Abstract
The correlation of structural assembly on a molecular level with macroscale properties such as accessibility and reactivity was investigated. A series of TCF-bleached E. globulus kraft dissolving pulps was prepared aiming at a specification suitable for viscose application. The removal of xylan to a comparable level was achieved by different pre- and post-treatments. Solid-state CP-MAS 13C NMR was used to determine the degree of order and the lateral fibril dimensions of cellulose fibrils. The results of the NMR measurements were related to the processability of these pulps during viscose manufacture, expressed in terms of filterability of the viscose dope and its amount of undissolved particles. The cellulose crystallinity did not affect the pulp reactivity. It was noticed that the cold caustic extracted (CCE) pulps revealed both large fibril aggregate width as determined from NMR data and low reactivity toward xanthation at the same time. These pulps exhibited significantly higher amounts of alkali-resistant xylan than those prepared by prehydrolysis kraft cooking.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
A low content of hemicelluloses is one of the characteristics of dissolving pulp. The hemicelluloses have to be largely removed since their presence impairs subsequent conversion processes such as viscose fiber, lyocell fiber and cellulose acetate production. In addition, also the quality of their final products is negatively affected by the presence of short-chain heteropolysaccharides. In conventional hardwood dissolving pulps, the hemicellulose content is typically reduced by acid hydrolytic degradation prior to kraft cooking or in combination with hot caustic extraction preceded by acid sulfite pulping and cold caustic extractions of both prehydrolysis kraft (PHK) and acid sulfite pulps. In all these cases, the residual xylan is characterized by rather low molecular weight. Upgrading of kraft paper pulps to dissolving pulps can be achieved by a post-extraction of xylan using an approximately 2 M sodium hydroxide solution at temperatures slightly above room temperature (cold caustic extraction or CCE, Jayme and Schenck 1949; Wallis and Wearne 1990). Here, the residual xylan is characterized by rather high molecular weight and a resistance to dissolution in alkali. The effects of the residual xylan in both conventional and post-extracted paper pulps are of interest to understand their behavior in subsequent conversion processes.
The reactions of pulp under heterogeneous conditions are dependent on the reactivity and the accessibility of the material. Accessibility of hydroxyl groups can be determined by deuterium exchange (Frilette et al. 1948; Rousselle and Nelson 1971), water sorption methods, by measuring the amount of restricted water in pulp by DSC (Maloney et al. 1998) or by determining the NMR T2 relaxation time of the water molecules (Carles and Scallan 1973; Ibbett et al. 2008). Ioelovich (2009) found full accessibility of water and restricted accessibility of organic molecules to non-crystalline domains as measured by X-ray diffraction. The phosphitylation of hydroxyl groups monitored by 31P NMR spectroscopy constitutes a relatively new way for the characterization of pulp accessibility (Filpponen and Argyropoulos 2008).
Pulp reactivity is affected not only by the accessibility but also by the reaction conditions as applied in the conversion processes. Methods to determine pulp reactivity comprise the dissolution of cellulose to a dope solution such as the conversion to viscose (Fock 1959; Treiber et al. 1962). Not only cellulose molecules in the amorphous state are accessible to chemical interactions, but also molecules that are located at the surface of the crystalline areas (Krässig 1993). A powerful tool to characterize the supramolecular structure of cellulose is the cross polarization-magic angle spinning (CP-MAS) 13C NMR spectroscopy (VanderHart and Atalla 1984; Newman and Davidson 2004; Larsson et al. 1997). Lateral fibril dimensions can be calculated from the NMR data (Wickholm et al. 1998) under the assumption that cellulose chains are organized in fibrils and fibril aggregates and that the contribution of the less-ordered signals comes from surface molecules (Newman and Hemmingson 1994; Larsson et al. 1997). This technique has been used to observe the changes in cellulose structure during pulping (Hult et al. 2000, 2001; Virtanen et al. 2008) where an increase in cellulose fibril aggregates was detected. This effect was explained by changes in the structure of the hemicelluloses, which were stripped of branches and acetyl groups, thus facilitating intermolecular aggregation. The additional freedom gained through the removal of lignin and hemicelluloses during cooking could also enhance cellulose fibril aggregation, which was observed by Duchesne et al. (2001) on spruce kraft pulps by FE-SEM and NMR.
