Abstract
Various studies have investigated the serum sclerostin and bone morphogenetic protein-2 (BMP-2) levels in patients with ankylosing spondylitis (AS), but the results were inconsistent. The aim of this meta-analysis was to synthetically assess the associations of serum levels of sclerostin and BMP-2 with AS. Multiple electronic databases were searched to locate relevant articles published before November 2018. Pooled standard mean difference (SMD) with 95% confidence interval (CI) was calculated by the random-effect model. Totally, 21 studies were included. Meta-analysis results showed no significant difference between AS group and control group in serum sclerostin levels (SMD = 0.098, 95% CI − 0.395 to 0.591, p = 0.697). Nevertheless, serum BMP-2 levels in AS patients were higher than that in controls (SMD = 1.184, 95% CI 0.209 to 2.159, p = 0.017). Subgroup analysis demonstrated that European and South American AS patients had lower serum levels of sclerostin than controls. AS patients with age ≥ 40 years, erythrocyte sedimentation rate (ESR) ≤ 20 mm/h and Bath Ankylosing Spondylitis Functional Index (BASFI) < 4 had statistically significant lower serum sclerostin concentrations compared to controls. Chinese and Korean AS patients as well as patients with lower CRP had higher serum BMP-2 levels than controls, and country may be a source of heterogeneity across the studies. No publication bias existed and sensitivity analysis confirmed the stability of results. Serum BMP-2, but not sclerostin levels may be closely related to the development of AS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ankylosing spondylitis (AS) is a chronic inflammatory autoimmune disease which predominantly involves the spinal column and sacroiliac joint [1]. AS is characterized by chronic inflammation and new bone growth which is usually associated with syndesmophyte formation and joint ankylosis, leading to restriction of mobility [2]. The existing relevant researches have made great progress, nevertheless, the exact pathogenesis of AS is not clear. Since new bone formation is confirmed to be the main cause of spinal deformity and function loss in AS, there has been substantial interest in investigating the role of biomarkers that are implicated in osteoblastogenesis, such as sclerostin and bone morphogenetic proteins (BMPs), in AS development [3, 4].
Sclerostin is primarily expressed and secreted by osteocytes and some terminally differentiated cells embedded within mineralized matrix such as cementocytes, chondrocytes, and osteocytes [5]. Sclerostin is regarded as a natural inhibitor of the Wnt/β-catenin signaling pathway, which plays a significant part in bone formation by regulating the development and differentiation of osteoblasts and osteoclasts [6]. Substantially, the activated Wnt proteins bind to the frizzled receptor and the low-density lipoprotein receptor-related protein 5 or 6 (LRP5/6), which makes part of β-catenin enter the cell nucleus and combine with the nuclear transcription factors, thus activating the transcription of downstream osteoblast-related genes and increasing bone formation [7]. Sclerostin is capable of competitively binding to LRP5/6, which restricts the Wnt proteins to reach LRP5/6, leading to the blocking of Wnt signaling pathway and consequently reducing the bone formation [6].
Bone morphogenetic proteins (BMPs), are crucial members of the transforming growth factor superfamily, which can induce embryonic development, cell lineage determination and osteoblastic differentiation [8]. Recombinant human BMPs have been demonstrated to be effective in enhancing bone healing and promoting spinal fusion [9]. BMP-2 is especially an important and active factor in the BMP family. BMP-2 is capable of independently inducing the expression of markers related to osteoblast and chondroblast differentiation by activating Smad signaling and regulating the transcription of osteogenic genes, thus promoting the formation of bone and cartilage [10, 11]. Furthermore, BMP-2 and Wnt/β-catenin signaling pathways have dependent and/or synergistic effects on regulating osteoblast differentiation and bone formation [12, 13]. BMP signaling regulates gene expression of the Wnt pathway, likewise, Wnt/β-catenin signaling can activate BMP-2 expression in osteoblasts [14].
Recently, the association of serum sclerostin or BMP-2 levels with AS have been investigated by plenty of studies, however, the results were inconsistent [4, 15,16,17]. Therefore, we conducted this meta-analysis to obtain the more comprehensive and accurate results quantificationally.
Materials and Methods
Publication Search
The current meta-analysis was carried out based on a standard guideline [18]. To obtain relevant publications, several electronic databases including PubMed, Medline, Embase, Cochrane Library, Web of Science, China National Knowledge Infrastructure (CNKI), WANFANG (Chinese Database) and VIP (Chinese Database) were retrieved until November 2018. Search keywords and strategy were as follows: (“sclerostin” or “SOST” or “bone morphogenetic protein-2” or “bone morphogenetic protein2” or “BMP-2” or “BMP2”) and (“ankylosing spondylitis” or “Bechterew’s disease” or “AS”). The corresponding Chinese terms were adopted in Chinese databases. Furthermore, we manually reviewed the references cited in the related articles.
