Abstract
Isolation of cellulose nanofibers was carried out from two diverse cellulosic sources, wood (poplar) and non-wood (wheat straw), by means of a developed chemo-mechanical treatment with emphasis on optimizing the bleaching step. During chemical treatments, the effect of the primary bleaching step on the quality of cellulose microfibers was investigated at different times and temperatures. Furthermore, to optimize bleaching conditions, Klason lignin was calculated, and the effect of bleaching steps on the Klason lignin was also studied. National renewable energy laboratory (NREL) procedure, Kappa number determination, Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy were conducted for chemical and morphological characterization of cellulose fibers during experiments, and transmission electron microscopy and amount of energy consumption were used to analyze cellulose nanofibers. The results obtained from NREL, Kappa number, and FTIR indicated that noncellulosic compounds were dramatically removed during chemical treatments, and the average diameters of cellulose nanofibers were obtained to be 43 and 45.2 nm for wheat straw and poplar wood. Finally, it was found that during mechanical treatment, the amount of energy consumed by poplar wood was higher than wheat straw.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decades, cellulose, the most abundant natural polymer around the world, has been considered as a promising natural fiber to reinforce composites and replace with conventional artificial fibers because it possesses specific properties including low price, light weight, and high mechanical properties. Moreover, cellulose can be formed easily during melt compounding processes like extrusion, compression molding, and pultrusion (Alemdar and Sain 2008b). Additionally, current developments in cellulose science have led to the emergence of cellulose nanofibers, which can be deemed as an enormous revolution in creating advanced biomaterials. Cellulose nanofibers have immense properties including outstanding morphology, superb geometrical dimensions, high crystallinity, specific surface area, and excellent mechanical properties, as well as biocompatibility, biodegradability, and lack of toxicity (Lin and Dufresne 2014). To isolate cellulose nanofibers, various methods have been proposed, such as biological method, which utilizes microorganisms, bacteria, or fungi to prepare nanofibers with a high crystallinity percentage between 84 and 89% (Behzad and Ahmadi 2016; Yamanaka et al. 1989). The second common way to extract cellulose nanofibers is mechanical method, isolating them from micro-size to nano-size mainly by disintegrating plant cell wall through mechanical treatments like high-pressure homogenization (Stelte and Sanadi 2009), cryocrushing (Alemdar and Sain 2008b), and ultrafine grinding (Iwamoto et al. 2007). Since high mechanical tension must be applied for individualizing cellulose boundless, nanofibers isolated by mechanical process possess low strength and small length-to-diameter ratio (Behzad and Ahmadi 2016). Another possible way to isolate cellulose nanofibers is the chemo-mechanical method. In this method, noncellulosic components are removed during various chemical treatments like bleaching, acid hydrolysis, or alkali treatment, helping destroy the integrity of fibers (Nasri‐Nasrabadi et al. 2014; Heidarian et al. 2016), and, as a result, during further mechanical stages less tension needs to be applied to fibers, and consequently, cellulose nanofibers with higher length-to-diameter ratios extract in comparison with those isolated from the mechanical method (Behzad and Ahmadi 2016). In the present work, cellulose nanofibers were isolated through a combination of chemical treatments followed by an ultrafine grinding process.
As a whole, cellulose is mainly extracted from wood (e.g., poplar, aspen) and non-wood resources (e.g., flax, hemp, jute, wheat straw, and cotton) (Richardson and Gorton 2003; Varshney and Naithani 2011). In fact, cellulose is a linear condensation biopolymer. Its backbone chain possesses D-anhydroglucopyranose units joined together by β-1, 4-glycosidic linkages (Ahola 2008; Alemdar and Sain 2008a; Bhatnagar 2004; Bhatnagar and Sain 2005; Kaushik et al. 2010; Richardson and Gorton 2003; Varshney and Naithani 2011), and its average degree of polymerization can be varied between 700 and 14,000 (Table 1; Bhatnagar and Sain 2005; Salameh 2009). As mentioned above, cellulose fibers can be utilized as a bio-reinforcement for manufacturing biocomposites. However, currently, as the cost of wood fibers are rising and demands are surpassing supply (Sathre and O’Connor 2010), using non-wood fibers for preparing cellulose fibers is increasing.
