Abstract
Circulating sphingosine 1-phosphate (S1P) levels may be a biomarker for osteoporotic fracture (OF). This study assessed whether the addition of S1P levels to the fracture risk assessment tool (FRAX) could improve predictability of OF risk. Plasma S1P concentrations and FRAX variables were measured in 81 subjects with and 341 subjects without OF. S1P levels were higher in subjects with than those without OF (3.11 ± 0.13 μmol/L vs. 2.65 ± 0.61 μmol/L, P = 0.001). Higher S1P levels were associated with a higher likelihood of OF (odds ratio [OR] = 1.33, 95% confidence interval [CI] = 1.05–1.68), even after adjusting for FRAX probabilities. Compared with the lowest S1P tertile, subjects in the middle (OR = 3.37, 95% CI = 1.58–7.22) and highest (OR = 3.65, 95% CI = 1.66–8.03) S1P tertiles had higher rates of OF after adjustment. The addition of S1P levels to FRAX probabilities improved the area under the receiver-operating characteristics curve (AUC) for OF, from 0.708 to 0.769 (P = 0.013), as well as enhancing category-free net reclassification improvement (NRI = 0.504, 95% CI = 0.271–0.737, P < 0.001) and integrated discrimination improvement (IDI = 0.044, 95% CI = 0.022–0.065, P < 0.001). Adding S1P levels to FRAX probabilities especially in 222 subjects with osteopenia having a FRAX probability of 3.66–20.0% markedly improved the AUC for OF from 0.630 to 0.741 (P = 0.012), as well as significantly enhancing category-free NRI (0.571, 95% CI = 0.221–0.922, P = 0.001) and IDI (0.060, 95% CI = 0.023–0.097, P = 0.002). S1P is a consistent and significant risk factor of OF independent of FRAX, especially in subjects with osteopenia and low FRAX probability.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is defined as a metabolic bone disease, characterized by decreased bone strength and predisposing to a high risk of osteoporotic fracture (OF) [1]. OF has major economic and social burden, and is associated with increased morbidity, disability, and mortality rates [2, 3]. Patients with osteoporosis and at high risk of OF should be treated pharmacologically to prevent OF [4,5,6]. The risk of OF has been estimated mainly by combination with bone mineral density (BMD) and/or clinical risk factors (CRFs) for OF [7]. The World Health Organization (WHO) working group has developed fracture risk assessment tools (FRAX) based on BMD and CRFs to enhance the ability to predict OF risk [7]. Current guidelines recommend that FRAX model probabilities should be used to select individuals needed to be treated with an anti-osteoporotic drug, especially individuals with BMD-determined osteoponia (‒ 2.5 < T-score < –1.0) [4, 5]. However, the overall ability of FRAX to predict fracture risk remains suboptimal [8, 9]. Thus, it is necessary to identify additional biomarkers that, when combined with FRAX, can better predict OF risk.
Sphingosine 1-phosphate (S1P) is a natural bioactive lipid molecule with various roles in bone metabolism, resulting in alterations of bone strength. S1P stimulates bone formation by enhancing the proliferation, survival, and migration of osteoblasts [10,11,12]. S1P also stimulates bone resorption, mainly by two mechanisms [13,14,15]. In the first mechanism, S1P stimulates osteoclast differentiation by increasing the secretion by osteoblasts of receptor activator of nuclear factor-κB ligand (RANKL) [15]. In the second mechanism, which depends on S1P concentration gradients between blood and bone, osteoclast precursors migrate from blood with high S1P concentrations to bone with low S1P concentrations, a migration facilitated by S1P receptor 2 (S1PR2)-mediated chemorepulsion [13, 14]. We previously reported that higher plasma S1P levels were associated with higher bone resorption marker such as the serum C-terminal telopeptide of type I collagen (CTX) and/or the urinary N-terminal telopeptide of type I collagen (NTX) and lower BMD, but not with a bone formation marker [16,17,18], suggesting that circulating S1P levels may be predominantly associated with bone resorption in humans.
Few clinical studies to date have assessed the role of S1P as a biomarker for OF. We previously reported that higher plasma S1P concentrations were associated with a higher risk of prevalent vertebral fractures (VFs) in postmenopausal women, with this risk being 9.33-fold higher in the highest than in the lowest S1P quartile [17]. Incident fractures also occurred more frequently in women with higher plasma S1P levels, independent of baseline BMD and CRFs [16]. A prospective longitudinal cohort study of 707 women in western Saudi Arabia studied over a mean 5.2 ± 1.3 years reported that OF incidence was 9.89-fold higher in subjects with higher plasma S1P levels, independent of BMD and CRFs [19]. Collectively, these results suggest that circulating S1P levels may be a new biomarker for OF risk [20]. To date, however, no study has assessed the association of S1P concentrations with OF risk independent of FRAX. We developed a S1P ELISA kit to measure plasma S1P concentrations as an in vitro diagnostic (IVD) medical device for clinical application. The present study investigated whether the addition of plasma S1P concentration, measured by ELISA, to the FRAX model could improve the prediction of OF risk. This study also assessed the clinical settings in which this kit may be particularly helpful.