It was the objective of the present study to relate the supramolecular structure of conventional and new types of Eucalyptus globulus dissolving pulps comprising comparable and different xylan levels to their behavior in viscose processing. A prerequisite for the comparability of the pulps was the adjustment of the intrinsic viscosity to a similar level. Alkali extraction, nitren extraction, enzymatic treatment with a monocomponent endoglucanase to improve the reactivity by enhancing the accessibility toward derivatizing chemicals (Henriksson et al. 2005; Kvarnlöf et al. 2007) and PHK cooking were applied to obtain pulps differing in process history, while the residual xylan was adjusted to a comparable level. The supramolecular structure was detected by CP-MAS 13C NMR measurements, and pulp reactivity was evaluated by the viscose filterability and the particle volume distribution.
Materials and methods
Cellulose substrates
The eucalypt pulp samples were prepared by different processing parameters: commercial kraft paper pulp (EK), kraft pulp with reduced hemicellulose content by cold caustic extraction (EK-CCE), kraft pulp with reduced hemicellulose content by nitren extraction (EK-Nitren), kraft pulp treated with CCE followed by an enzymatic treatment (EK-CCE-EG), prehydrolysis kraft pulp (PHK) subjected to prehydrolysis with a P-factor of 250, prehydrolysis kraft pulp with a P-factor of 1,500 followed by CCE treatment (CELL) and a reference sulfite dissolving pulp (SULF). All pulp samples were adjusted to a comparable intrinsic viscosity. Properties of the samples are listed in Table 1.
Prehydrolysis kraft cooks (PHK and CELL) were carried out in a 10-l digester with forced circulation and connected to three pressurized pre-heating tanks for the simulation of large-scale operation according to the VISCBC (continuous batch cooking) concept. The E. globulus wood chips (Uruguay, supplied from ENCE, Spain) were fractionated (>7 mm, 22.7% Klason lignin, 4.7% acid soluble lignin, 42.4% cellulose, 15.6% pentosan). The pulps (EK, EK-CCE, EK-CCE-EG, EK-Nitren) were prepared from E. globulus kraft pulp delivered by ENCE. A reference eucalypt sulfite pulp (SULF) was delivered by SAPPI Saiccor. Bleaching experiments were carried out in a medium-consistency high-shear mixer. The amount of ozone necessary to adjust final viscosity to a level of approximately 450 ml/g was determined by taking samples during the ozone (Z) step and measuring the intrinsic viscosity. Cold caustic extractions (CCE) were conducted with white liquor containing an effective alkali concentration of 100 g/l and a sulfidity of 25% at 25°C for 30 min and 10% pulp consistency. Nitren extraction was carried out according to Janzon et al. (2006). The pulp was extracted with a 4.6% nitren solution (determined by ICP) to reach a xylan content of about 5%. The enzymatic treatment was done according to Kvarnlöf et al. (2007) with Novozyme 476 (from Novozyme, Denmark), a monocomponent endoglucanase (activity: 4,500 ECU/g) at 40°C, pH 7.5, enzyme concentration 75 ECU/g pulp, 1.5 h and 3% pulp consistency. A temperature-controlled glass reactor with mechanical stirring was filled with the pulp suspension. The pH was regulated with NaOH or diluted sulfuric acid. The treatment was started with enzyme addition and terminated by filtration and immediate washing of the pulp with hot (>80°C) water.
