Abstract
The validity of the Kubelka–Munk (K-M) theory was investigated to determine the IR absorption spectra of wood based on diffuse reflection infrared Fourier transform (DRIFT) measurements taken on photodegraded samples. After analysing plenty of DRIFT spectra of wood and examining the shape of the K-M equation, it can be concluded that the measured K-M function can be used as an absorption spectrum if the values of the function are below 14 K-M units. Above this limit, the K-M theory, which was created for poorly absorbing materials, does not give the absorption of wood properly. If a matt aluminium plate is used as a background material and the values are between 14 and 40, absorption changes can be calculated after normalisation of the spectra. This normalising manipulation is only successful if there is an absorption peak close to the examined one which does not change its absorption during the photodegradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wood is a good light absorber for infrared (IR), visible and ultraviolet (UV) light. If the wood sample is lighted by UV light, it is totally absorbed in a 75–μm-thick layer (Hon and Ifju 1978). The depth profile and penetration of light into wood was investigated by Kataoka et al. (2004, 2005, 2007). The penetration depth depends on the wavelength of the light. The secondary effects of UV light irradiation can be detected in 400–500 μm depth for the aromatic skeletal vibration (1,510 cm−1) and in 600–700 μm depth for carbonyl (1,730 cm−1) in a long-term treatment (e.g. 1,500 h; Kataoka and Kiguchi 2001). This means wood is not transparent for light, and the usual transmission method is not suitable to measure the absorption properties of wood. For this purpose, powdered wood is usually mixed with light transparent material. This mixture is pressed to pellet, and the pellet is used to measure the wooden absorption using the transmission method. For measurements in the IR region, potassium bromide is a widely applied matrix material. This method was an excellent analytical tool to discover the chemical constitution of wood by IR spectroscopy. Since the change caused by irradiation appears in a thin surface layer, it is not easy to remove the proper thin layer for pellet preparation. One important example is the UV photodegradation of wood. It is difficult to remove a layer thinner than 80 μm which has suffered change during the treatment. Concerning this difficulty, many researchers apply the pellet method without indicating the thickness of the wood layer used for powder making (Hon and Feist 1986; Kosikova and Tolvaj 1998; Müller et al. 2003; Pandey 2005; Umemura et al. 2008).
The powerful Fourier transform IR technique gives the possibility to measure the diffusely reflected (DRIFT) intensity of a thin layer. This is a perfect technique to investigate the photodegradation because the information is given from the same layer which suffered photodegradation. The Kubelka–Munk (K-M) theory (Kubelka and Munk 1931; Kubelka 1948) was created to calculate the absorption properties of the surface layer using the reflected light. This theory was created for poorly absorbing materials. Since wood is a good light absorber, the K-M theory is widely applied to determine its light absorption (Owen and Thomas 1989; Horn et al. 1994; Tolvaj and Faix 1995; Craciun and Kamdem 1997; Takei et al. 1997; Zanuttini et al. 1998; Pandey 1999; Moore and Owen 2001; Freer et al. 2003; Weiland and Guyonnet 2003; Mitsui et al. 2003; Cui et al. 2004; Kishino and Nakano 2004; Tolvaj and Mitsui 2005). The situation is the same for pulp and paper research (Michell et al. 1989; Ristolainen et al. 2002). In many cases, even the K-M intensity data are not presented in the articles (Owen and Pawlak 1989; Zavarin et al. 1990; Pandey and Theagarajan 1997; Takei et al. 1997; Pandey and Khali 1998; Zhang and Kamdem 2000).
In many papers, milled wood or milled wood diluted with KBr powder is used to determine the absorption properties of wood by DRIFT technique (Berben et al. 1987; Faix and Böttcher 1992; Bouchard and Douek 1993; Stewart et al. 1995; Chang et al. 1998; Holmgren et al. 1999; Ferraz et al. 2000; Baldock and Smernik 2002; Vane 2003; Nuopponen et al. 2006; Toivanen and Alen 2007). Pandey and Theagarajan (1997) found that specular reflection causes distortion in the intensities of the bands in the range 950–1,150 cm−1, which can be minimised by diluting sample powder with KBr. Faix and Böttcher (1992) investigated DRIFT spectra of wood meal of increasing concentrations in KBr as well as increasing particle sizes and also that of solid wood surfaces. They concluded that in order to obtain reproducible FTIR spectra from wood surfaces, the roughness and the anatomical directions of the cut (tangential, radial and cross-cut) must be constant. It is difficult to compare the FTIR spectra of different solid wood surfaces because the natural roughness of the wood species varies widely.