Wheat straw, the dry stalks of wheat plants, is an agricultural by-product obtained after the removal of grain. It contains a compact structure of cellulose (35–40%) and hemicellulose (30–38%) in close association with lignin (14–17%). Likewise, poplar wood is one of the most common hardwoods in the world, and owing to its fiber properties, like flexibility and close grain, it can be considered as a distinctive wood for manufacturing biocomposites (Sun et al. 2004). Macroscopic image of wheat straw and poplar tree is shown in Fig. 1.
Recently, several studies have been conducted on optimizing chemo-mechanical treatments to extract cellulose nanofibers (Nasri‐Nasrabadi et al. 2014; Shamsabadi et al. 2015; Heidarian et al. 2016). Nasri-Nasrabadi et al. (2014) investigated the effect of optimizing acid hydrolysis conditions on isolating cellulose nanofibers from rice straw. Based on the results obtained from their study, it was indicated that acid hydrolysis treatment had a profound influence on increasing the crystallinity of fibers, and fibers treated with 2 M hydrochloric acid for 2 h possessed the highest crystallinity index. Shamsabadi et al. (2015) also evaluated the influence of optimizing hydrolysis conditions on wheat straw cellulose nanofibers based on the highest degree of crystallinity using the statistical response surface methodology. Just like Nasri-Nasrabadi et al. (2014), they observed that the best hydrolysis conditions, resulting in the highest crystallinity, were found to be 2.03 M acid concentration for 2.14-h treatment time. Heidarian et al. (2016) optimized the influence of pulping process on isolating cellulose nanofibers from sugarcane bagasse by response surface methodology. For this purpose, they selected alkali concentration and pulping time as the most important parameters in the delignification process. In addition, Kappa number, water retention value, α-cellulose, and hemicellulose were chosen as the model responses. According to their results, delignification at optimized conditions (alkali concentration of 17.5 wt% and pulping time of 1 h) led to highly delignified and fibrillated fibers in such a way that the facile isolation of cellulose nanofibers resulted in a high degree of final fibrillation. Although all of these studies cope well with the isolation of cellulose nanofibers, the investigation of bleaching conditions during the chemo-mechanical process of isolating cellulose nanofiber is still blurred. Moreover, as an efficient extraction of cellulose nanofibers enormously depends on the elimination of lignin (Behzad and Ahmadi 2016), bleaching can be considered as a critical step during chemo-mechanical treatment to isolate cellulose nanofibers.
In the present work, cellulose nanofibers were, therefore, extracted from poplar wood and wheat straw through a combination of chemical treatments followed by ultrafine grinding process, and in the chemical treatments, bleaching conditions were optimized using the response surface methodology. To the best of the authors’ knowledge, there is no investigation in the literature regarding the optimization of bleaching step for isolating cellulose nanofibers.
Materials and methods
Materials
Poplar and wheat straw were supplied from west of Iran (Hamedan). After washing with distillated water to remove impurities, all fibers were air-dried and cut into 1–2 cm lengths by a hammer mill. All chemical materials including sodium hydroxide (NaOH), sodium chlorite (NaClO2), hydrochloric acid (HCl), sulfuric acid (H2SO4), hydrogen peroxide (H2O2), and potassium permanganate (KMnO4) were of analytical grade, purchased from Merck Company, Germany.
Chemical treatments
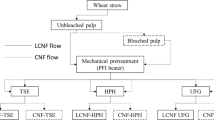
In this study, two different plants (wheat straw and poplar wood) were subjected to the same chemo-mechanical treatments for the isolation of cellulose nanofibers. For this purpose, chemical treatments were carried out simultaneously according to the following steps: Firstly, fibers were swelled by NaOH 17.5 wt% for 2 h at air temperature (25 °C) to remove extractives and other soluble components, like nonstructural materials or soluble lignin from their cell wall structures (Bhatnagar 2004). Afterward, the obtained pulps were bleached by H2O2 at optimized conditions obtained statistically through response surface methodology.
