Abstract
The aim of the current study is to improve the thermal stability of one-component moisture-curing polyurethane adhesives. The approach here tends to add suitable filler materials to the adhesive and to study the resulting effects. The investigation covers mechanical tests to determine the shear strength of the glued wood joints according to EN 302-1 (2004). Furthermore, the distribution of the filler material within the adhesive is shown by means of environmental scanning electron microscopy combined with energy-dispersive X-ray spectroscopy analysis. The thermal stability of the glued wood joints could be significantly improved by adding chalk with a volume fraction of 30% to the adhesive.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
One-component polyurethane adhesives (1C PUR) are increasingly used for the bonding of wood. The properties of the reacted polymers (like elasticity, strength, temperature, and moisture resistance) are influenced by the prepolymer as well as by additives like surfactant, catalyst, and especially filler material. Filler materials are non-volatile, non-gluing matters, which are insoluble in the adhesive. Common fillers are fibres (glass fibre, mica), powders (cellulose, aluminium oxide, silica), sheet-like materials (talc), cubic materials (chalk, barytes) (Zeppenfeld and Grunwald 2005) or nowadays nano-particles (Park et al. 2009) or functionalised nanoclays (Dodiuk et al. 2006).
In the past, several investigations on different types of adhesives and fillers have been carried out. The mechanical properties of polyvinyl acetate depending on morphology and chemical structure of the filler material (calcium carbonate) were investigated by Kovačević et al. (1996). The influence of the same filler on the rheological and adhesion properties of a water-based polyurethane dispersion was investigated by Muñoz Milán et al. (2005). Mansouri and Pizzi (2007) improved the performance of urea–formaldehyde and phenol–formaldehyde resin by adding micronised polyurethane powder. Sepulcre-Guilabert et al. (2001) proposed natural ultramicronised calcium carbonate and mixtures of fumed silica with natural ultramicronised calcium carbonate as filler for solvent-based PUR.
Investigations on the structure–property relationships of 1C PUR adhesives for wood, including adhesives with fibrous fillers, and their sensitivity to low wood moisture content (WMC) were carried out by Beaud et al. (2006). In contrast, Richter and Schierle (2002) and Schrödter and Niemz (2006) investigated the adhesive performance of 1C PUR under high moisture and temperature conditions. It can be concluded that the bonding strength of 1C PUR adhesives decreases with increasing WMC and temperature, respectively.
The investigations mentioned above show that the adhesion of joints produced with adhesives containing fillers was noticeably increased. The goal of this study is to investigate if comparable improvements are also achievable for the use of 1C PUR adhesives under high temperature exposure.
Materials and methods
Three laboratory adhesives were produced by Purbond (Sempach Station, Switzerland) with a varying filler material content. Thereby chalk was mixed into the adhesive using volume fractions of 15 and 30%. The adhesives’ parameters are listed in Table 1. All bondings were carried out with beech wood (Fagus sylvatica L.). The raw density ρ at an equilibrium moisture content ω of (12 ± 1) % amounted to (745 ± 34) kg/m3. The one-sided application of the adhesives was carried out with a spread of 150 g/m2 and a pressing pressure of 0.7 MPa. To investigate the influence of the filler material content on the shear strength, 15 specimens of each group were tempered in a drying chamber for 1 h at 100 and 150°C, respectively. Another group of specimens was conditioned at different relative humidities (35, 65, 85, 95% RH) at a temperature of 20°C.
The shear strength was determined according to EN 302-1 (2004). The specimens were tested using a displacement-controlled universal testing machine (Zwick Z100) under standard climatic conditions (20°C, 65% RH). The shear strain ε was evaluated with a video-extensometer. After recording the stress–strain curve until failure, the wood failure percentage was estimated visually in steps of 10%.
In addition, an environmental scanning electron microscope (ESEM) was used, and the bondline was analysed by means of energy-dispersive X-ray spectroscopy (EDX) to investigate the penetration depth and distribution of the adhesives within the wood. The EDX analysis allows for chemical characterisation of the specimens and thereby to distinguish between adhesive, wood and filler material, which contains a high amount of calcium.
Results and discussion
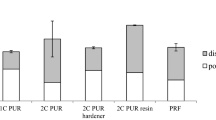
The shear strength of the glued wood joints increased significantly with a higher content of filler material. The graphs in Fig. 1 indicate an increase of strength at standard climatic conditions, but also after temperature exposure. The maximum increase amounted to 52% at 100°C using 30% filler. The wood failure percentage was also increased compared to adhesives without filler (Table 2) as a consequence of the better adhesion between wood and adhesive, which subsequently exceeded the wood strength.