Only some publications consider that the results calculated by K-M theory are questionable. Jones and Heitner (1973) found that the light scattering and absorption properties of radial wood sections using 456 and 495 nm light could be expressed mathematically by using K-M equations. The radial sections contain both earlywood and latewood, hence the photometric results would be similar for different samples from the same tree. However, every tangential section cut from the same tree would show different proportions of earlywood and latewood resulting in different photometric properties for different samples. From that finding, it was concluded that the tangential wood sections do not follow the K-M equations. These results only indicate that the diffuse reflection properties of earlywood and latewood are different because of their different porosity and chemical structure and not because of the invalidity of the K-M equations. Shen et al. (2000) reported that the scattering intensity distribution in the direction across the wood fibres is very close to perfect diffuse reflection, while the distribution in the direction along the fibres is a combination of diffuse and specular reflection at 632.8 nm wavelength. Hembree and Smyrl (1989) reported on the distortions of the K-M spectrum around intensive absorption bands of calcium carbonate and caffeine. In the case of wood sample, Faix and Németh (1988) pointed out that the DRIFT spectra evaluation of photodegraded wood is very difficult because the surface properties strongly influence the quality of the DRIFT spectra. Michell (1991) observed anomalous effects in the IR 950–1,200 cm−1 wavenumber region. Zavarin et al. (1990) reported on deviation in the intensity of the band at 1,166 cm−1 measured in directions parallel and perpendicular to the grain. Tolvaj and Mitsui (2004) found that the intensity of the peak around 1,173 cm−1 changed depending on the angle between the fibre direction and the incident IR light. The 950–1,200 cm−1 IR region is where the wood has the greatest absorption. Here, anomalous effects of DRIFT spectra were found as well. These experiences focused the attention of the authors on examining the validity of K-M theory in the investigated range.
Materials and methods
The investigated wood species were Japanese beech (Fagus crenata), poplar (Populus cauesceus) and Japanese cypress (Chamaecyparis obtuse). Two samples were cut with a size of 50 × 10 × 2 mm3 (long. × tang. × rad.). Planed surface of tangential cut was prepared (by industrial planer machine) containing only earlywood. After preparation, the air-dried samples were stored in an air-conditioned (20°C) laboratory (except irradiation). A strong UV light emitter, a mercury lamp, was applied. The total light emission of the mercury lamp was 320 W, and the samples were located 64 cm away from the lamp. (HAL 800NL, installed into a KBP.659 Nippon Denchi Co. Ltd. chamber.) The emission spectrum of the mercury lamp (supplied by the producer of the lamp) is located mainly in the UV region (80%). It contains 31% UV-A (380–315 nm), 24% UV-B (315–280 nm) and 25% UV-C (<280 nm) radiation. The irradiation was interrupted after 1, 3, 6, 12, 20, 24 and 36 h of irradiation in order to determine any changes. Diffuse reflectance Fourier-transformed infrared (DRIFT) spectroscopy was applied to determine the changes caused by the light irradiation. The 4,000–400 cm−1 wavenumber range was monitored. The IR measurements were repeated after each surface modification. The IR measurements were taken using a JASCO FTIR μ-8,900 double beam spectrometer equipped with a diffuse reflectance unit (JASCO: DR-81). The resolution was 4 cm−1, and 64 scans were averaged. Five different places were identified for measurement on each sample, and two samples were irradiated and measured parallel. Average spectra were calculated from ten individual spectra at all irradiation times and used for further analysis. The background spectra were obtained against a matt aluminium plate, because its reflection properties were stable during the 10-month examination period. The spectral intensities were calculated in K-M units. A new series of beech and Japanese cypress samples were irradiated for up to 36 h. After 36 h of irradiation, the surface of these samples was pressed between two iron plates at a pressure of 2.5 × 106 Pa for 10 s to reduce the roughness of the surface. For the difference spectra, the calculated K-M values were normalised to the peak at about 1,380 cm−1, and the normalised spectra were baseline corrected at 3,800 and 1,900 cm−1 with straight line method. The difference spectra were calculated as the spectrum after irradiation minus the bulk (non-irradiated sample) spectrum. The whole experiment was repeated once to confirm the results.