Optimization of primary bleaching parameters
Because there were no unique bleaching conditions to utilize as the best conditions for removing lignin in the literature, a design of experiment was applied to optimize the bleaching parameters. In this regard, Design Expert® software version 8.0.7.1 (Stat-Ease, Inc., Minneapolis, MN, USA) was utilized during all optimizing stages to statistically analyze the experimental data, for example, analysis of variance (ANOVA) and estimation of the response surface. To evaluate existing pertinent quadratic effects, F value was used, which compared experimental data with calculated responses at the center of the experimental domain. Two important factors, bleaching time and temperature, were chosen in three levels to obtain the number of runs required for the experimental design by determining Klason lignin as the model response. The swelled fibers, wood and non-wood, were bleached for different times and temperatures ranging between 60–90 min and 45–65 °C, respectively. Additionally, statistical ANOVA was used to determine the significance and adequacy of the model equations and terms. The coded values obtained by Design Expert® software are presented in Table 2.
Kappa number is an important parameter in pulp and paper industry and reveals the bleaching ability or degree of delignification of pulp (Shen and Chen 2009). By knowing Kappa number, the amount of residual lignin on fiber structure can easily be calculated. In this research, Kappa number determination was performed based on Chai and Zhu study (Chai and Zhu 1999), and it was applied as the response in the experimental design. By considering the amounts of H2O2 required for bleaching step, the swelled pulps were placed in hot water for different times and temperatures selected by design experiments. Finally, the pulps were washed with distilled water until a neutral pH was obtained, and then they were air-dried and prepared for the next step.
Acid hydrolysis conditions
After the primary bleaching step, fibers were hydrolyzed for 0.5 h in an autoclave by HCl solution (0.5 M) at a temperature and pressure of 121 ± 1 °C and 1 bar, respectively. Afterward, they were washed with distilled water until a neutral pH was obtained and then dried at 25 °C for 24 h. It was expected that noncellulosic materials, especially hemicellulose, would be removed from pulp structure by this step (Bhatnagar and Sain 2005).
Alkali treatment conditions
After hydrolyzing treatment, alkali treatment was utilized with 2 wt% NaOH solution at 80 ± 5 °C for 2 h, helping to remove noncellulosic components from the fiber structure (Alemdar and Sain 2008a; Bhatnagar and Sain 2005). Subsequently, the alkali-treated pulps were washed with distilled water until the pulp became neutral and then dried at room temperature for 24 h.
Secondary bleaching step
In the last step of chemical treatments, the obtained pulp was bleached by NaClO2, and Kappa number was again determined by measuring the oxidant demand of samples with KMnO4 (Chai and Zhu 1999). To perform the secondary bleaching process, a slurry of pulp was prepared in a blue cap with the mass ratio of 1:30 and then acidified to pH 2.3 using H2SO4, (10 wt%). Next, an amount of 0.15 g NaClO2 per 1 g sample was added to the blue cap and then placed in a water bath at the temperature of 55 °C (Rosa et al. 2011) and stirred with magnetic power. After 30 min, another 0.12 g NaClO2 was added to the mixture and agitated for another 30 min. At the end, the bleached pulp was washed with distilled water several times to reach neutral pH (Bhatnagar and Sain 2005). As this stage helped in reducing Klason lignin percentage to 1.066 and 0.923% in poplar wood and wheat straw, respectively, further bleaching was not performed.
Mechanical treatment
To prepare cellulose nanofibers with low consistency (~1 wt%), the chemically purified cellulose fibers were soaked in distilled water, and the diameter of fibers was reduced to nanoscale using an ultrafine grinder (MKCA6-3; Masuko Sangyo Co., Ltd., Japan). The grinding time required for preparing cellulose nanofibers was 11 min for wheat straw and 15 min for poplar wood. The total amount of cellulose nanofiber suspension ground per batch using the ultrafine grinder was 3 L. In addition, process temperature, rotational speed, and gap size were set at 50 °C, 1500 rpm, and −450 micrometers, respectively. Moreover, the number of grinding passes was repeated 3 times to obtain cellulose nanofiber slurry, and energy consumption during the process was calculated by power meter to evaluate the amount of energy required during cellulose nanofiber preparation.
Characterization
Optimization of primary bleaching step conditions
To optimize the primary bleaching parameters with H2O2, a design of experiment was carried out by employing Design Expert® software using response surface methodology. The actual experimental values and responses are presented in Table 3. As can be seen, Klason lignin indicates the model response for both wheat straw and poplar wood fibers.
