Abstract
A hemicellulose hydrolysate containing 19 g L−1 xylose was prepared from the culm of bamboo (Phyllostachys pubescens) by hydrolysis with 3 % sulphuric acid with a liquor to solid ratio of 10 (g g−1) at 121 °C for 1 h. After detoxification of the hydrolysate with a commercially available activated char followed by neutralisation with calcium carbonate, the resulting sugar solution was subjected to fermentation using the yeast, Candida magnoliae. The maximum xylitol production (10.5 g L−1) and the maximum xylitol volumetric productivity (0.42 g L−1 h−1) were attained under agitation set at 400 min−1 and aeration rate of 0.67 vvm (volume of air per volume of medium per minute). According to the results, a suitable control of the oxygen supply permits the xylitol formation from bamboo hemicellulose hydrolysate.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Xylitol, a naturally occurring five-carbon sugar alcohol, has received great attention in the food and odontological industries because of its high sweetening power, great negative heat of dissolution and anticariogenic properties. It is also used clinically as a sucrose substituent for obese people, diabetes and glucose-6-phosphate dehydrogenase (G6PD) deficient population. Xylitol is currently produced by a catalytic reduction in xylose present in the hemicellulose hydrolysates of hardwoods or agroindustrial wastes such as corn cobs. Because the hemicelluloses of hardwoods and agricultural wastes contain other monosaccharide units such as arabinose, galactose, glucose and mannose, extensive separation and purification steps are necessary to remove these contaminants from the hydrolysates before the chemical reduction. The inefficiency of current xylose preparation techniques affects seriously the recovery of xylose from lignocellulosic materials. The yields of xylitol correspond to only 50–60 % of xylan present in the raw materials (Hyvönen et al. 1982), and conventional xylitol production process is therefore relatively expensive (Nigam and Singh 1995). An alternative method for the xylitol production is microbial conversion of xylose in the lignocellulose hydrolysates (Winkelhausen and Kuzmanova 1998). Among the microorganisms that can assimilate xylose, the yeasts belonging to the genus Candida are the best xylitol producers (Onishi and Suzuki 1966; Gong et al. 1981; Meyrial et al. 1991).
Bamboos, belonging to the Gramineae, occur in natural vegetation of tropical, subtropical and temperate regions except Europe. They are the fastest growing plants and reach their full height of 15–35 m within a few months by diurnal growth rates of about 20 cm up to 100 cm (Liese 1987). Although the sprouts of bamboos have been used as a traditional foodstuff in East Asia, the culms are generally of little economic value in Japan. Because the hemicelluloses of bamboos are mainly composed of arabinoglucuronoxylan (Matsuzaki et al. 1962; Maekawa and Kitao 1973; Higuchi 1987), the culms of bamboos are a potential source of xylitol. To the authors’ knowledge, there is no report on the xylitol production from bamboos.
The microbial production of xylitol depends on the fermentation conditions employed (Silva et al. 1988). The oxygen transfer rate (OTR) is the most significant of all parameters that affect the microbial production of xylitol (Nolleau et al. 1995; Silva et al. 1997). Either high degree of aeration rate or high agitation rate even under limited oxygen supply conditions promotes cell propagation, while xylitol accumulation is repressed. In this study, the microbial conversion of hemicellulose hydrolysate prepared from Phyllostachys pubescens culm to xylitol by the yeast, Candida magnoliae, using two-phase aeration process was examined. The first step was carried out under aerobic conditions to improve glucose consumption through cell propagation in the medium (Nolleau et al. 1995; Saha and Bothast 1999; Ding and Xia 2006). The second step under limited oxygen conditions is intended to increase the xylitol accumulation. Before fermentation runs, detoxification of the hydrolysate with a commercially available activated char was also evaluated.