Analyses
CP-MAS-NMR measurements
The pulp samples were wetted with deionized water (approx. 50% water content) and packed into a 7-mm zirconium oxide rotor for solid-state CP-MAS 13C NMR measurement on a Bruker Avance DPX300 spectrometer operating at 75.46 MHz for 13C. All experiments were carried out at ambient temperature (26°C) using a Bruker 7-mm MAS probe. The MAS spinning rate was 4 kHz, the 1H decoupling field was 50 kHz, the contact time was 1 ms, and the delay between repetitions was 3 s; 2,048 transients were accumulated for each spectrum. The resulting FIDs were multiplied with an exponential window function (LB = 10 Hz) prior to Fourier transformation. Chemical shifts were referenced to the methylene signal in external adamantane (δ = 29.5 ppm).
Spectral deconvolution of the cellulose C4 region was performed following a procedure published by Larsson et al. (1997). Here, the intensities of nine lines with Lorentzian and Gaussian lineshape were adjusted using the Excel Solver tool, such that their superposition fits the experimental spectrum. Positions and widths (FWHM) of the fitted signals were kept constant and were taken as mean values from literature (Larsson et al. 1997; Wickholm et al. 1998; Nocanda et al. 2007), for enhanced comparability. The fibril lateral dimensions were calculated as described by Wickholm et al. (1998) according to the model of a fibril with a square cross-section given by the equation
Fibril dimensions are calculated with q (fibril) being the intensity of the signal of accessible and inaccessible surfaces and fibril aggregate dimensions with q (aggregate) as the intensity of the signal of accessible surfaces. The calculated number of cellulose chains n can be converted to a lateral dimension expressed in nm using a factor of 0.55 nm per chain (Krässig 1993).
The crystallinity index CrI was determined from the areas of the crystalline (93–86.5 ppm) and amorphous (86.5–80.6 ppm) C4 signals CrI = Acryst/(Acryst + Aamorph) (Newman and Hemmingson 1990).
Conventional pulp analysis
Kappa number was determined according to the Tappi method T236, intrinsic viscosity according to SCAN-CM 15:99, alkali resistance according to DIN 54355, carbohydrate composition by total hydrolysis and subsequent HPAEC-PAD (Sixta et al. 2001), Klason Lignin according to the Tappi method T222, acid soluble lignin according to the Tappi useful methods 250 and pentosan content according to Tappi T223.
Molecular weight distribution (MWD) was determined by size exclusion chromatography (SEC) with multi-angle laser light scattering (MALLS) detection in DMAc-LiCl solution according to Schelosky et al. (1999).
Pulp reactivity was determined according to Treiber et al. (1962) by measuring the filterability and the particles in viscose dope.
Enzymatic peeling was performed by treating the pulp sample with an enzyme mixture (Econase HC 400 and Celluclast 15L) and measuring the carbohydrate concentrations in the supernatant by HPLC according to Sjöberg et al. (2005).
The carboxyl content in the cellulosic materials was determined by fluorescence labeling with 9H-fluoren-2-yl-diazomethane (FDAM) combined with GPC with fluorescence detection according to Bohrn et al. (2006).
Acid hydrolysis of pulps
Hemicelluloses were chemically removed by acid hydrolysis in 2.5 M HCl at 100°C for 1 h and a consistency of 2% according to Liitiä et al. (2003). The hydrolyzed pulps were collected in a glass filter and washed neutral with demineralized water. NMR measurements were taken in the wet state.
T2 relaxation of water
The pulp sample was equilibrated in pH 7 buffer solution and prepared at saturated liquor content by centrifugation for 10 min at 5,500 rpm. The wet sample was packed into a 10-mm NMR tube, and the residual T2 relaxation time at 26°C was measured on a Bruker Avance 300-MHz instrument using a CPMG pulse program (Ibbett et al. 2008).