Chemical characterization of cellulose nanofibers
To investigate the chemical composition of fibers, high-performance liquid chromatography (HPLC) and UV spectrophotometer were selected based on national renewable energy laboratory (NREL/TP-510-42618) instruction (determination of structural carbohydrates and lignin in biomass) (Sluiter et al. 2005). Both raw and chemically treated fibers from poplar wood and wheat straw were subjected to this test. In this experiment, cellulose and hemicellulose were degraded to their glucose and xylose sugar units, and then their contents were characterized by HPLC. In addition, the amount of acid soluble lignin was determined by UV spectrophotometer.
Kappa number determination
Kappa number determination was performed by measuring the oxidant demand of pulp with KMnO4 under strongly acidic conditions and calculating the absorption coefficient of filtered fibers at the wavelength of 546 nm (UV–Vis Spectrophotometer, 1990 Shimidzu, Kyoto, Japan) (Chai and Zhu 1999).
Fourier transform infrared (FTIR) spectroscopy
To characterize the changes of chemical bonds, FTIR spectra were recorded on a FTIR instrument (Tensor27 model, Brucker Co, USA) in the range of 400–4000 cm−1 with a resolution of 4 cm−1. Dried raw and chemically treated fibers were subjected to this analysis.
Morphology analysis
The morphological changes during the chemical process were tracked by scanning electron microscopy (SEM) fully controlled by a computer work station. For this purpose, all samples were oven-dried at 105 °C after replacing water by ethanol. Then, they were mounted on an aluminum sample holder and coated by gold. Finally, images were taken by SEM (SIGMA model, Zeiss Co, Germany). The morphology of cellulose nanofibers was characterized by transition electron microscopy (TEM). A diluted suspension of cellulose nanofibers (0.1 wt%) was cast on a holey carbon-coated grid and studied by Zeiss, EM10C (Germany) at 80 kV. The TEM images of nanofibers from two sources were employed to measure their diameters and compare their dimension using an image analyzer program (Image J); at least 200 nanofibers were chosen for each measurement.
Results and discussion
Optimization of the primary bleaching conditions
The results obtained from response surface methodology for wheat straw and poplar wood are presented in Table 3. According to the results in Table 3, the F values for wheat straw (6.52) and poplar wood (5.1) imply that the proposed models are significant. In addition, B 2 and A 2 (p < 0.05) are the most acceptable parameters in both wheat straw and poplar wood between the model parameters, and lack of fit F values for both fibers indicate that the fitness of the models is significant. The other parameters obtained from statistical analysis confirm the reliability of the models.
From statistical analysis, Klason lignin changes versus bleaching time and temperature are mapped in Fig. 2a, b for both wheat straw and poplar wood. As can be noticed, after primary bleaching step, the minimum percentage of Klason lignin was obtained at 46 °C for 90 min in wheat straw (7.26%) and 64 °C for 90 min in poplar wood (7.38%). These optimized conditions were considered as the best bleaching conditions for further evaluation and characterization of fibers.
Chemical characterization of fibers
The chemical composition of untreated and chemically treated wheat straw and poplar wood fibers, obtained from NREL analysis and Kappa number determination, is presented in Table 4. As can be observed, after swelling and primary bleaching steps (at optimized conditions), the percentage of cellulose was increased from 47.6 to 63.4% and 52.63 to 72.6% in wheat straw and poplar wood, respectively. As a whole, perhydroxyl anions, released by H2O2, eliminate carbonyl groups which exist naturally in lignin structure (Renard et al. 1997). In addition, owing to the existing complex network bonds between lignin and polysaccharides, lignin cannot easily be removed from plant cell wall. As such, to destroy the integrity of lignin structure and facilitate the disintegration of fibers during mechanical stage, primary bleaching stage at optimized conditions can be deemed as a suitable way. Moreover, acid hydrolysis treatment is a common way to extract hemicellulose (Alemdar and Sain 2008a), mainly because of existing amorphous carbohydrate chains with a low degree of polymerization (degree of polymerization is <200) (Nasri‐Nasrabadi et al. 2014; Heidarian et al. 2016). It is also claimed that as the molecular bonds between lignin-hemicellulose are more likely to exist than lignin-cellulose, by dissolving hemicellulose, the structure of lignin is more accessible to NaClO2 bleaching stage (Nasri‐Nasrabadi et al. 2014). The NaClO2 bleaching stage, additionally, provides a high-quality bleached pulp by eliminating the residual phenolic molecules, such as lignin, polyphenol, and proteins (Dufresne 2012; Heidarian et al. 2016).