Methods
Study Participants and Protocol
The study population consisted of consecutive ambulatory men and postmenopausal women who visited the osteoporosis clinic of the Asan Medical Center (AMC; Seoul, Korea) between January 2010 and October 2017. All subjects had visited an osteoporosis clinic due to concerns about possible osteoporosis or were referred to the clinic for osteoporosis that had been detected during a routine examination. Menopause was defined as the absence of menstruation for at least 1 year and was confirmed by measuring serum concentrations of follicle stimulating hormone. Participants were excluded if they had taken medications that could affect bone metabolism within the past year or for more than 6 months (e.g. hormone-replacement therapy, systemic glucocorticoids, or bisphosphonates); if they had preexisting diseases that could affect bone metabolism (e.g. hyperthyroidism, hypothyroidism, hyperparathyroidism, hypoparathyroidism, rheumatoid arthritis, asthma, chronic obstructive pulmonary disease, solid or hematologic malignancy, diabetes, or major cardiovascular diseases); if they had osteophyte formation above Nathan classification grade 4 and/or severe facet joint osteoarthritis in lumbar spine radiographs; if they had a fever (oral temperature ≥ 38.0 °C) or an abnormal number of leukocytes (< 4.0 or > 10.0 × 109/L) or platelets (< 150 or > 350 × 109/L) on complete blood counts; or if they had abnormal liver, kidney, or thyroid function or abnormal serum concentrations of calcium, phosphorus, or alkaline phosphatase. This case–control study included 81 subjects with and 341 without OF.
Patient information was obtained using a self-administered questionnaire, which assessed smoking status (current smoker or not), alcohol use (≥ 3 or < 3 units/day), regular outdoor exercise (≥ 30 or < 30 min/day), history of medication use, previous medical or surgical procedures, and reproductive status (including menstruation). Information was also collected about parental history of fragility hip fractures to exclude all fractures that could be considered non-osteoporotic (i.e. fractures due to cancer or an accident, such as a motor vehicle accident, and all fractures of the fingers, face, skull, and toes). This study was approved by the AMC Ethics Review Committee, and written informed consent was obtained from all study subjects.
FRAX Probabilities
FRAX probabilities of major OF and hip fracture were calculated using Korea-specific models (FRAX v4.1) (www.shef.ac.uk/FRAX). FRAX probabilities from CRFs were calculated from sex, age, body mass index (BMI), prior fragility fracture, parental history of hip fracture, current smoking, long-term use of oral glucocorticoids, rheumatoid arthritis, other causes of secondary osteoporosis, and daily alcohol consumption of ≥ 3 units. FRAX probabilities from CRFs and BMD were calculated by the addition of BMD at the femoral neck to FRAX probabilities from CRFs.
Measurement of BMD and Fracture Assessment
Areal BMD (g/cm2) was measured at the lumbar spine (L1–4; LS-BMD), femoral neck (FN-BMD), and total hip (TH-BMD) using DXA (Lunar system running software version 9.30.044; Prodigy, Madison, WI). The precision of the equipment, as determined by its coefficient of variation (CV) in 17 volunteers who were not enrolled in the study, was calculated using root mean square standard deviation (SD). It was 0.67% for the LS-BMD and 1.25% for the FN-BMD. Each volunteer underwent five scans on the same day, and got off and on the table between scans.
Lateral thoracolumbar radiographs were obtained from all participants to determine the morphological VF. VF was assessed in accordance with the recommendations of the Working Group on Vertebral Fractures [21] and was quantitatively defined as a > 20% reduction in any vertebral height measurement (i.e. anterior, middle, or posterior) [22]. Non-vertebral fractures (NVFs) at major osteoporosis-associated locations (the hip, distal radius, and proximal humerus) were assessed using a self-administered questionnaire. Fractures that were clearly caused by major trauma, such as motor vehicle accidents or falls from higher than standing height, were excluded. Thus, low-trauma fractures after menopause in women or after age 50 years in men were included only if the report was definitive.