Results and discussion
Reactivity of pulp
The suitability of the eucalypt dissolving pulps for viscose application was tested by the determination of the filterability performance (FV) and the particle spectrum (Table 2) ranging from 3 to 150 μm (Part). The reference sulfite pulp SULF displays values for FV and particles typical for viscose pulps suitable for staple fiber production. The formation of alkali cellulose (AC, steeping with sodium hydroxide solution and subsequent pressing) is the first step in the Treiber procedure. During this step, the remaining xylan should be dissolved in the sodium hydroxide solution and be removed from the pulps.
As expected, the quality of viscose can be assessed as unacceptable for the hemicelluloses-rich pulp EK as indicated by poor filterability and high particle content. The viscose formation is preceded by an alkali activation step, and the residual (alkali-resistant) xylan in EK after steeping is high (5.2%). Even though CCE treatment significantly decreased the overall xylan content in EK-CCE, the quality of the viscose dope remains poor.
This result was not expected; earlier experiments (Sixta and Möslinger 2006) revealed higher filterability and significantly lower particle contents in the viscose dope produced from a CCE-treated pulp. The reactivity of kraft pulp toward acetylation is also reported to increase by alkali treatment (El-Din and El-Megeid 1994). The alkali cellulose (AC) made from the paper pulp EK as produced after steeping and pressing contains significant amounts of residual xylan. The enzymatic treatment (EK-CCE-EG) improves the filterability and lowers the amount of particles in the viscose dope, which is also reflected in lower xylan content in the AC. The increase in reactivity by enzymatic treatment has been observed by Henriksson et al. (2005), Köpcke et al. (2008) and Kvarnlöf et al. (2007). A possible explanation could be the removal of less-ordered cellulose between the fibrils cutting the fibrils and making them more accessible (Henriksson et al. 2005). Xylan removal by nitren extraction (EK-Nitren) has only minor effect on viscose filterability and on the particle content, and there is also residual xylan found in the AC. The prehydrolysis kraft pulp (PHK), the sulfite reference pulp (SULF) and the pure CCE-extracted prehydrolysis kraft pulp CELL result in a viscose dope with very good filterability and a low particle content, and the xylan in the pulps is soluble in alkali resulting in a nearly xylan-free pulp after steeping. This is a clear indication that alkali-resistant xylan lowers the reactivity of pulp in the viscose formation process.
Crystallinity and supramolecular structure
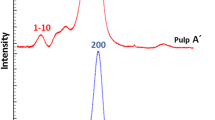
Spectral fitting analysis of the C4-region of CP-MAS 13C NMR spectra (Fig. 1) yields information on the relative amounts of different cellulose modifications. Lateral fibril dimensions can be calculated from the accessible and inaccessible surface signals. The calculation of lateral fibril dimensions can only be performed on hydrolyzed samples since it is necessary to remove the interfering signals from the hemicelluloses (Wickholm et al. 1998). The acid hydrolysis removes the hemicelluloses visible in the decrease in signal at 81.7 ppm (Fig. 2). However, in addition to the hemicelluloses, also some part of the less-ordered, amorphous cellulose is removed (VanderHart and Atalla 1984; Krässig 1993) as can be seen by the increase in crystallinity index (Table 3) after acid hydrolysis (AH). An influence of acid hydrolysis on the accessibility and reactivity cannot be excluded, and therefore, the crystallinity index of unhydrolyzed samples was determined by simple integration of crystalline and amorphous region of the spectrum (Newman and Hemmingson 1990) for comparison with reactivity and accessibility results.
The 13C NMR crystallinity index CrI of the pulps reveals a high crystallinity of EK-Nitren and PHK pulp indicating the removal of less-ordered material during processing (Table 3). Although both pulps have comparable crystallinity, the PHK pulp exhibits notably better viscose reactivity than EK-Nitren pulp. The equally reactive SULF reference pulp and the alkali-treated prehydrolysis kraft pulp CELL display a slightly lower CrI. The overall CrI of the pulp seems to have a minor effect on the pulp reactivity, indicating that the differences in supramolecular structure might be predominant in the process of pulp accessibility and reactivity. The enzymatic treatment slightly increases the CrI, which can be explained by the degradation of disordered domains by the endoglucanase. The crystallinity index of EK pulp is comparably low due to the high hemicellulose content.