Materials and methods
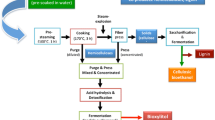
Preparation of bamboo culm hydrolysate
The air-dried culms of P. pubescens were ground in an Ultra Centrifugal Mill ZM1 (Retsch, Haan, Germany). The ground culms (P32 R82 mesh) were composed of 18.0 % pentosan (including 16.8 % xylan), 38.4 % glucan, 27.8 % lignin (including 1.4 % acid soluble lignin) and 1.3 % ash. The ground culms were hydrolysed with 3 % sulphuric acid with a liquid to solid ratio of 10 (g g−1) at 121 °C for 1 h. To remove toxic compounds released during the hydrolysis process, sorption experiments were conducted by agitating the hydrolysate with a steam-activated char (Shirasagi A, Japan EnviroChemicals, Ltd., Osaka, Japan) in a reciprocal shaker (160 strokes min−1) at 30 °C for 24 h. The resulting sugar solution was filtered and neutralised with calcium carbonate followed by centrifugation.
Microorganism and inoculum
Cells of C. magnoliae (FERM P-16522; AIST, Tsukuba) were grown on an agar slant containing malt extract (3 g L−1), yeast extract (3 g L−1), peptone (5 g L−1), d-glucose (10 g L−1) and agar (20 g L−1) at 4 °C for 3 day. A loop full of a slant culture was transferred to 5 mL of the pre-culture medium containing xylose (20 g L−1), Casamino acids (1 g L−1), Difco yeast nitrogen base without amino acids and ammonium sulphate (1.7 g L−1) and urea (2.27 g L−1), and cultivated at 30 °C for 24 h.
Experimental set-up
Batch fermentation runs were performed in a BMZ-P type culture installation (ABLE Corp., Tokyo, Japan) containing baffles and two sets of disc turbines with six and four flat blades and a working volume of 1.5 L of medium. This installation was equipped with pH, temperature, dissolved oxygen and aeration rate controllers. At the fixed temperature (30 °C), to consume glucose in the medium, the aerobic phase was applied in the first 10–12 h, and then the aeration rate was reduced. In the second aeration phase, the agitation was set at 300, 400 and 500 min−1 and the aeration was varied from 0.33 to 1.00 vvm (volume of air per volume of medium per minute).
The volumetric oxygen transfer coefficient (K L a) was determined under different aeration and agitation conditions. The dissolved oxygen concentration (DOC) of the medium was decreased to zero by nitrogen sparging and the K L a was calculated from the rate of DOC increase during subsequent aeration. The OTR was calculated from the relationship:
where C * and C are the saturated DOC and DOC, respectively.
Analytical methods
Neutral sugars, xylitol and ethanol were determined by high performance liquid chromatography (HPLC) with a refractive index detector. Tosoh CO-8020 HPLC system (Tosoh, Tokyo, Japan) was equipped with an Aminex HPX-87P column (300 × 7.8 mm, Bio-Rad, Richmond, VA) in combination with a Carbo-P microguard cartridge (4.6 × 30 mm, Bio-Rad, Richmond, VA). Ion exchanged, degassed and filtered water was used as mobile phase at a flow rate of 0.6 mL min−1 and at 85 °C. Cell concentrations were determined by absorbance at 660 nm. Dry cell weights were determined after two rounds of centrifugation and washing with distilled water and drying at 105 °C.
Results and discussion
Hemicellulose hydrolysates generally contain a range of toxic compounds generated during acid hydrolysis at elevated temperatures. Weak organic acids, furfural and 5-hydroxymethylfurfural originate from polysaccharides, and low molecular weight phenolics released from lignin are included in potential inhibitors of microbial metabolism (Palmqvist and Hahn-Hägerdal 2000). Although furfural and 5-hydroxymethylfurfural represent only a portion of the inhibitors present in the hydrolysates, the abundance of these furan derivatives serves as a useful marker to predict relative toxicity (Martinez et al. 2000). Because furfural and 5-hydroxymethylfurfural show strong absorption at 280 nm (Lai and Sarkanen 1971), relative toxicity of the hydrolysate can be evaluated by the absorbance at 280 nm (A 280) (Tada et al. 2004).
When the culm of P. pubescens was hydrolysed with 3 % sulphuric acid with a liquid to solid ratio of 10 (g g−1) at 121 °C for 1 h, the resulting hydrolysate contained 19 g L−1 xylose, as shown in Table 1. The A 280 value of the hydrolysate (145.8) was, however, too high to perform microbial conversion of solubilised sugars. Tada et al. (2004) reported that successful xylitol production from corn cob hydrolysates by C. magnoliae required the A 280 value to be less than 20 (corresponding to 0.13 g L−1 as furfural).