Results and discussion
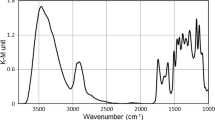
The DRIFT spectrum gives only information about a thin surface layer. Using this method, the diffusely reflected light is collected, and the absorption is calculated by the Kubelka–Munk equation
where k, s and R are the absorption coefficient, the scattering coefficients and the reflectance of a thick enough layer, respectively. Fortunately, the scattering coefficient hardly depends on the wavenumber of the IR light, so the shape of K-M function is similar to the wavenumber dependence of the absorption coefficient. This K-M function is usually used as the IR absorption spectrum. The K-M function is shown in Fig. 1. From IR spectrum of wood, the measured K-M values are usually higher than five units. This means that less than 10% of the incident light can be collected from the surface of the wood by the detector. The collected energy quantity is highly important in the case of background spectrum as well. The light source—sample—detector geometry is always designed, such that only the diffusely reflected light can reach the detector. For background spectrum, the proper material has bad mirror reflection and excellent diffuse reflectance properties. Metal mirror is never used for this purpose. Rough surface is recommended with particles as small as possible. Therefore, KBr powder is often used for this purpose. The K-M function is almost linear below 14 K-M units, while between 14 and 40 units, it is a curve. Above 40 units, the function is highly sheer, so a small decrease in the reflection causes a large change in the k/s values. In this region, the change of K-M units is not proportionate to the absorption change. This happens if the surface roughness increases by roughening the surface with sandpaper. The rough surface elevates the light scattering, and the collected light intensity decreases. The DRIFT spectrum increases enormously not because of the absorption increase. (Details are written in Tolvaj and Mitsui 2004). This finding indicates that the DRIFT spectra cannot be used automatically as absorption spectra. The K-M units of a DRIFT spectrum are calculated by comparison of the reflected intensity data of the background material with that of the sample. High intensities in background spectrum generate high intensities in DRIFT spectrum of the sample too. That is why a proper, good quality background spectrum is important for getting high-quality DRIFT spectrum. The limits of K-M intensities defined in this study are only valid if the background is a matt aluminium plate.
Similar phenomenon occurs if the wooden surface is exposed to strong UV light for a long time. The DRIFT technique is usually applied for monitoring the chemical changes in this case using the K-M equation. The phenomenon is well demonstrated in Fig. 2. This figure represents the K-M functions of beech wood after 0, 12, 24 and 36 h of irradiation using a mercury lamp. The band assignment can be found in a previous work (Tolvaj and Faix 1995).
The main change is the overall intensity increase caused by UV irradiation. This increase is not proportionate with the irradiation time. This change cannot represent the absorption increase because the absorption increase is due to the creation of new chemical groups. The creation of new absorbing groups may happen only after degradation of other chemical compounds. This degradation should be visible as an absorption decrease; however, there is no absorption decrease visible in Fig. 2. The UV treatment increases the roughness of the sample surface and therefore changes the scattering properties. In consequence, less light reaches the detector, and the K-M values increase according to the K-M function (Fig. 1). This increase is enormously fast if the K-M units exceed 40. This phenomenon is visible in Fig. 2, especially in 3,100–3,600 cm−1 region.
The poor reflection creates the automatic signal amplification of the spectrophotometer, which amplifies the noises as well. This is well visible in Fig. 2 in 3,100–3,600 cm−1 region. This example represents clearly that the K-M theory cannot be applied automatically to determine light absorption of wood.