As can be seen in Table 4, based on the NREL results, the percentage of total lignin decreased from 20.3 and 12.2% to 7.19 and 8.49%, and the percentage of hemicellulose declined from 31.2 and 34.6% to 7.83 and 8.85% in wheat straw and poplar wood, respectively. Furthermore, the final percentage of cellulose after chemical treatments was found to be 82.3% and 84.67% in poplar and wheat straw fibers. Approximately the same percentage of cellulose was found during the optimization of hydrolysis stage for wheat straw studied by Shamsabadi et al. (2015).
For bleached and unbleached fibers, Klason lignin decreased from 11.05 and 10.8% to 0.962 and 1.04% in wheat straw and poplar wood, respectively. The observed discrepancies between NREL and Kappa number measurements are rooted in the fact that the latter do not reveal any information regarding the soluble lignin percentage, while NREL results, as mentioned above, calculate both percentages of Klason lignin and soluble lignin, called total lignin. According to Table 4, cellulose fibers were successfully isolated for the next mechanical treatment.
Fourier transform infrared (FTIR) spectroscopy
Figure 3 shows the FTIR spectra of raw and chemically treated fibers. According to FTIR spectra, the peak at 3334 cm−1 for both fibers (wheat straw and poplar wood) corresponded to O–H stretch band due to the vibrations of hydroxyl groups (Kaushik et al. 2010; Sain and Panthapulakkal 2006; Sun et al. 2005; Xiao et al. 2001). Moreover, for both wheat straw and poplar wood, peaks in the areas of 2930 and 2989 cm−1 were due to the aliphatic saturated C–H stretching vibration in lignin and polysaccharides (cellulose and hemicelluloses), respectively. Furthermore, the prominent peaks attributed to acetyl and uronic ester groups and ester linkage of carboxylic groups were recorded at 1734 and 1739 cm−1 for raw wheat straw and poplar wood fibers (Alemdar and Sain 2008a; Sain and Panthapulakkal 2006; Suna et al. 2001; Xiao et al. 2012). These peaks almost faded in fibers after secondary bleaching step indicating almost removal of hemicellulose and lignin. Additionally, peaks at 1524 and 1529 cm−1 corresponded to the aromatic rings available in lignin structure in untreated wheat straw and poplar wood fibers, respectively (Alemdar and Sain 2008a; Cherian et al. 2008; Waleed and Maha 2003). The intensity of peaks in the area of 1238 and 1234 cm−1 sharply decreased after chemical treatments in wheat straw and poplar wood, indicating the removal of hemicelluloses from fiber structure. C−O stretch band and deformation band in cellulose, lignin, and hemicellulose were presented at 1029 and 1030 cm−1 for wheat straw and poplar wood (Kaushik et al. 2010; Sun et al. 2005). Other peaks in the area of 896 and 894 cm−1 represented the C−H bond available in cellulose structure for wheat straw and poplar wood (Alemdar and Sain 2008a; Kaushik et al. 2010).
Morphology and dimension of cellulose nanofibers
To investigate the morphological changes during the isolation of cellulose nanofibers, SEM and TEM images were taken for both fibers (Figs. 4, 5). As can be observed from SEM images, cellulose was completely inaccessible and packed in a complex structure of lignin (Fig. 4a, d) which was disintegrated partially after primary bleaching process (Fig. 4b, e). In addition, the obtained cellulose microfibers after chemical treatments are shown in Fig. 4c, f. These images show that plant cell wall was destroyed after extracting noncellulosic components (Fig. 4c, f).
Furthermore, the TEM images (Fig. 5) indicate that nanofibers were successfully isolated after mechanical treatment. Moreover, image analyzing program (Image J) revealed that the average diameter of nanofibers was obtained to be 43 nm (Fig. 5a) and 45.2 nm (Fig. 5b) in wheat straw and poplar wood, respectively.