Activated charcoal treatment is an efficient method of reduction in the amounts of these inhibitors (Gong et al. 1993; Lee et al. 1999). In this study, to minimise the inhibition, the hydrolysate was treated with a steam-activated char at 30 °C. Because 1 day of contact time was enough to attain the sorption equilibrium between the inhibitors and activated charcoal (Parajó et al. 1996), 24 h of the contact time was fixed throughout the sorption experiments. The A 280 value of the hydrolysate decreased as an increase in the dose of activated char, while the monosaccharide concentrations stayed almost constant (Table 1). When 20 mL of the hydrolysate was treated with 0.4 g of activated char (20 g L−1 of carbon dose), the A 280 value was reduced from 145.8 (0.93 g L−1 as furfural) to 5.6 (0.04 g L−1 as furfural). An additional charge did not result in significant improvement. These results indicate that furfural, 5-hydroxymethylfurfural and low molecular weight phenolics are selectively removed from the hydrolysate by sorption onto the activated char. Although acetic acid released from the xylan backbone is a potential inhibitor of microbial metabolism, it can be removed during neutralisation of the hydrolysate with calcium carbonate.
The concentration profiles of xylose, glucose, xylitol, ethanol and biomass as a function of fermentation time are shown in Fig. 1. Although small quantities of glucose (3.5–3.9 g L−1) were present in the fermentation media, it was completely consumed during the first 12 h of the fermentation process. A slow rate of xylose consumption was observed before all the glucose was completely assimilated. After complete consumption of glucose, C. magnoliae metabolized xylose at higher rates. When the aeration rate was set at 0.67 vvm and the agitation rate was varied 300–500 min−1, the xylose consumption rate of C. magnoliae increased with increasing agitation rate. In contrast, it stayed almost constant, when the aeration rate varied from 0.33 to 1.00 vvm at a constant agitation rate of 400 min−1.
Table 2 presents the values of the fermentation parameters obtained in experiments conducted with the hydrolysates containing about 19 g L−1 xylose and supplemented with nutrients. C. magnoliae grew in the hydrolysate media and accumulated xylitol at different rates under all aeration conditions employed. The highest level of biomass (17.4 g L−1) was attained with the highest K L a value, while the xylitol secretion was strongly repressed. Under the aeration conditions, C. magnoliae mainly produces cell mass through the xylose metabolism. Xylose is first reduced to xylitol by mainly NADPH-linked xylose reductase. Xylitol is either secreted from cell or oxidised by NAD-dependent xylitol dehydrogenase. The first two reactions are a rate limiting step in xylose fermentation. The OTR strongly affects the regeneration of NADPH and NAD+. Under oxygen limited conditions, the by-products such as xylitol and ethanol are formed. In these experiments, the maximum xylitol production (10.5 g L−1) and the maximum xylitol volumetric productivity (0.42 g L−1 h−1) were attained under agitation set at 400 min−1 and aeration rate of 0.67 vvm. These values are rather low, compared with those from the concentrated hydrolysates of rice straw, sugarcane bagasse, eucalyptus wood and corn cob (Parajó et al. 1998). When the ground culm was hydrolysed with 3 % sulphuric acid with a liquid to solid ratio of 3 (g g−1) at 121 °C for 1 h, a sugar solution containing 59.9 g L−1 xylose could be obtained (results not shown). Xylitol production from bamboo hemicellulose hydrolysate can be improved by the use of concentrated hydrolysate as a substrate since low xylose concentration leads to biomass increase without xylitol production (Meyrial et al. 1991).
Conclusion
A fermentable substrate with a relatively high xylose concentration (19 g L−1) could be prepared from the culm of P. pubescens by acid hydrolysis with dilute sulphuric acid under mild operating conditions. Inhibitors of microbial metabolism, such as dehydration products from solubilised sugars and phenolics released from lignin, were successively removed from the hydrolysate by treatment with a commercially available steam-activated char. A suitable control of the oxygen supply permits successful xylitol production from the hydrolysate. Bamboo culms are a potential source of substrate that can be converted to xylitol by xylose-fermenting yeasts.