To define the limits of the K-M theory, more detailed treatments are needed. For this purpose, the treatment was interrupted after 1, 3, 6, 12 and 20 h of UV irradiation. The changes of a poplar sample are presented in Fig. 3. These are the measured data without baseline correction. Poplar was chosen because its K-M function was the most intensive one in the important fingerprint (900–1,900 cm−1) region. The intensity increase is similar here to the one in Fig. 2. The change of K-M functions should represent both changes caused by alteration of scattering and absorption. Unfortunately, the change of absorption is not visible at all. The great changes by roughness alteration overlap the smaller changes of absorption. Fortunately, the scattering is not wavenumber dependent in a small wavenumber region in contrast to the absorption, which is characteristically wavenumber dependent. This fact gives the possibility to diminish the effect of scattering mathematically. For this purpose, an internal wavenumber point is needed where almost no absorption change is caused by the examined treatment. If the spectra are normalised to the unit at this wavenumber, this mathematical process can minimise the effect of scattering. Certainly, the change here is determined only by the scattering because there is no absorption change. Experiments on photodegradation confirm that the CH deformation in polysaccharides absorbing around 1,380 cm−1 is intact during photodegradation (Tolvaj and Faix 1995; Forsskåhl and Janson 1995; Kataoka and Kiguchi 2001). So this peak intensity can be used as an internal standard in case of photodegradation. The mathematical process of normalising is that all K-M data are divided by the value at 1,380 cm−1, and the results are expressed in relative units and not in K-M units anymore.
The difference spectra were calculated after baseline correction and normalising the spectra at 1,380 cm−1 (Tolvaj and Faix 1995). These difference spectra are presented in Fig. 4. After irradiation, the carbonyl band between 1,680 and 1,850 cm−1 increased, and the peak of the aromatic skeletal vibration originating from lignin (1,510 cm−1) decreased together with the guaiacyl vibrations at 1,275 cm−1 as has been noted in previous studies (Kosikova and Tolvaj 1998; Müller et al. 2003; Horn et al. 1994; Pandey and Theagarajan 1997; Ohkoshi 2002; Sudiyani et al. 2003; Mitsui and Tsuchikawa 2005). Usually two peaks develop in 1,680–1,850 cm−1 region during the exposure of wood to UV radiation. After irradiation for 1 h using a mercury lamp, the two bands are well separated; however, after 6 h, they have already merged to create one peak. The absorption decrease of guaiacyl vibrations at 1,275 cm−1 is well demonstrated after 1 h of irradiation. However, this negative peak is lifted up because of the overall increase in 950–1,300 cm−1 region. More pronounced is the behaviour of the absorption decrease at 1,140 cm−1. This decrease originates from the splitting of ether bonds caused by UV irradiation. This large negative peak is also lifted so high that after 12 h of irradiation, no one can expect that this is a negative peak. It seems like a minimum between two positive peaks. However, it should be a negative peak if the abnormal overall increase has not lifted that part of the spectrum.
As shown in Fig. 3, the intensity of K-M function exceeds the 40 units level between 1,040 and 1,280 cm−1 at 6 h of irradiation. At 12- and 20-h irradiations, this range is between 990 and 1,400 cm−1, and the peak at 1,740 cm−1 also overhangs this limit. The difference spectra (Fig. 4) do not show distortion in the 1,300–1,700 cm−1 interval, where the K-M intensities are below (or close to) 40 units. Here, only the changes caused by the photodegradation are visible. But if the K-M value considerably exceeds 40 units (1,000–1,300 cm−1 interval), normalising, as a mathematical manipulation, is unable to correct the distortion caused by the K-M function (Fig. 4). Comparison of Fig. 3 with Fig. 4 demonstrates that normalising the spectra at an internal wavenumber can only partly reduce the distortion of the K-M function.
