Abstract
The diffusion model based on Fick’s second law was applied to the moisture absorption process in wood fiber-based composites. The data of moisture absorption process on wood fiberboard and wood fiber/polymer composites with different densities were analyzed using the diffusion model. Specimens were subjected to different relative humidities and temperatures. The diffusion coefficients (D) and surface emission coefficients (S) along the thickness direction were calculated by the unsteady-state method using a nonlinear curve fitting algorithm. The experimental results showed that the S obtained from Fick’s second law diffusion model did not provide an external resistance when the moisture is absorbed into the wood. While the diffusion model did not provide the best fit of the moisture absorption data, the calculated internal moisture transfer rate, D, still shows physical meanings. The temperature had a significant effect on the D. The higher the temperature, the greater the D obtained. The D showed a decreased trend as the RH (or EMC) increased. No specific trends were found on the D as a function of board density. Other limitations of the diffusion model for the moisture movement in wood are also discussed.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Wood fiber-based composites are hygroscopic and hydrophilic materials. Moisture content in wood and wood composites significantly affects their physical and mechanical properties. Moisture transfer in wood and wood composites influences dimensional stability, the viscoelastic properties and durability. Below the fiber saturation point (FSP), moisture transport through the wood is controlled by diffusion. Bound moisture diffusion is a combination of two movements: the vapor diffusion through the void structure, and the bound water diffusion through the cell wall (Skaar 1958). A diffusion model based on Fick’s second law has been developed to describe this mass transfer process (Skaar 1958).
The unsteady-state moisture diffusion in the direction perpendicular to the surface of wood can be expressed by the following partial differential equation:
where C is the moisture concentration, t is the time, x is the distance from the centerline of a symmetrical specimen in the direction of moisture flow, and D the diffusion coefficient.
Initial and boundary conditions for moisture evaporation at the surface are given by:
and
where C e is the moisture concentration in equilibrium with the water vapor pressure in the surrounding air, C a is the actual moisture concentration in the surface at any time, a is the half-thickness of the specimen, and S is the surface emission coefficient.
A theoretical solution for Eq. 1 is (Crank 1986):
where \( \overline{C} \) is the average value of C across the specimen thickness, β n are the positive roots of
where the transport ratio
and
As we see from the diffusion model, there are two coefficients, diffusion coefficient (D) and surface emission coefficient (S), of which D is for characterizing the internal moisture transfer rate in the materials and S is for characterizing the resistance when the water molecules are moving through the material surface (Skaar 1958). Siau and Avramidis (1996) called S the external resistance in the moisture diffusion process.
Previous publications showed some limitations for the diffusion model based on Fick’s second law. Some concerns are in regard to the driving force assumptions. The diffusion model assumes that the moisture movement is driven by the moisture concentration differences. However, moisture normally exists in wood with three phases: bound water, liquid water, and water vapor (Pang 1996). These three phases are subject to different driving forces. Liquid water may be driven by capillary action (Spolek and Plumb 1981) or water potential (Cloutier and Fortin 1993). Bound water may be driven by chemical potential (Stanish 1986), and water vapor may be driven by vapor pressure difference. Therefore, there must be deviations on the assumption of the specific driving force for the moisture transfer process in wood materials, especially at high relative humidity. Crank (1986) explained the deviation between the model prediction and the measured values from the experiment to be related to the “non-Fickian” or “anomalous” sorption. Wadso (1993) showed that the absorption in wood from 75 to 84% relative humidity was non-Fickian. Therefore, part of the prediction deviation of the model for this experiment may come from the non-Fickian behavior.
Despite the above concerns and limitations, this diffusion model based on Fick’s law showed successful for the moisture de-sorption process in wood, such as wood drying (Skaar 1958; Comstock 1963; Droin-Josserand et al. 1988; Avramidis 1987; Liu 1989; Chen et al. 1995). However, for modeling the moisture absorption process of wood or wood-based products, some researchers raised additional concerns (Nakano 1995; Wadso 1993). The moisture movement into wood consists of two types of diffusion: the pore diffusion and the diffusion into wood substances (Nakano 1995). For the latter, the swelling of the wood substance should be considered, which creates the secondary adsorptive sites. Also, swelling causes the change of the shape of pores. Therefore, the pore diffusion may also be influenced. Because of the stress relaxation that occurs during moisture penetration, wood composites can have greater swelling than wood, from which more prediction errors by the diffusion model would occur.