Energy consumption of cellulose nanofibers
Regarding the amount of energy consumed during fibrillation process, it was found that wheat straw fibers had lower energy consumption (around 1.2 kWh/kg) compared with poplar wood fibers, having an average energy consumption of 1.4 kWh/kg, which proved the more facile isolation of cellulose nanofibers in wheat straw than poplar wood fibers during the mechanical treatment. Moreover, in comparison with the energy consumption of sludge cellulose nanofibers (1.3 kWh/kg) and cellulose nanofibers (1.7 kWh/kg) studied by Jonoobi et al. (2012), results were more promising for facile preparation of cellulose nanofibers from both wooden and non-wooden sources. Therefore, it can be claimed that optimization process had a positive effect on isolating nanofibers.
Conclusion
In this work, cellulose nanofibers were extracted from wheat straw and poplar wood by a chemo-mechanical method. Response surface methodology was applied to evaluate the optimum bleaching conditions. The best conditions for time and temperature were found to be 90 min and 46 °C for wheat straw fibers and 90 min and 64 °C for poplar wood fibers. The chemical composition and morphology of cellulose fibers were investigated by NREL procedure, Kappa number, SEM, and FTIR. In addition, TEM and energy consumption was used to evaluate cellulose nanofibers. According to the obtained results, the average diameter of nanofibers in wheat straw and poplar wood was found to be 43 and 45.2 nm, respectively. NREL procedure, Kappa number determination, and FTIR showed noncellulosic materials were mainly removed from fibers, and the final percentage of cellulose was obtained to be 84.67 and 82.3% for wheat straw and poplar wood fibers, respectively. Finally, the amount of energy consumed by poplar wood to isolate cellulose nanofibers was higher than wheat straw.
References
Ahola S (2008) Properties and interfacial behaviour of cellulose nanofibrils. Dissertation, Helsinki University of Technology
Alemdar A, Sain M (2008a) Isolation and characterization of nano fibers from agricultural residues—wheat straw and soy hulls. Bioresour Technol 99:1664–1671
Alemdar A, Sain M (2008b) Biocomposites from wheat straw nanofibers: morphology, thermal and mechanical properties. Compos Sci Technol 68:557–565
Behzad T, Ahmadi M (2016) Nanofibers. In: Rahman M, Asiri M (eds) Nanofiber research—reaching new heights. Intech, New York, pp 14–24
Bhatnagar A (2004) Isolation of cellulose nanofibers from renewable feed stocks and root crops. Dissertation, University of Toronto
Bhatnagar A, Sain M (2005) Processing of cellulose nanofiber-reinforced composites. J Reinf Plast Compos 24:1259–1268
Chai XS, Zhu JY (1999) Rapid and direct pulp Kappa number determination using spectrophotometry. J Pulp Pap Sci 25:387–392
Cherian BM, Pothan LA, Nguyen-Chung T, Mennig G, Kottaisamy M, Thomas S (2008) Novel method for the synthesis of cellulose nanofibril whiskers from banana fibers and characterization. J Agric Food Chem 56:5617–5627
Dufresne A (2012) Nanocellulose: from nature to high performance tailored materials. Walter de Gruyter, Berlin
Heidarian P, Behzad T, Karimi K (2016) Isolation and characterization of bagasse cellulose nanofibrils by optimized sulfur-free chemical delignification. Wood Sci Technol 50:1071–1088
Iwamoto S, Nakagaito AN, Yano H (2007) Nano-fibrillation of pulp fibers for the processing of transparent nanocomposites. Appl Phys A Mater 89:461–466
Jonoobi M, Mathew AP, Oksman K (2012) Producing low-cost cellulose nanofiber from sludge as new source of raw materials. Ind Crops Prod 40:232–238
Kaushik A, Singh M, Verma G (2010) Green nanocomposites based on thermoplastic starch and steam exploded cellulose nanofibrils from wheat straw. Carbohydr Polym 82:337–345
Lin N, Dufresne A (2014) Nanocellulose in biomedicine: current status and future prospect. Eur Polym J 59:302–325