The surface roughness can be diminished by pressing the surface with a flat metal plate. While pressure reduces the roughness of the surface, this manipulation hardly changes the chemical bond system. Small alterations in the chemical bond system are not visible in the IR spectrum, but the decrease of roughness reduces the scattering. Thus, values of the K-M function can be diminished by this method, and these reduced values are able to satisfy the limits of the K-M theory. The effect of pressing is visible in Fig. 5. The treated Japanese cypress sample (after irradiation for 36 h with a mercury lamp) was pressed at a pressure of 2.5 × 106 Pa for 10 s with a metal plate in a pressure apparatus, and the sample was measured before and after pressing. It is visible that the pressure reduces the intensity values of the K-M function considerably. Figure 6 presents the difference spectra (created from baseline corrected spectra, normalisation was not applied) of cypress sample after 36 h of irradiation and after pressing this irradiated surface. Figure 6 demonstrates that pressing makes visible the absorption decrease of aromatic ring splitting at 1,510 cm−1 and the decrease of the guaiacyl vibration at 1,275 cm−1, which are not visible on the difference spectrum before pressing. Moreover, the absorption decrease of the OH groups around 3,620 cm−1 will be visible by pressing, as reported in previous works (Tolvaj and Faix 1995; Papp et al. 2005; Barta et al. 2005). Based on Figs. 5 and 6, it can be stated that pressing can reduce the scattering effect of the UV light irradiated surface of wood, but the changes caused by the photodegradation remain visible.
Conclusion
After analysing plenty of DRIFT spectra of wood and examining the shape of the K-M equation (Fig. 1), it can be concluded that the measured K-M function can be used as an absorption spectrum if the values of the function are below 14 K-M units. Above this limit, the K-M theory, which was created for poorly absorbing materials, does not indicate the absorption of wood properly. If a matt aluminium plate is used as a background material and the values are between 14 and 40, the absorption changes can be calculated after normalisation of the spectra. This normalising manipulation is only successful if there is an absorption peak close to the examined one which does not change its absorption during the examined treatment.
References
Baldock JA, Smernik RJ (2002) Chemical composition and bioavailability of thermally altered Pinus resinosa (Red pine) wood. Org Geochem 33:1093–1109
Barta E, Papp G, Preklet E, Tolvaj L, Berkesi O, Nagy T, Szatmári S (2005) Changes of absorption in infrared spectra of softwood materials irradiated by UV-laser as a function of energy. Acta Silvatica et Lignaria Hungarica 1(1):83–91 (http://www.aslh.nyme.hu/)
Berben SA, Rademacher JP, Sell LO, Easy DB (1987) Estimation of lignin in wood pulp by diffuse reflectance fourier-transform infrared spectroscopy. Tappi J 70:129–131
Bouchard J, Douek M (1993) Structural and concentration effects on the diffuse reflectance FTIR spectra of cellulose, lignin and pulp. J Wood Chem Technol 13:481–499
Chang ST, Wu CL, Wang SY, Chang HT (1998) Influence of concentration and particle size on the DRIFT spectroscopy of wood. Taiwan J For Sci 13:1–18
Craciun R, Kamdem PD (1997) XPS and FTIR applied to the study of waterborne copper naphthenate wood preservatives. Holzforschung 51:207–213
Cui W, Kamdem D, Rypstra T (2004) Diffuse reflectance infrared Fourier transform spectroscopy (DRIFT) and color changes of artificial weathered wood. Wood Fiber Sci 36:291–301
Faix O, Böttcher JH (1992) The influence of particle size and concentration in transmission and diffuse reflectance spectroscopy of wood. Holz Roh Werkst 50:221–226
Faix O, Németh K (1988) Monitoring of wood photodegradation by DRIFT-spectroscopy. Holz Roh Werkst 46:112