Based on all the previous discussions, it would be interesting to find out how the prediction of the diffusion model deviates from the moisture absorption process. The objective of this study is to evaluate the accuracy of prediction for Fick’s second law diffusion model for the moisture absorption process for wood fiberboard and wood fiber/polymer composites.
Materials and methods
Compression molded specimens of wood fiberboard and wood fiber-polymer composites were used in this study (Shi 1997). Wood fiberboard samples were manufactured from hardwood fibers (75% aspen, 25% other hardwood). The wood fiber/polymer composite samples were manufactured with the same hardwood fibers and reclaimed automobile polymer particle mixture of 35 meshes (0.5 mm) with a loading of 30% by weight. The polymer mixture mainly consisted of about 27% polyurethane, 17% polypropylene, 12% polyvinyle chloride (PVC), 9% acrylonitrile butadiene styrene (ABS), and 35% other polymers. Polymeric diphenylmethane diisocyanate (pMDI) resin was applied at 4% level to all the boards. Specimens with four target densities, 550, 750, 900, and 1,000 kg/m3, were manufactured. Most specimens were cut to 76.2 × 76.2 × 3.18 mm3 size. Specimens with a target density of 900 kg/m3 were cut to 50.7 × 50.7 × 3.18 mm3 size. These specimens were subjected to four relative humidities, 66, 75, 93, and 100% at room temperature (measured as 17 ± 3°C), and four temperatures at 100% relative humidity. Since this study was to investigate the moisture absorption process, the lowest RH was selected as 66% to ensure that the moisture concentration in the environment was higher than that in the specimens. The relative humidities were controlled by using chemical solvents shown in Table 1. The specimens were equilibrated in a sealed desiccator with distilled water or saturated salt solutions to control the relative humidity. An oven was used to control the temperatures. The edges of the specimens were sealed by aluminum foil bonded with epoxy to limit the moisture transport into the composites in one direction only. To ensure the same moisture content for all the specimens, all the specimens were oven dried at 103 ± 2°C before testing. The oven-dry board densities of all the specimens were measured. Two or three specimens were prepared for the moisture absorption testing at each condition. The average of the weight gain as a function of time for the 2–3 replications was used for the diffusion model fit.
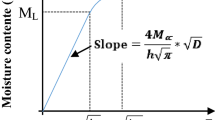
The unsteady-state method was used for all the specimens in this study to determine both D and S by measuring the change of the specimen weight as a function of time until reaching equilibrium weight (Eqs. 4–7). Because the diffusion coefficient in wood based products is generally a function of moisture concentration, the D value calculated from Eq. 4 represents an average value over the moisture concentration range examined. Several methods have been developed to determine D and S (Choong and Skaar 1969, 1972; Liu 1989). The calculation of D and S in these methods was carried out at one point and the calculated values are the average value within a certain range of moisture contents. In this study, a nonlinear curve fitting algorithm method was used to determine D and S. The D and S obtained by the curve fitting method will account for all the data in the whole moisture transfer process, and therefore are more accurate than the previous methods.
To apply the non-linear curve fitting algorithm, the predicted fractional moisture change (F) can be obtained by:
The F value obtained through the experiment (F′) can be calculated by:
The criterion used in the nonlinear curve fitting is the mean of the square of the difference of F value:
where N is number of data points.
A program was written in Matlab® software in which the gradient-based optimum method was used to find the D and S at the minimum prediction error. The bisection method was used to find the positive root β (Cohen et al. 1973).
Results and discussion
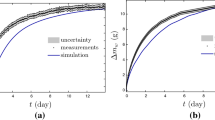
Figures 1 and 2 show the moisture transfer processes at 100% relative humidity and different temperatures for both wood fiberboard and wood fiber/polymer composites. Figures 3 and 4 show the moisture transfer processes for both wood fiberboard and wood fiber/polymer composites at 17°C and different relative humidities. Figures 5 and 6 show the moisture transfer process for wood fiberboard and wood fiber/polymer composites manufactured with different densities. From Figs. 1, 2, 3, 4, 5 and 6 it is seen that systematic errors occur between the measured values and model prediction.
