Abstract
Crude Manganese Peroxidase (MnP) preparations from the fungus Bjerkandera sp. BOS55 were tested for their abilities to bleach and delignify oxygen delignified Eucalyptus Kraft pulp in a sequence combining a chelating and a peroxide stage. The inclusion of the enzymatic treatment in the bleaching sequence helped considerably to improve the final brightness. Other interesting results included moderate Kappa reduction, slight yield loss and paper sheets with an acceptable breaking length and burst index.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
From all the processes involved in the manufacture of paper, the bleaching stage has one of the highest contributions to environmental pollution (Brunner, Pulliam 1993). Legislative tightening has led to the almost complete abandonment of elemental chlorine and to the adoption of other agents resulting in new bleaching processes, termed 'elemental chlorine free' (ECF) or 'totally chlorine free' (TCF) with lower environmental impacts (Johnston et al. 1996). A great part of the effort put into these environmental actions in the last few years has been directed towards the reduction of chemical reagents in the pulp bleaching.
The application of biotechnology to the pulp and paper industry has been an object of many research studies (Eriksson 1998; Tortter 1990). Years ago, microorganisms began to be used in the treatment of effluents, the fermentation of sulphate liquors, the preparation of starch for paper sizing and the prevention/control of slime buildup on paper machines. Nowadays, research is more focused on improving tree species, pulping, modifying fibres and bleaching. An interesting approach is the use of lignin-degrading fungi, not in a prebleaching stage but as an alternative bleaching process. This alternative has already been proven to reduce by 72% the required bleaching agents for Kraft pulp (Fujita et al. 1991).
The investigation of how these microorganisms degrade a polymer of the structural complexity of lignin has been the object of many research studies. The lignin-degrading capacity of these fungi is now known to be due to extracellular oxidative enzymes that function together with low molecular weight cofactors (Barr and Aust 1994). Secreted by the fungi in response to low levels of key nutrients such as C, N or S, these enzymes are mainly lignin peroxidase (LiP) (Tien and Kirk 1983), manganese peroxidase (MnP) (Glenn and Gold 1985), manganese-independent peroxidase (MIP) (de Jong et al. 1992), laccase (Eggert et al. 1995) and H2O2-generating oxidases (Kuwahara et al. 1984). The role of each of these enzymes is still unclear since the lignin-degrading species differ in the range of ligninolytic enzymes they produce.
MnP activity is detected in active biobleaching cultures of different strains (Moreira et al. 1997), and has been correlated with the biobleaching ability of different white-rot fungi. Kondo et al. (1994) proved the bleaching ability of purified MnP in in vitro systems, provided that Mn2+, Tween 80, malonate and H2O2 were supplied in adequate concentrations. Moreover, the use of MnP avoids operational problems related to the need of a mediator, as in the case of a laccase-based bleaching, with the larger associated benefits related to economical and environmental points of view. However, an application of MnP on a pilot or an industrial scale is still lacking, which would be the first step to involve industrial partners in the uses of this enzyme.
The main aim of this work is to introduce a MnP-based enzymatic stage in a bleaching sequence on a pilot scale and analyse its effect on the bleaching and delignification of oxygen pre-delignified kraft pulp (OKP) of Eucalyptus globulus. The effect on the physical properties of the handsheets made after the bleaching is also considered.
Materials and methods
Microorganism and culture conditions
The enzyme manganese peroxidase (MnP) used in all the experiments was isolated from the white-rot fungus Bjerkandera sp. strain BOS55 (ATCC 90940), which was cultured in malt extract plates at 30º C for 5 days (Mester et al. 1996). After this first period of growth, four or five 6 mm agar plugs were transferred to Fernsbach flasks containing 200 ml of a medium composed of glucose (10 g/l), peptone (5 g/l), CaCl2 (0.1 g/l), salts in traces (100 ml/l) (Tien and Kirk 1988) and thiamine (to reach a final concentration of 2 mg/l). The microorganism was then set to grow in static conditions for 5 days at 30º C.