Measurement of S1P
Fasting venous blood samples were centrifuged to obtain plasma. All samples showing hemolysis or clotting were discarded. All plasma samples were obtained at the baseline visit and stored at − 80 °C before being assayed to determine the S1P concentration. There was no significant difference in S1P levels from baseline to 1 year (n = 9, P = 0.083), from baseline to 2 or 3 years (n = 6, P = 0.053), and from baseline to 4 or 5 years (n = 3, P = 0.157) in paired t-test with Bonferroni correction. Stable S1P levels in stored human plasma were noted in 11 patients, with serial samples from various storage periods (Supplementary Fig. S1), which is consistent with previous results [19]. Samples were only used if they were collected more than 12 months after an NVF. To evaluate the performance of S1P ELISA kits, S1P concentrations in 84 samples were measured by competitive enzyme-linked immunosorbent assay (ELISA) using kits from Echelon Biosciences Inc. (Salt Lake City, UT) [16,17,18,19] and SEJONG BIOMED CO., LTD (Paju City, Korea) according to the manufacturers’ instructions. There was no significant difference in S1P levels between the two kits (P = 0.706) in paired t-tests. The Bland–Altman plot revealed a mean difference of 0.008 in S1P between the two kits, with an SD of 0.050 (Supplementary Fig. S2) and limits of agreement (LOA) within 2 SDs at − 0.09 and + 0.10. The lower limit of detection of the kits from Echelon Biosciences Inc. was 0.06 μmol/L, with intra- and inter-assay CVs of 6.4% and 6.1%, respectively. In comparison, the lower limit of detection of the kits from SEJONG BIOMED CO., LTD. was 0.008 μmol/L, with intra- and inter-assay CVs of 4.9% and 4.7%, respectively.
S1P concentrations in the 81 subjects with and the 341 subjects without OF were measured using the S1P ELISA kits from SEJONG BIOMED CO., LTD. Each plasma sample was assayed in duplicate, with the results for each plasma sample reported as means.
Statistical Analysis
Data are presented as mean ± SD, median and interquartile range (IQR), or number (percentage), unless otherwise specified. Baseline characteristics of the cases and controls were compared using Student’s t-tests for continuous variables and χ2 tests for categorical variables. The Kolmogorov–Smirnov test showed the normality of the distributions of plasma S1P concentrations (P = 0.167). S1P stability was investigated by comparing its levels in 11 patients from various storage periods (at baseline, 1 year, 2 or 3 years, and 4 or 5 years) using paired t-test with Bonferroni correction. Differences in S1P levels between the two kits were determined using paired t-tests. Bland–Altman Plots with LOA calculations were used to provide insights into systematic biases between the measurement methods [23]. Multiple logistic regression analyses were performed to generate odds ratios (ORs) with a 95% confidence interval (CI) that compared the odds of OF according to S1P levels or S1P tertiles. Receiver-operating characteristics (ROC) analysis was performed, with the areas under the RUC curves (AUCs) compared to evaluate the ability of S1P concentrations, FRAX from CRFs, and FRAX from CRFs and BMD, alone or in combination, to predict the likelihood of OF. The cut-off for plasma S1P concentration predictive of OF was calculated using Youden’s index [24]. A likelihood ratio test was used to compare the goodness-of-fit of the model adding the S1P concentration to the FRAX probability with that of the FRAX probability‐alone model [25]. The net reclassification improvement (NRI) and integrated discrimination improvement (IDI) were estimated to test the additive value of S1P to FRAX probability from CRFs and BMD predictive of OF occurrence [26, 27]. All statistical analyses were performed with SPSS statistical software (SPSS Inc., Chicago, IL) and the open-source programming language R, with P < 0.05 considered statistically significant.
Results
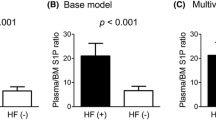
Characteristics of the study subjects are listed in Table 1. Of the 81 subjects with OF, 50 had VFs and 31 had NVFs. Median (range) time from NVF occurrence was 3 years (1–20 years). Subjects with OF were older and had a higher BMI than those without OF. Their mean ages of subjects with and without OF were 62.9 ± 7.1 years and 57.6 ± 6.4 years, respectively. No significant differences in smoking, drinking, history of premature menopause, parental history of hip fracture, and serum calcium and phosphorus concentrations were observed between the two groups. All BMD values were significantly lower in subjects with than those without OF (all, P < 0.001). Rates of osteoporosis and FRAX probabilities were higher in subjects with OF than in controls. The FRAX probabilities calculated from CRFs and BMD in subjects with and without OF were 6.6 ± 3.9% and 4.4 ± 1.5%, respectively, for major OF, and 2.0 ± 2.8% and 0.7 ± 0.6%, respectively, for hip fracture (all, P < 0.001). Plasma S1P concentrations were significantly higher in subjects with than those without OF (3.11 ± 0.13 μmol/L and 2.65 ± 0.61 μmol/L, P = 0.001). S1P concentrations were positively correlated with FRAX probabilities before and after the addition of BMD to CRFs (Supplementary Table S1).