References
Ding X, Xia L (2006) Effect of aeration rate on production of xylitol from corncob hemicellulose hydrolysate. Appl Biochem Biotechnol 133:263–270
Gong CS, Chen LF, Tsao GT (1981) Quantitative production of xylitol from D-xylose by a high-xylitol producing yeast mutant Candida tropicalis HXP2. Biotechnol Lett 3:130–135
Gong CS, Chen CS, Chen LF (1993) Pretreatment of sugar cane bagasse hemicellulose hydrolyzate for ethanol production by yeast. Appl Biochem Biotechnol 39–40:83–88
Higuchi T (1987) Chemistry and biochemistry of bamboo. Bamboo J 4:132–145
Hyvönen L, Koivistoinen P, Voirol F (1982) Food technological evaluation of xylitol. Adv Food Res 28:373–403
Lai YZ, Sarkanen KV (1971) Isolation and structural studies. In: Sarkanen KV, Ludwig CH (eds) Lignins -occurrence, formation, structure and reactions. Wiley-Interscience, New York, p 192
Lee WG, Lee JS, Shin CS, Park SC, Chang HN, Chang YK (1999) Ethanol production using concentrated oak wood hydrolysates and methods to detoxify. Appl Biochem Biotechnol 77–79:547–559
Liese W (1987) Research on bamboo. Wood Sci Technol 21:189–209
Maekawa E, Kitao K (1973) Isolation and constitution of a xylan from bamboo. Agric Biol Chem 37:2073–2081
Martinez A, Rodriguez ME, York S, Preston JF, Ingram LO (2000) Use of UV absorbance to monitor furans in dilute acid hydrolysates of biomass. Biotechnol Prog 16:637–641
Matsuzaki K, Moriya M, Sobue H (1962) Structure of bamboo hemicellulose. Kogyo Kagaku Zasshi 65:987–989
Meyrial V, Delgenes JP, Moletta R, Navarro JM (1991) Xylitol production from D-xylose by Candida guilliermondii: fermentation behaviour. Biotechnol Lett 13:281–286
Nigam P, Singh D (1995) Processes for fermentative production of xylitol–a sugar substitute. Process Biochem 30:117–127
Nolleau V, Preziosi-Belloy L, Navarro JM (1995) The reduction of xylose to xylitol by Candida guilliermondii and Candida parapsilosis: incidence of oxygen and pH. Biotechnol Lett 17:417–422
Onishi H, Suzuki T (1966) The production of xylitol, L-arabitol and ribitol by yeasts. Agric Biol Chem 30:1139–1144
Palmqvist E, Hahn-Hägerdal B (2000) Fermentation of lignocellulosic hydrolysates. II: inhibitors and mechanisms of inhibition. Biores Technol 74:25–33
Parajó JC, Domínguez H, Domínguez JM (1996) Charcoal adsorption of wood hydrolysates for improving their fermentability: influence of the operational conditions. Biores Technol 57:179–185
Parajó JC, Domínguez H, Domínguez JM (1998) Biotechnological production of xylitol. Part 3: operation in culture media made from lignocellulose hydrolysates. Biores Technol 66:25–40
Saha BS, Bothast RJ (1999) Production of xylitol by Candida peltata. J Ind Microbiol Biotechnol 22:633–636
Silva SS, Felipe MGA, Mancilha IM (1988) Factors that affect the biosynthesis of xylitol by xylose-fermenting yeasts. A review. Appl Biochem Biotechnol 70–72:331–339
Silva SS, Riberio JD, Felipe MGA, Vitolo M (1997) Maximizing the xylitol production from sugar cane bagasse hydrolysate by controlling the aeration rate. Appl Biochem Biotechnol 63–65:557–564
Tada K, Horiuchi J, Kanno T, Kobayashi M (2004) Microbial xylitol production from corn cobs using Candida magnoliae. J Biosci Bioeng 98:228–230
Winkelhausen E, Kuzmanova S (1998) Microbial conversion of D-xylose to xylitol. J Ferment Bioeng 86:1–14
Acknowledgments
This work was supported by a grant from Satellite Venture Business Laboratory of Kitami Institute of Technology.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Miura, M., Watanabe, I., Shimotori, Y. et al. Microbial conversion of bamboo hemicellulose hydrolysate to xylitol. Wood Sci Technol 47, 515–522 (2013). https://doi.org/10.1007/s00226-012-0501-z
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-012-0501-z