Based on the discussion in the introduction, we know that some limitations for the diffusion model based on Fick’s second law exist for modeling the moisture transfer process in wood and wood-based products. These limitations could contribute to the overall prediction errors shown in Figs. 1, 2, 3, 4, 5 and 6. However, it is interesting to see that the greatest systematic errors for the model prediction happened at the initial portion of the moisture absorption process. The predicted moisture content values by the diffusion model were consistently lower than the measured values from the experiment. Tables 2 and 3 show the equilibrium moisture content (EMC), calculated diffusion coefficient (D), surface emission coefficient (S), transport ratio (F), and error of F value for the wood fiberboard and wood fiber/polymer composites used in the experiments at different conditions. From Tables 2 and 3, the calculated transport ratios are fairly larger (185–1966) for all the specimens. It indicates that the surface emission coefficient in the diffusion model did not have a significant influence on the diffusion process of the specimens. Also, the F error increases as the temperature increases (Table 2). This may be due to greater measurement errors at high temperature. Finally, Table 2 shows that the F error decreases with an increase in RH (or EMC). This may be because the lower the RH, the less the total moisture uptake, and the higher the measurement sensitivity on the relative moisture change.
The surface emission coefficient represents the rate at which moisture is absorbed or lost from the surface of the board. It can be regarded as the external resistance (Siau and Avramidis 1996). In the case of wood drying (moisture de-sorption), because of the hydrogen bonds on the wood surfaces, the water molecules will need to break the bond to escape into the ambient environment, which the surface emission coefficient in the diffusion model describes as external resistance. Because of this reason, usually for wood drying, the initial weight loss of wood specimens is delayed due to the external resistance. For the moisture absorption process, on the other hand, the hydroxyl groups on the wood surface will attract the water molecules from the environment much faster at the initial stage. Therefore, during the moisture uptake measurement, unlike the moisture de-sorption process, there are more weight gains at the initial stage of the moisture absorption process. Therefore, in the case of moisture absorption of wood and wood-based products, the surface emission coefficient in the diffusion model does not appear to play a role of external resistance.
While the surface emission coefficient in the diffusion model does not appear to have influence in the model prediction for the moisture absorption process, the calculated internal moisture transfer rate, diffusion coefficient, may still show the physical meanings. The effects of the temperature, relative humidity, and the density on the diffusion coefficient are analyzed as below.
As expected, the temperature has a significant effect on the internal moisture transfer rate (D) in the composites. The D increases as the temperature increases. Usually, the effect of temperature on the mass transfer should follow the following Arrhenius equation:
where A is a constant, R is gas constant (8.314 J/mol K), E a is the activation energy, and T is the temperature (K).
Arrhenius plot of ln D versus 1/T for both wood fiberboard and wood fiber/polymer composites was developed and is shown in Fig. 7. The higher the temperature, the higher the speed of the movement for the water molecules, and the faster the moisture penetration rate into the board. The activation energies (E a) were calculated as 9.34 kJ/mole for wood fiberboard, and 12.35 kJ/mole for wood fiber/polymer composites.
The effect of moisture concentration in the environment (relative humidity) on the diffusion coefficient is shown in Fig. 8. Since the relative humidity is directly related to the EMC of the specimen, the plots of D versus EMC was used. The internal moisture transfer rate is dependent on the EMC and the moisture concentration potential in the board. The lower the RH, the lower the amount of water vapor in the ambient environment, and the faster the specimens will reach equilibrium. On the other hand, the higher the RH, the higher the gradient of moisture concentration in the specimen, and the higher the moisture transfer rate. Therefore, it is found from Fig. 8 that the correlation between the EMC and D (R 2 is 0.69 for WFB and 0.77 for WFPC) is not very good. However, the overall trend was that D increases as the EMC (or RH) decreases.
Figure 9 shows the relationship between the diffusion coefficient and oven-dry density. Often it is expected that with lower density materials, the moisture transfer rate is faster (Sekino 1994). However, from the results obtained in this study, no specific trend is found for the diffusion coefficient versus density based on the results of this experiment.
Conclusions
This study suggests that the diffusion model based on Fick’s second law with both surface emission coefficient and diffusion coefficient used, may not accurately describe the moisture absorption process for the wood fiber-based composites. Systematic prediction errors for the moisture absorption process showed in the prediction, especially at the initial stage of the moisture uptake process, indicating that, different from the moisture de-sorption process (drying), the surface emission coefficient does not provide the external resistance when the moisture is absorbed into the wood. While the surface emission coefficient does not play a role for modeling the moisture absorption process, the internal moisture transfer rate (diffusion coefficient) still shows physical meanings. The temperature had a significant effect on the internal moisture transfer rate as expected with the higher the temperature, the greater the diffusion coefficient. The diffusion coefficient showed a decreasing trend as the RH (or EMC) increased. No specific trends were found on the diffusion coefficients as a function of board density.