Ferraz A, Baeza J, Rodriguez J, Freer J (2000) Estimating the chemical composition of biodegraded pine and eucalyptus wood by DRIFT spectroscopy and multivariate analysis. Bioresource Technol 74:201–212
Forsskåhl I, Janson J (1995) Sequential treatment of mechanical and chemimechanical pulps with light and heat. Part 2. FTIR and UV-VIS absorption-scattering spectra. Nord Pulp Pap Res J 7:48–54
Freer J, Ruiz J, Peredo MA, Rodriguez J, Baeza J (2003) Estimating the density and pulping yield of E-globulus wood by DRIFT-MIR spectroscopy and principal components regression (PCR). J Chilean Chem Soc 48:19–22
Hembree DM, Smyrl HR (1989) Anomalous dispersion effects in diffuse reflectance infrared fourier transform spectroscopy: a study of optical geometries. Appl Spectrosc 43:267–274
Holmgren A, Bergström B, Gref R, Ericsson A (1999) Detection of pinosylvins in solid wood of scots pine using fourier transform raman and infrared spectroscopy. J Wood Chem Technol 19:139–150
Hon DNS, Feist WC (1986) Weathering characteristics of hardwood surfaces. Wood Sci Technol 20:169–183
Hon DNS, Ifju G (1978) Measuring penetration of light into wood by detection of photo-induced free radicals. Wood Sci 11:118–127
Horn BA, Qiu J, Owen NL, Feist WC (1994) FT-IR studies of weathering effects in western redcedar and southern pine. Appl Spectrosc 48:662–668
Jones HG, Heitner C (1973) Optical measurement of absorption and scattering properties of wood using the Kubelka-Munk equations. Pulp Pap Mag Can 74:T182–T186
Kataoka Y, Kiguchi M (2001) Depth profiling of photo-induced degradation in wood by FT-IR microspectroscopy. J Wood Sci 47:325–327
Kataoka Y, Kiguchi M, Evans PD (2004) Photodegradation depth profile and penetration of light in Japanese cedar earlywood (Criptomeria japonica D. Don) exposed to artificial solar radiation. Surf Coatings Int Part B-coatings Trans 87(3):187–193
Kataoka Y, Kiguchi M, Fujiwara T, Evans PD (2005) The effects of within-species and between-species variation in wood density on the photodegradation depth profiles of sugi (Cryptomeria japonica) and hinoki (Chamaecyparis obtusa). J Wood Sci 51(5):531–536
Kataoka Y, Kiguchi M, Williams RS, Evans PD (2007) Violet light causes photodegradation of wood beyond the zone affected by ultraviolet radiation. Holzforschung 61(1):23–27
Kishino M, Nakano T (2004) Artificial weathering of tropical woods. Part 1: Changes in wettability. Holzforschung 58:552–557
Kosikova B, Tolvaj L (1998) Structural changes of lignin- polysaccharide complex during photodegradation of Populus grandis. Drev Vysk 43:37–46
Kubelka PJ (1948) New contributions to the optics of intensely light-scattering materials. Part I. J Opt Soc Am 38:448–457
Kubelka PJ, Munk F (1931) Ein Beitrag zur Optik der Farbanstriche. Zeitschrift für Technische Physik 11a:593–601
Michell AJ (1991) An anomalous effect in the DRIFT spectra of woods and papers. J Wood Chem Technol 11:33–40
Michell AJ, Nelson PJ, Chin CWJ (1989) Diffuse reflectance spectroscopic studies of the bleaching and yellowing of eucalyptus regnans cold soda pulp. Appita J 42:443–448
Mitsui K, Tsuchikawa S (2005) Low atmospheric temperature dependence on photodegradation of wood. J Photochem Photobiol B: Biol 81:84–88
Mitsui K, Murata A, Tolvaj L (2003) Investigation of the change in the DRIFT spectra of light-irradiated wood with heat treatment. Holz Roh Werkst 61:82
Moore AK, Owen NL (2001) Infrared spectroscopic studies of solid wood. Appl Spectrosc Rev 36:65–86
Müller U, Rätzsch M, Schwanninger M, Steiner M, Zöbl H (2003) Yellowing and IR-changes of spruce wood as result of UV-irradiation. J Photochem Photobiol B: Biol 69:97–105
Nuopponen MH, Birch GM, Sykes RJ, Lee SJ, Stewart D (2006) Estimation of wood density and chemical composition by means of diffuse reflectance mid-infrared Fourier transform (DRIFT-MIR) spectroscopy. J Agric Food Chem 54:34–40
Ohkoshi MJ (2002) FTIR-PAS study of light-induced changes in the surface of acetylated or polyethylene glycol-impregnated wood. J Wood Sci 48:394–401