The lateral fibril dimensions (Table 4) calculated with Eq. 1 after spectral fitting (Fig. 1) of the C4 region of the CP-MAS 13C spectra are comparable to the values found in the literature (fibril width 4–5 nm, aggregate width 15–25 nm). The pulp samples have similar fibril widths but differences are found in the fibril aggregates. The CCE-extracted pulps EK-CCE and EK-CCE-EG have larger fibril aggregates than EK, EK-Nitren, PHK, CELL and SULF pulp. Larger fibril aggregates could be one contributing reason for a decreased reactivity. The poor reactivity behavior of EK and EK-Nitren is not reflected in the fibril aggregate widths. Although the reactivity behavior of PHK and EK-Nitren is different, the lateral fibril dimensions are similar.
It must be noted that the fitting of nine lines into the C4-resonance leaves some questions about the uniqueness of the solution. A poor spectral resolution can add to the uncertainties. However, the employed method is believed to be robust enough to give results representative for the cellulose structure, yielding at least relevant relative comparisons.
Accessibility
The interaction between molecules in non-crystalline regions is related to the accessibility of the material. A common method to measure water accessibility is the water retention value (WRV), which comprises the weight gain of a sample after swelling in water and centrifugation under defined conditions. The measurement of the T2 relaxation time of water by NMR is another method for studying water behavior in cellulosic materials (Carles and Scallan 1973). The relaxation time is influenced by the interactions of the water molecules with the accessible hydroxyl groups of cellulose, and the method allows the determination of the amount of “restricted” or “bound” water. The amorphous phase for these pulps was obtained by simple integration of the crystalline and the amorphous region of the C4 signal in the CP-MAS-NMR spectrum. The fourth method applied was the determination of the accessible surface by deconvolution of the CP-MAS 13C NMR spectra of the samples after acid hydrolysis. The results are summarized in Table 5 and clearly show that a comparison of accessibility obtained with different methods poses difficulties. In terms of WRV, EK and CELL show a slightly better accessibility than the other pulps. The amount of surface water obtained by NMR-T2-relaxation is higher in the alkali-treated pulps EK-CCE, EK-CCE-EG and CELL. Only EK shows a more pronounced amorphous region compared to the other samples probably because of its higher content of hemicelluloses. The amount of accessible surfaces indicates a good accessibility of cellulose fibrils in the pulps SULF, CELL and PHK, which were treated with acid at some point of their production history and in post-extracted EK-Nitren. The high xylan content in EK causes the good water retention and a large amorphous phase in the pulp but the reactivity is low. The alkali treatment seems to lead to a larger amount of restricted water (Ws) in pulp, maybe indicating a structural change in the crystalline regions but has no effect on the formation of viscose. The closest relationship between reactivity and accessibility is found in the amount of accessible surfaces, respectively, and the lateral fibril aggregate dimensions determined by deconvolution of CP-MAS 13C NMR spectra.
Hemicelluloses
The physico-chemical properties of the hemicelluloses are affected by the pulping processes. The hemicelluloses are deacetylated under alkaline conditions and dependent on the sodium hydroxide conditions and the temperature undergo dissolution with largely preserved molecular weight. Under acidic conditions, they are severely degraded and soluble only as oligo- and/or monomeric sugars, while the residual xylan remains partly acetylated. Consequently, the remaining xylan in the pulp is expected to differ in molecular weight, local distribution, degree of acetylation or number of side chains. The samples were examined by the method of enzymatic peeling (Sjöberg et al. 2005), by GPC and by the method of carboxyl labeling with FDAM (Bohrn et al. 2006) in order to obtain information about the residual xylan.