The effect of the filler material decreased with increasing WMC. At 6.5% wood moisture, the maximum overall increase of shear strength amounted to 31% at 30% filler material. Schrödter and Niemz (2006) determined maximum compression shear strength at about 12% WMC within a similar investigation on commercially available 1C PUR adhesives. From this, it follows that after the drying process, internal compression stresses arise within the bondline, which have a positive effect in the case of tensile load.
In contrast to the specimens exposed to high temperatures, the average increase in shear strength at 21.4% WMC was relatively low (15%); however, there was no significance at the 5% level (Fig. 2). This means that the filler material had no substantial effect on the shear strength at high WMC. The limiting factor for the adhesive bond is the moisture resistance of the adhesive itself, independent of its filler material content. Hydrolytic effects are a possible explanation for the lower shear strength.
The main reason for the increased shear strength is the reduced penetration into the cell lumina, which is clearly shown by the combined ESEM/EDX micrograph (Fig. 3). On the left side (30% filler), a completely filled bondline and empty pores document a good bond. The adhesive without filler (right side) on the other hand, shows a poorly bonded adherend. The adhesive filled out pores even 500 μm away from the bondline; however, the joint starved instead. Already Suchsland (1958) advised that there is no relationship between the penetration depth and the bonding quality as long as the adhesive fills out the topmost surface forming cell layer.
Because calcium carbonate was used as filler material, the element calcium can be easily used for detecting the substance with EDX. It turned out that the filler material was homogeneously dispersed within the adhesive matrix (Fig. 3, picture detail) and no separations could be detected.
Conclusion
Chalk turned out to be a suitable filler material, which is easily addable to the adhesive, well miscible and cost efficient and it significantly improves the thermal stability of glued wood joints in the aimed temperature range. For future studies, it would be of particular interest to find suitable alternative filler materials and to determine the optimal filler material content regarding costs and bonding properties.
References
Beaud F, Niemz P, Pizzi A (2006) Structure-property relationships in one-component polyurethane adhesives for wood: sensitivity to low moisture content. J Appl Polym Sci 101(6):4181–4192
Dodiuk H, Belinski I, Dotan A, Kenig S (2006) Polyurethane adhesives containing functionalized nanoclays. J Adhes Sci Technol 20(12):1345–1355
EN 302-1 (2004) Adhesives for load−bearing timber structures—test methods—Part 1: determination of bond strength in longitudinal tensile shear strength
Kovačević V, Lučić S, Hace D, Cerovečki Ž (1996) Tensile properties of calcium carbonate reinforced poly(vinyl acetate). J Adhes Sci Technol 10(12):1273–1285
Mansouri HR, Pizzi A (2007) Recycled micronized polyurethane powders as active extenders of UF and PF wood panel adhesives. Holz Roh Werkst 65(4):293–299
Muñoz Milán AB, Pérez-Limiñana A, Arán-Aís F, Torró-Palau A, Orgilés-Barceló AC (2005) Effect of the amount of calcium carbonate as filler on the rheological and adhesion properties of a water-based polyurethane dispersion. Macromol Symp 221:33–41
Park SW, Kim BC, Lee DG (2009) Tensile strength of joints bonded with a nano-particle-reinforced adhesive. J Adhes Sci Technol 23(19):95–113
Richter K, Schierle M (2002) Behaviour of 1 K PUR adhesives under increased moisture and temperature conditions. Lignovisionen 4:149–154
Schrödter A, Niemz P (2006) Investigations on the failure behaviour of glue joints at high temperatures and relative humidity. Holztechnologie 47(1):24–32
Sepulcre-Guilabert J, Ferrándiz-Gómez TP, Martín-Martínez JM (2001) Properties of polyurethane adhesives containing natural calcium carbonate plus fumed silica mixtures. J Adhes Sci Technol 15(2):187–203
Suchsland O (1958) On the penetration of glue in wood gluing and the significance of the penetration depth for the strength of glue joints. Holz Roh Werkst 16(3):101–108
Zeppenfeld G, Grunwald D (2005) Klebstoffe in der Holz- und Möbelindustrie. DRW-Verlag, Leinfelden-Echterdingen
Acknowledgments
The authors would like to thank Gabriele Peschke from the Fracture Mechanics of Concrete Group at the Institute for Building Materials (ETH Zurich) for her support at the ESEM.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Clauβ, S., Allenspach, K., Gabriel, J. et al. Improving the thermal stability of one-component polyurethane adhesives by adding filler material. Wood Sci Technol 45, 383–388 (2011). https://doi.org/10.1007/s00226-010-0321-y
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-010-0321-y