Owen NL, Pawlak Z (1989) An infrared study of the effect of liquid ammonia on wood surfaces. J Mol Struct 198:435–449
Owen NL, Thomas DW (1989) Infrared studies of “Hard” and “Soft” woods. Appl Spectrosc 43:451–455
Pandey KK (1999) A study of chemical structure of soft and hardwood and wood polymers by FTIR spectroscopy. J Appl Polym Sci 71:1969–1975
Pandey KK (2005) Study of the effect of photo-irradiation on the surface chemistry of wood. Polym Degr Stab 90:9–20
Pandey KK, Khali DP (1998) Accelerated weathering of wood surfaces modified by chromium trioxide. Holzforschung 52(5):467–471
Pandey KK, Theagarajan KS (1997) Analysis of wood surfaces and ground wood by diffuse reflectance (DRIFT) and photoacustic (PAS) Fourier transform infrared spectroscopic techniques. Holz Roh Werkst 55:383–390
Papp G, Barta E, Tolvaj L, Berkesi O, Nagy T, Szatmári S (2005) Changes in DRIFT spectra of wood irradiated by UV laser as a function of energy. J Photochem Photobiol A: Chem 173(2):137–142
Ristolainen M, Alén R, Malkavaara P, Pere J (2002) Reflectance FTIR microspectroscopy for studying effect of xylan removal on unbleached and bleached birch kraft pulp. Holzforschung 56(5):513–521
Shen J, Zhou JQ, Vazquez O (2000) Experimental study of optical scattering and fiber orientation determination of softwood and hardwood with different surface finishes. Appl Spectrosc 54:1793–1804
Stewart D, Wilson HM, Hendra PJ, Morrison IM (1995) Fourier-transform infrared and raman-spectroscopic study of biochemical and chemical treatments of oak wood (Quercus-Rubra) and barley (Hordeum-Vulgare) straw. J Agric Food Chem 43:2219–2225
Sudiyani Y, Imamura Y, Doi S, Yamauchi S (2003) Infrared spectroscopic investigations of weathering effects on the surface of tropical wood. J Wood Sci 49:86–92
Takei T, Hamajima M, Kamba N (1997) Fourier transform infrared spectroscopic analysis of the degradation of structural lumber in Horyi-ji temple. Mokuzai Gakkaishi 43:285–294
Toivanen TJ, Alen R (2007) A.FTIR/PLS method for determining variations in the chemical composition of birch (Betula pendula/B-publescens) stem wood. Appita J 60:155–160
Tolvaj L, Faix O (1995) Artifical ageing of wood monitored by DRIFT spectroscopy and CIE L*a*b* color measurements. I. Effect of UV light. Holzforschung 49:397–404
Tolvaj L, Mitsui K (2004) Surface preparation and direction dependence of DRIFT spectra of wood. Appl Spectrosc 58:1137–1140
Tolvaj L, Mitsui K (2005) Light source dependence of the photodegradation of wood. J Wood Sci 51:468–473
Umemura K, Yamauchi H, Ito T, Shibata M, Kawai S (2008) Durability of isocyanate resin adhesives for wood V: changes of color and chemical structure in photodegradation. J Wood Sci 54:289–293
Vane CH (2003) Monitoring decay of black gum wood (Nyssa sylvatica) during growth of the shiitake mushroom (Lentinula edodes) using diffuse reflectance infrared spectroscopy. Appl Spectrosc 57:514–517
Weiland JJ, Guyonnet R (2003) Study of chemical modifications and fungi degradation of thermally modified wood using DRIFT spectroscopy. Holz Roh Werkst 61:216–220
Zanuttini M, Citroni M, Martinez MJ (1998) Application of diffuse reflectance infrared Fourier transform spectroscopy to the quantitative determination of acetyl groups in wood. Holzforschung 52:263–267
Zavarin E, Jones SJ, Cool LG (1990) Analysis of solid wood surfaces by diffuse reflectance infrared Fourier transform (DRIFT) spectroscopy. J Wood Chem Technol 10:495–513
Zhang J, Kamdem DP (2000) FTIR characterization of cooper ethanilamine–wood interaction for wood preservation. Holzforschung 54(2):119–122
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Tolvaj, L., Mitsui, K. & Varga, D. Validity limits of Kubelka–Munk theory for DRIFT spectra of photodegraded solid wood. Wood Sci Technol 45, 135–146 (2011). https://doi.org/10.1007/s00226-010-0314-x
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-010-0314-x