1999) SPSS reference guide. SPSS, Chicago
Urian KW, Wells RS, Scott MD, Irvine AB, Read AJ, Hohn AA (1998) When the shark bites: an analysis of shark bite scars on wild bottlenose dolphins (Tursiops truncatus) from Sarasota, Florida (abstract). In: World marine mammals conference. Monaco, p 139
Watts P, Gaskin DE (1985) Habitat index analysis of the harbor porpoise (Phocoena phocoena) in the southern coastal Bay of Fundy, Canada. J Mamm 66:733–744
Wells RS, Norris KS (1994) The island habitat. In: Norris KS, Würsig B, Wells RS, Würsig M (eds) The Hawaiian spinner dolphin. University of California Press, Berkeley, pp 31–53
Wells RS, Irvine AB, Scott MD (1980) The social ecology of inshore odontocetes. In: Herman LM (ed) Cetacean behavior: mechanisms and processes. Wiley, New York, pp 263–317
Wells RS, Hansen LJ, Baldridge A, Dohl TP, Kelly DL, Defran RH (1990) Northward extension of the range of bottlenose dolphins along the California coast. In: Leatherwood S, Reeves RR (eds) The bottlenose dolphin. Academic, San Diego, pp 421–431
Würsig B (1986) Delphinid foraging strategies. In: Schusterman RJ, Thomas JA, Wood FG (eds) Dolphin cognition and behavior: a comparative approach. Lawrence Erlbaum, Hillsdale, N.J., pp 347–359
Würsig B, Würsig M (1979) Behavior and ecology of the bottlenose dolphin, Tursiops truncatus, in the South Atlantic. Fish Bull (Wash DC) 77:399–412
Würsig B, Würsig M (1980) Behavior and ecology of the dusky dolphin, Lagenorhynchus obscurus, in the South Atlantic. Fish Bull (Wash DC) 77:871–890
Acknowledgements
We thank the many volunteers for their essential help on the boat and in the laboratory as well as the Frasers, the van Berkels, the Würsigs, S. Johnson, the Dusky Dolphin Research Project (Earthwatch), University of Canterbury (Edward Percival Field Station and Mason Research Facility) and Department of Conservation (South Westland Field Centre and West Coast Conservancy) for accommodation and local support. This paper was improved by comments from L. Ballance, S. Dawson, L. McDonald, L. Sadler, M. Thiel, B. Würsig and two anonymous reviewers. Special thanks to G. Trounson and T. Cole for help with the production of the figures. The study was supported by Cetacean Society International, Department of Conservation, Marine Mammal Research Program of Texas A&M University, New Zealand Whale and Dolphin Trust, Reckitt and Colman and the University of Otago (Postgraduate Scholarship and Divisional Grant). The research was conducted under a Marine Mammal Permit issued to S.B. by the New Zealand Department of Conservation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by G.F. Humphrey, Sydney
Rights and permissions
About this article
Cite this article
Bräger, S., Harraway, J.A. & Manly, B.F.J. Habitat selection in a coastal dolphin species (Cephalorhynchus hectori). Marine Biology 143, 233–244 (2003). https://doi.org/10.1007/s00227-003-1068-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-003-1068-x