Provided that the enzymes are too voluminous to enter the pores of the cellulose sample, the xylose-to-glucose ratio in the peeled mass of a pulp sample subjected to an enzyme mixture for a predefined time is indicative for the xylan distribution. An enrichment of the xylan on the surface is characterized by a high initial xylan-to-glucan ratio followed by a steep decrease to the average xylan-to-glucan ratio level in the inner cell wall layers.
The results (Fig. 3) of the enzymatic peeling experiments show nearly similar behavior of the EK-CCE and the EK-CCE-EG samples. The endoglucanase treatment after the cold caustic extraction has no effect on the xylan distribution across the cell wall. The xylan in both samples seems to be enriched on the surface, probably because of the re-precipitation of the hemicelluloses during kraft cooking. The surface layers of the PHK pulp are characterized by a lower xylan-to-glucan ratio despite a comparable xylan content of 5%, which indicates a more even distribution of the xylan. The post-extracted EK-Nitren pulp with a slightly lower average xylan content of 4% reveals a lower xylan-to-glucan ratio at the surface layers. The more even distribution of xylan is probably due to the ability of nitren to dissolve the reprecipitated surface xylan. The enzymatic peeling of the sulfite pulp (SULF) suggests a relatively high xylan-to-glucan ratio in the outer cell wall layers, which may be explained by the ability of the enzymes to penetrate into the inner cell wall regions due to the high surface porosity of acid sulfite dissolving pulps. Thus, the degraded xylan is not representative for the outer surface layers of the pulp only.
The molecular mass distribution was determined by GPC using DMAc-LiCl as a solvent. The molar masses Mn and Mw are listed in Table 1. The pulp samples were adjusted to a comparable intrinsic viscosity to exclude influences of molecular weight on the accessibility and reactivity. The distribution of the post-extracted pulps EK-CCE, EK-CCE-EG, EK-Nitren and CELL is fairly narrow (Fig. 4 left), and the residual xylan is mostly included in the cellulose peak. The paper pulp EK displays a distinct shoulder in the low molecular weight region caused by the hemicelluloses. The acid-treated pulps PHK (prehydrolysis kraft) and SULF (acid sulfite) reveal a small amount of relatively low molecular weight fraction (Fig. 4, right), indicating a lower molecular weight of the hemicelluloses. The FDAM labeling (Table 6) confirms an enrichment of carboxyl groups in the low molecular weight fraction below 32 kDa for the acid-treated PHK and SULF pulps.
Carboxyl groups in pulp constituents originate predominantly from uronic acid side chains in the xylan fraction and to a smaller extent also from oxidation processes in alkaline pulping and bleaching processes. The carboxyl group profile in dependence of molecular weight fractions can be determined by means of the FDAM-labeling method (Bohrn et al. 2006). The procedure includes labeling of carboxyl groups and subsequent GPC analysis with a fluorescence detector. The carboxyl group content of the post-extracted pulps EK-CCE, EK-CCE-EG, EK-Nitren and CELL is accumulated in the high molecular weight region (Table 6), indicating the existence of high molecular weight xylan. In contrast, the sulfite pulp SULF contains more carboxyl groups in the low molecular weight fraction due to the acidic degradation of the hemicelluloses during pulping.
Conclusion
The physico-chemical properties of the residual xylan in a dissolving pulp are highly affected by the xylan removal process applied. Pulp reactivity, expressed as viscose processability, is impaired by the presence of alkali-resistant and thus high molecular weight xylan. These pulps, prepared solely by alkaline processes, such as kraft pulping combined with CCE post-treatment, are characterized by larger fibril aggregate dimensions than those pulps exhibiting an acid pre-treatment, PHK, CELL and SULF, which in turn signal high reactivity toward xanthation. Endoglucanase treatment of a CCE-treated kraft pulp causes an increase in viscose reactivity in line with a decrease in the amount of alkali-resistant xylan. The pulp reactivity seems to be related to the amounts and properties of the residual xylan in the pulp rather than to indicators representing the fiber wall supramolecular structure. This suggests an indirect role of the fiber wall supramolecular structure as a limiting factor for hemicellulose removal by restricting accessibility.