Higher plasma S1P levels were significantly associated with major OF, both before and after adjustment for FRAX probabilities determined from CRFs and/or BMD of the femur neck (ORs = 1.33–1.43; Table 2). To further test this association, the subjects were divided into tertiles based on plasma S1P concentrations. The rates of OF in the lowest (< 2.15 μmol/L), middle (≥ 2.15 μmol/L to < 3.16 μmol/L), and highest (≥ 3.16 μmol/L) S1P tertiles were 10.0%, 22.7%, and 24.8%, respectively (P for trend = 0.003). Compared with the lowest tertile, the ORs for OF in the middle and highest tertiles were 3.35 (95% CI = 1.65–6.81) and 4.55 (95% CI = 2.17–9.54), respectively. The significant differences persisted even after adjustment for FRAX probabilities (Table 2). Separate analyses of VF and NVF (Supplementary Table S2) showed that, relative to the lowest tertile, the ORs for VF in the middle and highest tertiles were 3.58 (95% CI = 1.51–8.49) and 3.94 (95% CI = 1.59–9.77), respectively. Similarly, relative to the lowest tertile, the ORs for NVF in the middle and highest tertiles were 3.13 (95% CI = 1.02–9.56) and 6.20 (95% CI = 1.92–20.01), respectively. These higher ORs for both NVF and VF persisted even after adjustment for FRAX probabilities.
The discriminatory ability of plasma S1P concentration was assessed by ROC analysis (Table 3). Calculations using Youden’s index showed that the optimal cut-off value of plasma S1P concentration for OF was 2.19 μmol/L [24], with a sensitivity of 72.8% and a specificity of 53.4%. The AUC of S1P > 2.19 μmol/L alone was comparable to that of the FRAX probability from CRFs and BMD at the femur neck (0.673 vs. 0.708, P = 0.451). The addition of plasma S1P concentration to the FRAX probability significantly improved the AUC from 0.708 to 0.769 (P = 0.013). Likelihood ratio tests also indicated that the addition of plasma S1P concentration > 2.19 μmol/L to FRAX probability was a significantly better fit than FRAX probability alone (P < 0.001). Category-free NRI (0.504, 95% CI = 0.271–0.737, P < 0.001) and IDI (0.044, 95% CI = 0.022–0.065, P < 0.001) analyses also confirmed that the addition of plasma S1P concentration substantially improved the discriminatory power of the FRAX probability for OF (Supplementary Table S3).
To clarify the clinical association of plasma S1P concentration with OF, the subjects were divided into tertiles according to FRAX probability. The FRAX probabilities for major OF calculated from CRFs and BMD for the lowest, middle, and highest tertiles were 1.82–3.65%, 3.66–4.91%, and 4.92–25.28%, respectively, and the rates of OF were 7.9%, 17.7%, and 31.9%, respectively (P for trend < 0.001). Figure 1 showed the OF prevalence in each group assorted by tertiles of S1P and FRAX probability. Assessment of subjects in the lowest FRAX probability tertile showed no association between S1P tertile and OF (P = 0.962). By contrast, assessment of women in the highest S1P tertile showed that the OF rate was significantly higher in the middle FRAX tertile (P for trend = 0.008) and tended to be higher in the highest FRAX tertile (P for trend = 0.068) relative to the lowest FRAX tertile. These findings suggest that plasma S1P concentration may be useful in predicting OF, especially in women with a FRAX probability ≥ 3.66%.