Abbreviations
- a :
-
half-thickness of the specimen
- A:
-
constant in the Arrhenius equation
- ABS:
-
acrylonitrile butadiene styrene
- C :
-
moisture concentration
- C 0 :
-
initial moisture concentration
- C e :
-
moisture concentration in equilibrium
- C a :
-
actual moisture concentration
- \( \overline{C} \) :
-
average value of C
- E a :
-
activation energy
- EMC:
-
equilibrium moisture content
- F :
-
fractional moisture changes
- FSP:
-
fiber saturation point
- L :
-
transport ratio
- N :
-
number of data points
- pMDI:
-
polymeric diphenylmethane diisocyanate
- PVC:
-
polyvinyle chloride
- R :
-
gas constant (8.314 J/mol K)
- RH:
-
relative humidity
- S :
-
surface emission coefficient
- t :
-
time
- Temp:
-
temperature
- WFB:
-
wood fiberboard
- WFPC:
-
wood fiber/polymer composites
- x :
-
distance from the centerline of a symmetrical specimen in the direction of moisture flow
- β n :
-
positive root of β n tan β n = L
References
Avramidis ST, Siau JF (1987) An investigation of the external and internal resistance to moisture diffusion in wood. Wood Sci Technol 21:249–256
Chen Y, Choong ET, Wetzel DM (1995) Evaluation of diffusion coefficient and surface emission coefficient by an optimization technique. Wood Fiber Sci 27(2):178–182
Choong ET, Skaar C (1969) Separation internal and external resistance to moisture removal in wood drying. Wood Sci 1(4):200–202
Choong ET, Skaar C (1972) Diffusivity and surface emissivity in wood drying. Wood Fibre 4(2):80–86
Cloutier A, Fortin Y (1993) A model of moisture movement in wood based on water potential and determination of the effective water conductivity. Wood Sci Technol 27:95–114
Cohen AM, Cutts JF, Fielder R, Jones DE, Ribbans J, Stuart E (1973) Numerical analysis. Wiley, New York
Comstock GL (1963) Moisture diffusion coefficients in wood as calculated from adsorption, desorption, and steady state data. Forest Prod J March 97–103
Crank J (1986) The mathematics of diffusion. 2nd edn. Clarendon Press, Oxford
Droin-Josserand AJ, Taverdet L, Vergnaud JM (1988) Modeling the absorption and desorption of moisture by wood in an atmosphere of constant and programmed relative humidity. Wood Sci Technol 22:299–310
Liu JY (1989) A new method for separating diffusion coefficient and surface emission coefficient. Wood Fiber Sci 21(2):133–141
Nakano T (1995) Note on a formulation of a water adsorption process of wood. Wood Sci Technol 29:231–233
Pang S (1996) Relationship between a diffusion model and a transport model for softwood drying. Wood Fiber Sci 29(1):58–67
Sekino N (1994) Humidity control efficiency of low-density particleboard for interior walls III moisture absorption rates and moisture conductivities. Mokuzai Gakkaishi 40(5):519–526
Shi SQ (1997) Composites processed from wood fibers and automobile polymer fluff. Ph.D. Dissertation. Michigan Technological University
Siau JF, Avramidis S (1996) The surface emission coefficient of wood. Wood Fiber Sci 28(2):178–185
Skaar C (1958) Moisture movement in beech below the fiber saturation point. Forest Prod J 8:352–357
Spolek GA, Plumb OA (1981) Capillary pressure of woods. Wood Sci Technol 15:189–199
Stanish MA (1986) The roles of bound water chemical potential and gas diffusion in moisture transport through wood. Wood Sci Technol 20:53–70
Wadso L (1993) Measurements of water vapor sorption in wood Part 2. Results. Wood Sci Technol 28:59–65
Acknowledgments
The data used in this paper was from Shi’s Ph.D. dissertation at Michigan Tech. Special thanks should be given to Dr. Douglas Gardner and Dr. John Wang for their guidances. The author would also like to thank Dr. Yuliu Zheng for his help on the data analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
This manuscript is approved for publication as Journal Article No. FP 393 of the Forest and Wildlife Research Center, Mississippi State University.
Rights and permissions
About this article
Cite this article
Shi, S.Q. Diffusion model based on Fick’s second law for the moisture absorption process in wood fiber-based composites: is it suitable or not?. Wood Sci Technol 41, 645–658 (2007). https://doi.org/10.1007/s00226-006-0123-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-006-0123-4