Erlenmeyer 250 ml flasks each containing 81 ml of culture medium were inoculated with 9 ml of the blended 5-day culture grown fungus. The culture medium was composed of glucose (10 g/l), peptone (2 g/l), sodium acetate (1.64 g/l, pH 4.5), BIII medium (15) (100 ml/l), thiamine to reach a final concentration of 2 mg/l (added once the medium was sterilised), Tween 80 (0.01%), MnSO4 (84.51 mg/l) and glycolic acid (380.25 mg/l). The cultures were incubated in an orbital shaker at 30º C and 150 rpm to favour the formation of pellets. After 4 or 5 days, when the MnP activity was between 200 and 400 U/l, these cultures were used to inoculate the fermenter.
The fermenter, a BIOSTAT(r)E B. Braun-Biotech International with a 10 l capacity, was equipped with pH, Redox, temperature and pO2 sensors, a 6-blade propeller agitator and internal aeration loops. The acquisition of data was computerised. Nine litres of culture medium (previously described) were added to the fermenter, which was then inoculated with 1 l of the agitated cultures obtained in the Erlenmeyer flasks. The temperature was set to 30º C, the agitation rate was set to 150 rpm and the aeration rate was supplied to maintain oxygen saturation. Once the peak production of MnP was detected, decreasing temperature stopped the fermentation and the extracellular liquid was vacuum filtered and ultrafiltrated through a semipermeable membrane, which permitted the separation of the solvent from larger enzyme molecules. Then, the enzyme was concentrated by ultrafiltration. Finally, the enzyme was stabilised by the addition of MnSO4 to reach a final concentration of 500 μM.
Before its use in delignification assays, the enzyme was subjected to dialysis to eliminate all the salts remaining in the extracellular fluid. The cellulose membrane used was from Sigma Chemicals. The dialysis was carried out for 15 h against 10 mM sodium acetate at pH 4.5 and 4º C with agitation.
Measurement of MnP activity
The MnP activity was determined spectrophotometrically (Shimadzu UV-160A) at 30º C and 468 nm, as described by Field et al. (1992). The method was based on the oxidation of 2,6-dimethoxyphenol (2,6-DMP) by the MnP system to form a quinone dimer. The molar extinction coefficient at this wavelength was 49600 M-1cm-1.
The MnP assays were carried out with 200 μl of 250 mM sodium malonate at pH 4.5, 50 μl of 20 mM 2,6-DMP, 50 μl of 20 mM MnSO4, 50 μl of the sample whose MnP activity was to be determined and 550 μl of water. The reaction was started by the addition of 100 μl of 4 mM H2O2, ending up with a volume of 1 ml. One unit of activity was considered as the amount of the enzyme, which oxidised a micromol per minute.
Characterisation of the paper pulp
The Eucalyptus globulus paper pulp used in this work was obtained from a Kraft mill in Spain and was previously subjected to oxygen delignification. The pulp, provided in 60 g/m2 handsheets, was characterised by a Kappa number of 8.3. The starting materials and the products obtained from them were characterised according to the following standard methods (ISO, 1998; TAPPI, 1992;): Kappa number (TAPPI T236), Hemicellulose content (TAPPI T450), Brightness (TAPPI T452), Reversion (ISO 5262), Breaking length and Tensile Index (ISO 1924–1), Burst index (ISO 2758), Tear index (ISO 1974), Porosity (ISO 5636–5) and Density (ISO 534).
Bleaching assays
A bleaching sequence of X-Q-P (MnP-chelating-peroxide stage) was evaluated on oxygen delignified Eucalyptus Kraft pulp. The experimental assays were performed in 4 l stainless steel reactors, fitted with stirrers. The temperature inside was reached by injecting steam into a chamber surrounding the reactor. The assay conditions were as follows: X stage: consistency (10%), time (540 min), room temperature, MnSO4 (100 μM), sodium malonate (50 mM), hydrogen peroxide (0.5 mM every hour), pH (4.5); Q stage: consistency (10%), time (60 min), temperature (85º C), DTPA dosage (0.4% on BD pulp), pH (5.5); P stage: consistency (10%), time (180 min), temperature (98º C), NaOH dosage (1.7% on BD pulp), H2O2 (2.8%), MgSO4 (0.11%), sodium silicate (0.3%).
With the purpose of reliably assessing the extent of delignification attributable to the enzyme, reference samples with no enzyme addition were used as controls. The samples were replicated three times.