References
Bohrn R, Potthast A, Schiehser S, Rosenau T, Sixta H, Kosma P (2006) The FDAM method: determination of carboxyl profiles in cellulosic materials by combining group-selective fluorescence labeling with GPC. Biomacromolecules 7:1743–1750
Carles JE, Scallan AM (1973) The determination of the amount of bound water within cellulosic gels by NMR spectroscopy. J Appl Polym Sci 17:1855–1865
Duchesne I, Hult EL, Molin U, Daniel G, Iversen T, Lennholm H (2001) The influence of hemicellulose on fibril aggregation of kraft pulp fibres as revealed by FE-SEM and CP/MAS 13C-NMR. Cellulose 8:103–111
El-Din NMS, El-Megeid FFA (1994) The effect of cold alkali pretreatment on the reactivity of some cellulosic pulps towards acetylation. Holzforschung 48:496–500
Filpponen I, Argyropoulos DS (2008) Determination of cellulose reactivity by using phosphitylation and quantitative 31P NMR spectroscopy. Ind Eng Chem Res 47(22):8906–8910
Fock W (1959) Eine modifizierte Methode zur Bestimmung der Reaktivität von Zellstoffen für die Viskoseherstellung. Das Papier 13:92–95
Frilette VJ, Hanle J, Mark H (1948) Rate of exchange of cellulose with heavy water. J Am Chem Soc 70:1107–1113
Henriksson G, Christiernin M, Agnemo R (2005) Monocomponent endoglucanase treatment increases the reactivity of softwood sulphite dissolving pulp. J Ind Microbial Biotechnol 32:211–214
Hult EL, Larsson PT, Iversen T (2000) A comparative CP/MAS 13C-NMR study of cellulose structure in spruce wood and kraft pulp. Cellulose 7:35–55
Hult EL, Larsson PT, Iversen T (2001) A CP/MAS 13C-NMR study of supermolecular changes in the cellulose and hemicellulose structure during kraft pulping. Nordic Pulp Pap Res J 16(1):33–39
Ibbett RN, Schuster KC, Fasching M (2008) The study of water behaviour in regenerated cellulosic fibres by low-resolution proton NMR. Polymer 49:5013–5022
Ioelovich M (2009) Accessibility and crystallinity of cellulose. BioResources 4:1168–1177
Janzon R, Puls J, Saake B (2006) Upgrading of paper-grade pulps to dissolving pulps by nitren extraction: optimisation of extraction parameters and application to different pulps. Holzforschung 60:347–354
Jayme G, Schenck U (1949) Die Auswirkung verschiedener alkalischer Veredlungsbedingungen auf das Verhalten von Zellstoffen bei der Acetylierung. Das Papier 23:469–476
Köpcke V, Ibarra D, Ek M (2008) Increasing accessibility and reactivity of paper grade pulp by enzymatic treatment for use as dissolving pulp. Nordic Pulp Pap Res J 23:363–368
Krässig HA (1993) Cellulose: structure, accessibility and reactivity. Polymer monographs, vol 11. Gordon and Breach Science Publishers, Switzerland
Kvarnlöf N, Germgard U, Jönsson LJ, Söderlund CA (2007) Optimization of the enzymatic activation of a dissolving pulp before viscose manufacture. Tappi J 6(6):14–19
Larsson PT, Wickholm K, Iversen T (1997) A CP/MAS 13C NMR investigation of molecular ordering in celluloses. Carbohydr Res 302:19–25
Liitiä T, Maunu SL, Hortling B, Tamminen T, Pekkala O, Varhimo A (2003) Cellulose crystallinity and ordering of hemicelluloses in pine and birch pulps as revealed by sold-state NMR spectroscopic methods. Cellulose 10:307–316
Maloney TC, Johansson T, Paulapuro H (1998) Removal of water from the cell wall during drying. Pap Technol 39:39–47