OF prevalence based on tertiles of plasma S1P concentrations and tertiles of FRAX probability in all study participants (N = 422). FRAX probability was defined as the 10 year probability of major OF based on calculations using CRFs and BMD at the femur neck. CRFs were sex, age, body mass index, current smoking, alcohol intake (≥ 3 U/day), other causes of secondary osteoporosis, and parental history of hip fracture. BMD Bone mineral density, CRFs clinical risk factors, FRAX fracture risk assessment tool, OF osteoporotic fracture, S1P sphingosine 1-phosphate
Many clinical guidelines indicate that FRAX probability for major OF ≥ 20% is the cut-off for treatment with anti-osteoporotic drugs of subjects with osteopenia, as determined by BMD criteria (− 2.5 < T-score < –1.0) [4, 5]. However, OF can occur in osteopenic subjects with FRAX probability for major OF < 20%, resulting in under-treatment [28, 29]. All 39 OFs in subjects with osteopenia by BMD criteria had a FRAX probability for major OF < 20%. Based on current clinical guidelines [4, 5] as well as our findings, we determined the discriminatory ability of plasma S1P concentration for OF in osteopenic women with a FRAX probability for major OF of 3.66–20.0%. The addition of plasma S1P concentration to the FRAX probability significantly improved the AUC from 0.630 to 0.741 (P = 0.012; Table 4). Likelihood ratio tests also indicated that the model combining plasma S1P concentration > 2.19 μmol/L and FRAX probability was a significantly better fit than the model using FRAX probability alone (P < 0.001). Category-free NRI (0.571, 95% CI = 0.221–0.922, P = 0.001) and IDI (0.060, 95% CI = 0.023–0.097, P = 0.002) analyses also confirmed that the addition of plasma S1P concentration significantly improved the discriminatory power of the FRAX probability for OF (Supplementary Table S4).
Discussion
To our knowledge, this study is the first to investigate the relationship between circulating S1P levels and OF, independent of FRAX probability, in human subjects. Plasma S1P concentration, as measured using a new S1P ELISA kit, was associated with the OF risk, alone and combined with FRAX probabilities from BMD and CRFs. Moreover, higher plasma S1P concentration was significantly associated with OF risk independent of FRAX probability. The addition of plasma S1P levels to FRAX significantly improved the discriminatory performance of the latter for OF, as shown by a 6.2% increase in AUC and by NRI and IDI. The optimal plasma S1P cut-off concentration was 2.19 μmol/L. The determination of plasma S1P concentration was especially useful in osteopenic individuals with FRAX probabilities of 3.66–20.0% for major OF, with an 11.1% increase in AUC. These findings indicate that the S1P ELISA kit are clinically applicable to the selection of individuals at high risk of OF and can reduce the rate of under-treatment of patients against osteoporosis.
The association between circulating S1P levels and OF risk independent of FRAX probability remains unclear. OF risk is determined by both skeletal and non-skeletal factors [5]. Skeletal factors include bone mass and quality, and non-skeletal factors include fall risk. Of FRAX components, BMD is a reliable measure of bone mass, whereas CRFs for fracture are incomplete and indirectly associated with bone quality and non-skeletal factors [30]. Thus, in addition to CRFs, circulating S1P levels may further reflect bone quality and non-skeletal factors. For example, S1P was found to directly and indirectly stimulate bone resorption [13,14,15], leading to higher bone loss. Therefore, higher S1P levels can not only result in the deterioration of bone mass but also the deterioration of bone quality and microarchitecture, which can contribute to higher OF risk. Higher circulating S1P levels may also be associated with higher fall risk. S1P affects the cellular biology of many tissues [31, 32], which can influence systemic fragility and lead to higher fall risk. For example, S1P has been implicated in systemic inflammation [33], which has been associated with higher fall risk [34]. Additional studies of the relationships of circulating S1P levels with bone quality (for example, trabecular bone score) and fall incidence are needed to clarify which components of fracture risk are influenced by S1P levels.
Most importantly, this study identified a subgroup of subjects in which circulating S1P levels should be measured, i.e. osteopenic subjects with a FRAX probability for major OF of 3.66–20.0%. To date, there has been no specific intervention threshold based on FRAX to guide treatment for preventing fractures in Korea. Although age-specific intervention thresholds based on FRAX probability are employed in the UK [35] and in some European countries [36, 37], FRAX probability for major OF of ≥ 20%, which is recommend by many clinical guidelines [4, 5] including the National Osteoporosis Foundation, has been proposed in Korea. In the present study, however, 39 (11.7%) of the 334 osteopenic subjects with a FRAX probability < 20% experienced an OF, raising an under-treatment issue, in agreement with the previous reports [28, 29]. It means that the current guidelines based on FRAX probability identify a large portion of osteopenic subjects who will experience OF as being at low risk for OF (false-negatives). The present study demonstrated that measuring circulating S1P levels might be useful to enhance OF risk prediction in osteopenic subjects with a FRAX probability for major OF of 3.66–20.0%. The addition of S1P measurements enhanced AUC in all study women by 6.1% (from 0.708 to 0.769) and in osteopenic women by 11.1% (from 0.630 to 0.741). These findings indicate that the determination of OF risk based on circulating S1P levels in addition to FRAX probabilities can minimize under-treatment of osteoporosis.