Results and discussion
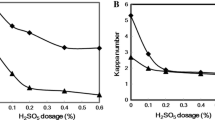
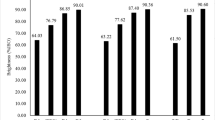
Table 1 shows the evolution of brightness, brightness reversion, viscosity and the final Kappa number at different enzyme dosages in the pre-enzymatic stage incorporated into the bleaching sequence. The final brightness of pulp increased with an increased enzyme dosage, reaching a maximum and then remaining constant. An enzyme dosage of 20 U/g gave very good results and a higher concentration did not improve bleaching efficiency. This fact is very interesting for application purposes, as, obviously, a low charge of enzyme is desirable. The enhancement in pulp brightness (6.1% units ISO higher than in the control assay) was quite relevant after a single enzymatic treatment, considering that at these high brightness values (close to 90%) an appreciable bleaching is often achieved only by using chemical reagents. The increase in brightness was correlated with a reduction in the final Kappa number, which decreased relatively less markedly with the increase in enzyme dosage. The major portion of the Kappa number of the pulp used in this study can be attributed to the presence of hexenuronic acids (Teleman et al. 1996). Hexenuronic acids could only have been removed by acid treatment, but not by treatments conducted here (Vuorinen et al. 1996). This is the reason why the level of the Kappa number remained so stable. Hexenuronic acids originated from the conversion of 4-O-methylglucuronic acid substituents of xylan under alkaline conditions. Other pulp parameters such as viscosity, an indirect measurement of cellulose content, and reversion were comparable to the reference sample and practically unaltered by the enzymatic treatment, which indicated that the quality of the paper is quite unaffected.
Table 2 shows the variation in fibre length, the yield and the hemicellulose content after the sequence with the selected dosage of enzyme (20 U/g). The fibre length was slightly diminished after the enzymatic stage due to the higher proportion in fibre length, smaller than 0.45 mm. The yield and hemicellulose content showed a slight diminution in the case of the pulp treated with MnP. Nevertheless, the very small differences found for all these variables is presumed to be due to the fact of the higher brightness level achieved and this decrease can be considered in the admissible range. Since the enzyme preparations were virtually free of cellulase activity, the decrease in yield was possibly mainly due to the parallel decrease in hemicellulose rather than a decrease in cellulose content. This hypothesis is also supported by the negligible loss in viscosity in comparison with control assays.
After performing the bleaching sequence, the pulps were introduced into a PFI mill to determine conventional papermaking properties, such as the tear index, the tensile index, the porosity, the density, the burst index and freeness. The average results for experiments carried at different enzyme dosage are presented in Figs. 1 , 2 and 3. These parameters showed no significant differences between control and optimum enzymatic assays. In addition, the slight differences between them were favourable in the case of the enzymatic assays, as observed in the tensile index, the burst index, the porosity and the freeness. The evolution of the burst index was similar to the tensile index, due to the fact that both properties are based on the interfibre bonding potential. Concerning the energy consumption, some energy savings during the refining stage could be expected by the decrease in freeness. The tear index was slightly superior in the case of the reference at a PFI beating time of 2.5 min; this tendency was the opposite, at 4.5 min, indicating that there was no significant difference for both samples. Only the density was slightly reduced (less than 1%) in comparison with the reference sample. These results showed that the enzymatic step had no negative effect on the physical properties of the pulp, compared to the reference values.
Conclusions
Although the gain in brightness and the loss of lignin by an enzymatic MnP treatment has been previously reported in the literature, so far only laboratory scale experiments were carried out and no further scaleup was attempted. The inclusion of the enzymatic treatment in a bleaching sequence on a large scale produced promising results with the final brightness (6.1% units ISO higher than in control assay). Moreover, good paper physical properties, such as the tear index, the tensile index, the burst index and freeness may make it possible to transfer this technology from the laboratory to the paper mill. Further research will be focused on the development of bleaching sequences considering the inclusion of sequential enzymatic treatments and chemical stages.