Newman RH, Davidson TC (2004) Molecular conformations at the cellulose-water interface. Cellulose 11(1):23–32
Newman RH, Hemmingson JA (1990) Determination of the degree of cellulose crystallinity in wood by carbon-13 nuclear magnetic resonance spectroscopy. Holzforschung 44(5):351–355
Newman RH, Hemmingson JA (1994) Carbon-13 NMR distinction between categories of molecular order and disorder in cellulose. Cellulose 2:95–110
Nocanda X, Larsson PT, Spark A, Bush T, Olsson A, Madikane M, Bissessur A, Iversen T (2007) Cross polarisation/magic angle spinning 13C-NMR spectroscopic studies of cellulose structural changes in hardwood dissolving pulp process. Holzforschung 61:675–679
Rousselle MA, Nelson ML (1971) Accessibility of cotton cellulose by deuterium exchange. Text Res J 41(7):599–604
Schelosky N, Röder T, Baldinger T (1999) Molmassenverteilung cellulosischer Produkte mittels Größenausschlußchromatographie in DMAc/LiCl. Das Papier 53(12):728–738
Sixta H, Möslinger R (2006) Internal report, Lenzing AG
Sixta H, Schelosky N, Milacher W, Baldinger T, Röder T (2001) Characterization of alkali-soluble pulp fractions by chromatography. In Proceedings of the 11th ISWPC. Nice, France, pp 655–658
Sjöberg J, Rosenau T, Potthast A, Kosma P (2005) Cross-sectional analysis of the polysaccharide composition in cellulosic fiber materials by enzymatic peeling/high-performance capillary zone electrophoresis. Biomacromolecules 6:3146–3151
Treiber E, Rehnstroem J, Ameen C, Kolos F (1962) Über die Laboratoriumsviskosekleinstanlage zur Testung von Chemiefaserzellstoffen- A small scale laboratory viscose plant for testing rayon grade pulps. Das Papier 16:85–94
VanderHart DL, Atalla RH (1984) Studies of microstructure in native cellulose using solid-state 13C NMR. Macromolecules 17:1465–1472
Virtanen T, Maunu SL, Tamminen T, Hortling B, Liitiä T (2008) Changes in ultrastructure during various kraft pulping conditions evaluated by 13C CPMAS NMR spectroscopy. Carbohydr Polym 73:153–163
Wallis AFA, Wearne RH (1990) Chemical cellulose from radiata pine kraft pulp. Appita 43(5):355–366
Wickholm K, Larsson PT, Iversen T (1998) Assignment of non-crystalline forms in cellulose I by CP/MAS 13C NMR spectroscopy. Carbohydr Res 312:123–129
Acknowledgments
We gratefully acknowledge Dr. Antje Potthast for the measurement of FDAM-labelled samples. Financial support was provided by the Austrian government, the provinces of Lower Austria, Upper Austria, and Carinthia as well as by the Lenzing AG. We also express our gratitude to the Johannes Kepler University, Linz, the University of Natural Resources and Applied Life Sciences, Vienna, and the Lenzing AG for their in-kind contributions. Author P.T. Larsson acknowledges the Wallenberg Wood Science Centre in Sweden for financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is dedicated to Gerd Wegener on the occasion of his retirement as professor at the Technische Universität München.
Rights and permissions
About this article
Cite this article
Wollboldt, R.P., Zuckerstätter, G., Weber, H.K. et al. Accessibility, reactivity and supramolecular structure of E. globulus pulps with reduced xylan content. Wood Sci Technol 44, 533–546 (2010). https://doi.org/10.1007/s00226-010-0370-2
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-010-0370-2