This study did not evaluate whether the determination of circulating S1P levels could reduce over-treatment of patients identified by the current FRAX model. FRAX probability in the study population was rather low, even in the group with fractures; thus, we could not evaluate the usefulness of S1P in the subjects with higher FRAX probability. In the present study, subjects with secondary osteoporosis caused by any disease or by drugs including steroids, as well as subjects with any abnormal laboratory findings, were excluded, resulting in the limited inclusion of the subjects with high FRAX probability. Because of a small number of subjects with high FRAX probability, the subgroup analysis about an over-treatment issue was impossible.
An IVD medical device to measure S1P levels is needed for diagnosis. S1P levels measured by our ELISA kit for an IVD medical device were highly correlated with S1P levels measured by an ELISA kit for research use alone. In addition, consistent with the previous results [16, 17, 19], plasma S1P concentrations measured with this kit were associated with OF risk independent of BMD and CRFs. The combination of plasma S1P concentrations with FRAX showed greater association with OF than FRAX alone by multiple analytic methods, such as AUC, NRI, and IDI. Because treatment decisions are based on the prediction of absolute OF risk, the present findings indicate that our ELISA kit measuring plasma S1P concentration may be useful in osteoporosis management.
This study had several potential limitations. First, this was a cross-sectional case–control study, not a longitudinal study. Therefore, we could not assess whether plasma S1P concentration was predictive of future OF occurrence. However, the similar results of our case–control study [16] and another prospective study [19] suggest the likelihood that plasma S1P concentration can predict future OF occurrence. Second, the study population was limited to subjects without well-known risk factors for OF, including steroid use, rheumatoid arthritis, and secondary osteoporosis. Thus, additional studies are needed to extend our findings to other patient populations. Third, the study population including cases with fractures was small size and was not representative of the general population of Korea. Thus, there is a need to confirm our results in further studies with larger cohorts. Fourth, we did not measure the levels of 25-hydroxyvitamin D and bone resorption markers, and the case–control groups were not matched for age. Therefore, we cannot exclude the possibility that these unmeasured factors and the undetermined age-associated factors may have influenced the observed results, although the FRAX parameters, including age, were controlled in several analyses and strict exclusion criteria were adopted to minimize this possibility. In addition, previous studies have shown that positive association of S1P with OF was independent of the levels of 25-hydroxyvitamin D [19] and those of CTX and/or NTX [16, 17]. Furthermore, a previous study noted that S1P levels better predicted OF risk than NTX and CTX levels [19]. These suggested that the addition of SIP to FRAX can be valuable in clinical settings.
In conclusion, S1P was shown to be a consistent and significant risk factor of OF independent of FRAX, suggesting that the addition of circulating S1P levels to the FRAX model can improve the prediction of OF. The measurement of circulating S1P levels was especially helpful in assessing OF risk in osteopenic subjects with low FRAX probability.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795. https://doi.org/10.1001/jama.285.6.785
Lee YK, Yoon BH, Koo KH (2013) Epidemiology of osteoporosis and osteoporotic fractures in South Korea. Endocrinol Metab (Seoul) 28:90–93. https://doi.org/10.3803/EnM.2013.28.2.90
Sattui SE, Saag KG (2014) Fracture mortality: associations with epidemiology and osteoporosis treatment. Nat Rev Endocrinol 10:592–602. https://doi.org/10.1038/nrendo.2014.125
Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, Kleerekoper M et al (2016) American Association of Clinical Endocrinologists and American College of Endocrinology Clinical Practice Guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016–executive summary. Endocr Pract 22:1111–1118. https://doi.org/10.4158/EP161435.ESGL
National Osteoporosis Foundation (2014) Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation, Washington, DC