Nasri‐Nasrabadi B, Behzad T, Bagheri R (2014) Extraction and characterization of rice straw cellulose nanofibers by an optimized chemomechanical method. J Appl Polym Sci. doi:10.1002/app.40063
Renard C, Rohou Y, Hubert C, Della Valle G, Thibault J-F, Savina J-P (1997) Bleaching of apple pomace by hydrogen peroxide in alkaline conditions: optimisation and characterisation of the products. LWT-Food Sci Technol 30:398–405
Richardson S, Gorton L (2003) Characterisation of the substituent distribution in starch and cellulose derivatives. Anal Chim Acta 497:27–65
Rosa MLS, Rehman N, Miranda M, Nachtigal S, Bica C (2011) Chlorine-free extraction of cellulose from rice husk and whisker isolation. Carbohydr Polym 87:1131–1138
Sain M, Panthapulakkal S (2006) Bioprocess preparation of wheat straw fibers and their characterization. Ind Crops Prod 23:1–8
Salameh YFA (2009) Method of extracting cellulosic material from olive pulp. Dissertation, An-Najah National University
Sathre R, O’Connor J (2010) Meta-analysis of greenhouse gas displacement factors of wood product substitution. Environ Sci Policy 13:104–114
Shamsabadi MA, Behzad T, Bagheri R (2015) Optimization of acid hydrolysis conditions to improve cellulose nanofibers extraction from wheat straw. Fibers Polym 16:579–584
Shen W, Chen X (2009) Measuring and controlling model of pulp kappa number with spectroscopy during batch sulfite pulping process. Ind Eng Chem Res 48:8980–8984
Sluiter A, Hames B, Ruiz R, Scarlata C, Sluiter J, Templeton D, Crocker D (2005) Determination of structural carbohydrates and lignin in biomass. National renewable energy laboratory (NREL). http://www.nrel.gov/biomass/analytical_procedures.html. Accessed 17 July 2005
Stelte W, Sanadi AR (2009) Preparation and characterization of cellulose nanofibres from two commercial hardwood and softwood pulps. Ind Eng Chem Res 48:11211–11219
Sun XF, Sun RC, Tomkinsonb J, Bairdd MS (2004) Degradation of wheat straw lignin and hemicellulosic polymers by a totally chlorine-free method. Polym Degrad Stab 83:47–57
Sun XF, Sun RC, Fowler P, Baird MS (2005) Characteristics of degraded cellulose obtained from steam-exploded wheat straw. Carbohydr Res 340:97–106
Suna RC, Fanga JM, Tomkinsona J, Gengb ZC, Liu CL (2001) Fractional isolation, physico-chemical characterization and homogeneous esterification of hemicelluloses from fast-growing poplar wood. Carbohyd Polym 44:29–39
Varshney VK, Naithani S (2011) Chemical functionalization of cellulose derived from nonconventional sources. In: Kalia S, Kaith BS, Kaur I (eds) Cellulose fibers: bio- and nano-polymer composites. Springer, Berlin, pp 43–60
Waleed KEZ, Maha MI (2003) Synthesis and characterization of cellulose resins. Polym Adv Technol 14:623–631
Xiao B, Sun XF, Run CS (2001) Chemical, structural, and thermal characterizations of alkali-soluble lignins and hemicelluloses, and cellulose from maize stems, rye straw, and rice straw. Polym Degrad Stab 74:307–319
Xiao W, Yin W, Xia S, Ma P (2012) The study of factors affecting the enzymatic hydrolysis of cellulose after ionic liquid pretreatment. Carbohydr Polym 87:2019–2023
Yamanaka S, Watanabe K, Kitamura N, Iguchi M, Mitsuhashi S, Nishi Y, Uryu M (1989) The structure and mechanical properties of sheets prepared from bacterial cellulose. J Mater Sci 24:3141–3145
Acknowledgements
The authors would like to thank Nano Novin Polymer Co. for assisting in cellulose nanofibers isolation using their ultrafine grinder.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shahbazi, P., Behzad, T. & Heidarian, P. Isolation of cellulose nanofibers from poplar wood and wheat straw: optimization of bleaching step parameters in a chemo-mechanical process by experimental design. Wood Sci Technol 51, 1173–1187 (2017). https://doi.org/10.1007/s00226-017-0929-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-017-0929-2