References
Barr DP, Aust SD (1994) Mechanisms white-rot fungi use to degrade pollutants. Environ Sci Technol 28:78A-87A
Brunner FL, Pulliam TL (1993) An analysis of the environmental impact on pulping and bleaching technologies. Tappi J 76 (7):65–74
de Jong E, Field JA and de Bont JAM (1992) Evidence for a new extracellular peroxidase: manganese inhibited peroxidase from the white-rot fungus Bjerkandera sp. BOS55. FEBS Lett 299:107–110
Eggert C, Temp U, Dean JFD and Eriksson KE (1995) A fungal metabolite mediates degradation of non-phenolic lignin structures and synthetic lignin by laccase. FEBS Lett 391:144–148
Eriksson KEL (1998) Past successes and future possibilities for biotechnology in the pulp and paper industry. In: Proceedings of the 7th International Conference on Biotechnology in the Pulp and Paper Industry, Vol. A, pp. 1–4, Vancouver, Canada 16-19 June 1998
Field JA, de Jong E, Feijoo G and de Bont JAM (1992) Biodegradation of polycyclic aromatic hydrocarbons by new isolates of white-rot fungi. Appl Environ Microbiol 58:2219–2226
Fujita K, Kondo R, Sakai K, Kashino Y, Nishida T and Takahara Y (1991) Biobleaching of kraft pulp using white-rot fungus IZU-154. Tappi J 74:123–127
Glenn JK, Gold MH (1985) Purification and properties of an extracellular Mn(II)-dependent peroxidase from the lignin-degrading basidiomycete, Phanerochaete chrysosporium. Arch Biochem Biophys 242:329–341
ISO (1998) Paper, board and pulps. ISO Standard Handbook, 2nd edition, Geneva
Johnston PA, Stringer RL, Santillo D, Stephenson AD, Labounskaia I and McCartney HMA (1996) Towards zero-effluent pulp and paper production: the pivotal role of totally chlorine free bleaching. Greenpeace Technical Report 7/96, November 28
Kondo R, Harazono K and Sakai K (1994) Bleaching of hardwood Kraft pulp with manganese peroxidase secreted from Phanerochaete sordida YK-624. Appl Environ Microbiol 60:4359–4363
Kuwahara M, Glenn JK, Morgan MA and Gold MH (1984) Separation and characterization of two extracellular H2O2-dependent oxidases from ligninolytic cultures of Phanerochaete chrysosporium. FEBS Lett 169:247–250
Mester T, Peña M and Field JA (1996) Nutrient regulation of extracellular peroxidases in the white rot fungus Bjerkandera sp. strain BOS55. Appl Microbiol Biotechnol 44:778–784
Moreira MT, Feijoo G, Sierra-Alvarez R, Lema JM and Field JA (1997) Biobleaching of oxygen delignified kraft pulp by several white rot fungal strains. J Biotechnol 53:237–251
Technical Association of the Pulp and Paper Industry (TAPPI) (1992) TAPPI Press , Atlanta, GA
Teleman A, Hausalo T, Tenkanen M and Vuorinen T (1996) Identification of the acidic degradation products of hexenuronic acid and characterization of hexenuronic acid-substituted xylooligosaccharides by NMR spectroscopy. Carbohydr Res 280:197–208
Tien M, Kirk TK (1983) Lignin-degrading enzyme from the hymenomycete Phanerochaete chrysosporium Burds. Science 221:661–663
Tien M, Kirk TK (1988) Lignin peroxidase of Phanerochaete chrysosporium. Meth Enzymol 161:238–248
Tortter PC (1990) Biotechnology in the pulp and paper industry: a review (Part I) TAPPI J 73:198–204
Vuorinen T, Buchert J, Teleman A, Tenkanen M and Fagerstrom P (1996) Selective hydrolysis of hexenuronic acid groups and its application in ECF and TCF bleaching of kraft pulps. In: Proceedings of the International Pulp Bleaching Conference Vol. 1. Atlanta, GA, 14 April, 1996
Acknowledgements
This work was funded by the Spanish Commission of Science and Technology (CICYT) (1FD97–0854).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
T. Moreira, M., Feijoo, G., Canaval, J. et al. Semipilot-scale bleaching of Kraft pulp with manganese peroxide. Wood Sci Technol 37, 117–123 (2003). https://doi.org/10.1007/s00226-003-0175-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-003-0175-7