Orimo H, Nakamura T, Hosoi T, Iki M, Uenishi K, Endo N, Ohta H et al (2012) Japanese 2011 guidelines for prevention and treatment of osteoporosis—executive summary. Arch Osteoporos 7:3–20. https://doi.org/10.1007/s11657-012-0109-9
Kanis JA, Harvey NC, Johansson H, Oden A, McCloskey EV, Leslie WD (2017) Overview of fracture prediction tools. J Clin Densitom 20:444–450. https://doi.org/10.1016/j.jocd.2017.06.013
Bolland MJ, Siu AT, Mason BH, Horne AM, Ames RW, Grey AB, Gamble GD et al (2011) Evaluation of the FRAX and Garvan fracture risk calculators in older women. J Bone Miner Res 26:420–427. https://doi.org/10.1002/jbmr.215
Hillier TA, Cauley JA, Rizzo JH, Pedula KL, Ensrud KE, Bauer DC, Lui LY et al (2011) WHO absolute fracture risk models (FRAX): do clinical risk factors improve fracture prediction in older women without osteoporosis? J Bone Miner Res 26:1774–1782. https://doi.org/10.1002/jbmr.372
Grey A, Chen Q, Callon K, Xu X, Reid IR, Cornish J (2002) The phospholipids sphingosine-1-phosphate and lysophosphatidic acid prevent apoptosis in osteoblastic cells via a signaling pathway involving G(i) proteins and phosphatidylinositol-3 kinase. Endocrinology 143:4755–4763. https://doi.org/10.1210/en.2002-220347
Grey A, Xu X, Hill B, Watson M, Callon K, Reid IR, Cornish J (2004) Osteoblastic cells express phospholipid receptors and phosphatases and proliferate in response to sphingosine-1-phosphate. Calcif Tissue Int 74:542–550. https://doi.org/10.1007/s00223-003-0155-9
Roelofsen T, Akkers R, Beumer W, Apotheker M, Steeghs I, van de Ven J, Gelderblom C et al (2008) Sphingosine-1-phosphate acts as a developmental stage specific inhibitor of platelet-derived growth factor-induced chemotaxis of osteoblasts. J Cell Biochem 105:1128–1138. https://doi.org/10.1002/jcb.21915
Ishii M, Egen JG, Klauschen F, Meier-Schellersheim M, Saeki Y, Vacher J, Proia RL et al (2009) Sphingosine-1-phosphate mobilizes osteoclast precursors and regulates bone homeostasis. Nature 458:524–528. https://doi.org/10.1038/nature07713
Ishii M, Kikuta J, Shimazu Y, Meier-Schellersheim M, Germain RN (2010) Chemorepulsion by blood S1P regulates osteoclast precursor mobilization and bone remodeling in vivo. J Exp Med 207:2793–2798. https://doi.org/10.1084/jem.20101474
Ryu J, Kim HJ, Chang EJ, Huang H, Banno Y, Kim HH (2006) Sphingosine 1-phosphate as a regulator of osteoclast differentiation and osteoclast-osteoblast coupling. EMBO J 25:5840–5851. https://doi.org/10.1038/sj.emboj.7601430
Bae SJ, Lee SH, Ahn SH, Kim HM, Kim BJ, Koh JM (2016) The circulating sphingosine-1-phosphate level predicts incident fracture in postmenopausal women: a 3.5-year follow-up observation study. Osteoporos Int 27:2533–2541. https://doi.org/10.1007/s00198-016-3565-z
Kim BJ, Koh JM, Lee SY, Lee YS, Lee SH, Lim KH, Cho EH et al (2012) Plasma sphingosine 1-phosphate levels and the risk of vertebral fracture in postmenopausal women. J Clin Endocrinol Metab 97:3807–3814. https://doi.org/10.1210/jc.2012-2346
Lee SH, Lee SY, Lee YS, Kim BJ, Lim KH, Cho EH, Kim SW et al (2012) Higher circulating sphingosine 1-phosphate levels are associated with lower bone mineral density and higher bone resorption marker in humans. J Clin Endocrinol Metab 97:E1421–1428. https://doi.org/10.1210/jc.2012-1044
Ardawi MM, Rouzi AA, Al-Senani NS, Qari MH, Elsamanoudy AZ, Mousa SA (2018) High plasma sphingosine 1-phosphate levels predict osteoporotic fractures in postmenopausal women: the center of excellence for osteoporosis research study. J Bone Metab 25:87–98. https://doi.org/10.11005/jbm.2018.25.2.87
Garnero P (2014) New developments in biological markers of bone metabolism in osteoporosis. Bone 66:46–55. https://doi.org/10.1016/j.bone.2014.05.016
Kiel D (1995) Assessing vertebral fractures. National osteoporosis foundation working group on vertebral fractures. J Bone Miner Res 10:518–523. https://doi.org/10.1002/jbmr.5650100403
Genant HK, Wu CY, van Kuijk C, Nevitt MC (1993) Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res 8:1137–1148. https://doi.org/10.1002/jbmr.5650080915
Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1:307–310
Perkins NJ, Schisterman EF (2005) The Youden Index and the optimal cut-point corrected for measurement error. Biom J 47:428–441. https://doi.org/10.1002/bimj.200410133
Lewis F, Butler A, Gilbert L (2011) A unified approach to model selection using the likelihood ratio test. Methods Ecol Evol 2:155–162. https://doi.org/10.1111/j.2041-210X.2010.00063.x
Pencina MJ, D'Agostino RB Sr, D'Agostino RB Jr, Vasan RS (2008) Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med 27:157–172. https://doi.org/10.1002/sim.2929(Discussion 207–212)
Pencina MJ, D'Agostino RB Sr, Steyerberg EW (2011) Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med 30:11–21. https://doi.org/10.1002/sim.4085
Cheung E, Cheung CL, Kung AW, Tan KC (2014) Possible FRAX-based intervention thresholds for a cohort of Chinese postmenopausal women. Osteoporos Int 25:1017–1023. https://doi.org/10.1007/s00198-013-2553-9
Su Y, Leung J, Hans D, Lamy O, Kwok T (2017) The added value of trabecular bone score to FRAX® to predict major osteoporotic fractures for clinical use in Chinese older people: the Mr. OS and Ms. OS cohort study in Hong Kong. Osteoporos Int 28:111–117. https://doi.org/10.1007/s00198-016-3741-1
Kanis JA, Hans D, Cooper C, Baim S, Bilezikian JP, Binkley N, Cauley JA et al (2011) Interpretation and use of FRAX in clinical practice. Osteoporos Int 22:2395–2411. https://doi.org/10.1007/s00198-011-1713-z
Maceyka M, Harikumar KB, Milstien S, Spiegel S (2012) Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22:50–60. https://doi.org/10.1016/j.tcb.2011.09.003
Rosen H, Goetzl EJ (2005) Sphingosine 1-phosphate and its receptors: an autocrine and paracrine network. Nat Rev Immunol 5:560–570. https://doi.org/10.1038/nri1650
Kunkel GT, Maceyka M, Milstien S, Spiegel S (2013) Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov 12:688–702. https://doi.org/10.1038/nrd4099
Soysal P, Stubbs B, Lucato P, Luchini C, Solmi M, Peluso R, Sergi G et al (2016) Inflammation and frailty in the elderly: a systematic review and meta-analysis. Ageing Res Rev 31:1–8. https://doi.org/10.1016/j.arr.2016.08.006
Kanis JA, McCloskey EV, Johansson H, Strom O, Borgstrom F, Oden A, National Osteoporosis Guideline Group (2008) Case finding for the management of osteoporosis with FRAX—assessment and intervention thresholds for the UK. Osteoporos Int 19:1395–1408. https://doi.org/10.1007/s00198-008-0712-1
Briot K, Cortet B, Thomas T, Audran M, Blain H, Breuil V, Chapuis L et al (2012) 2012 update of French guidelines for the pharmacological treatment of postmenopausal osteoporosis. Joint Bone Spine 79:304–313. https://doi.org/10.1016/j.jbspin.2012.02.014
Kanis JA, McCloskey EV, Johansson H, Cooper C, Rizzoli R, Reginster JY, Scientific Advisory Board of the European Society for Clinical, and Economic Aspects of Osteoporosis, and Osteoarthritis (ESCEO), and the Committee of Scientific Advisors of the International Osteoporosis Foundation (IOF) (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24:23–57. https://doi.org/10.1007/s00198-012-2074-y
Funding
This study was supported by grants from the Asan Institute for Life Sciences, Seoul, Republic of Korea (Project No. 2019IP0862) and from the Korea Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (Project No. HI15C2792). The funding organization had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
SHL and J-MK contributed to the conception and design of study. Material preparation and data collection were performed by SHL, JYL, K-HL, Y-SL, S-HK, SC, S-HC, and J-MK. Analysis and interpretation of data were performed by SHL and J-MK. The first draft of the manuscript was written by SHL and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of Interest
Seong-Hee Kim, Sooyoung Choi, and Seong-Hwan Cho have patents registered in Korea (KR 10-1486368) and patent applications in the USA (US 15/927,459) for sphingosine-1-phosphate. They provided the S1P ELISA kits, but were not involved in the design and conduct of the study (i.e. the management, analysis, and interpretation of the data). Seung Hun Lee, Jee Yang Lee, Kyeong-Hye Lim, Young-Sun Lee, and Jung-Min Koh state that they have no conflicts of interest.
Ethics Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was approved by the Asan Medical Center Ethics Review Committee (2018–0556).
Informed Consent
Written informed consent was obtained from all study subjects.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lee, S.H., Lee, J.Y., Lim, KH. et al. High Circulating Sphingosine 1-Phosphate is a Risk Factor for Osteoporotic Fracture Independent of Fracture Risk Assessment Tool. Calcif Tissue Int 107, 362–370 (2020). https://doi.org/10.1007/s00223-020-00731-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00731-1