Inclusion Criteria and Exclusion Criteria
Included study must meet the following criteria: (1) used a case–control, cohort or cross-sectional design; (2) provided detailed data regarding serum concentrations of sclerostin or BMP-2 in AS patients and healthy controls; (3) published in English or Chinese. If there were duplicate studies in different publications, only one was selected according to the sample size and publication date.
Excluded points were (1) case reports, letters, editorials, meeting abstracts, reviews or other non-original articles, (2) studies without available or extractable requisite information, (3) animal or in vitro studies.
Literature Quality
The Newcastle-Ottawa Quality Assessment Scale (NOS) was used by two researchers (JY and SX) to independently assess and score the selected studies. NOS consists of eight questions with nine items which evaluate participants' selection, group comparability and ascertainment for the exposure. The NOS score ranges from 0 to 9, and a higher score means better quality in methodology.
Data Extraction
The following characteristics were collected from each eligible articles: name of first author, publication year, country, sample size, mean and standard deviation (SD) of serum sclerostin and BMP-2 levels in AS group and control group. When significant information was missing in the original articles, we tried to send an email to the corresponding authors for available data.
Statistical Analysis
Because of the discrepant units of serum sclerostin concentrations, the standardized mean difference (SMD) with its confidence interval (CI) was calculated for every study, and described by a forest plot. Mean values and standard deviations (SDs) were provided in most studies, but in a minority of articles, only the median values with maximum and minimum values or the median values with 25th and 75th percentiles were presented. In such case, we transformed initial data to the estimated mean and SD through the latest and accurate methods [19, 20] (http://www.comp.hkbu.edu.hk/~xwan/median2mean.html). Cochrane Q test (Chi square test, χ2) and I2 test (I2 = [(Q − df)/Q] × 100%) were used to evaluate the statistical heterogeneity amongst the incorporated studies. The random-effect model was used if there was significantly statistical heterogeneity (p < 0.10 for the Q test or I2 > 50%), otherwise the fixed-effect model was selected. To ascertain the source and effect of heterogeneity, we conducted subgroup analyses and meta-regression analyses. Begg’s and Egger’s tests were performed to detect the potential publication bias within studies. Sensitivity analysis was adopted to assess the reliability and robustness of the overall result if there was a high heterogeneity. All statistical analyses were carried out using Stata 14.0 (StataCorp, College Station, TX, USA) software. Statistical significance was set at a two-sided p < 0.05.
Results
Publication SEARCH and Study Characteristics
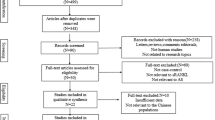
Initially, 327 publications were retrieved, of which 22 articles (included 17 articles for sclerostin [3, 15, 16, 21,22,23,24,25,26,27,28,29,30,31,32,33,34], four articles for BMP-2 [4, 17, 35, 36], and one article covers both sclerostin and BMP-2 [37]) met the inclusion criteria and were incorporated in the present meta-analysis (Fig. 1). Totally, 18 studies including 1186 AS patients and 719 controls researched the serum sclerostin levels, and five studies investigated the serum levels of BMP-2 in 300 AS patients and 155 controls. All the studies were published from 2008 to 2018. Patients were diagnosed with AS according to the modification of the New York criteria (19 studies) and Assessment of SpondyloArthritis International Society (ASAS) criteria (one study), and one study included AS patients based on their clinical and imaging characteristics. Serum levels of sclerostin and BMP-2 were measured by enzyme-linked immunosorbent assay (ELISA) in most studies except one with an enzyme immunoassay (EIA). The NOS scores of all studies ranged from 6 to 8, meaning the satisfactory quality in methodology. Table 1 details the general characteristics of all included studies.
Results of Meta-Analysis
Test of Heterogeneity
Significant heterogeneity was observed among the studies of sclerostin and BMP-2, respectively (sclerostin: I2 = 95.7%, p < 0.001; BMP-2: I2 = 94.7%, p < 0.001), thus the random-effect models were applied (Figs. 2, 3).
Overall Results
The overall pooled results indicated that there were no statistical difference in serum sclerostin concentrations between AS patients and controls (SMD = 0.098, 95% CI − 0.395 to 0.591, p = 0.697) (Fig. 2). Nevertheless, AS group had significant higher serum levels of BMP-2 compared to control group (SMD = 1.184, 95% CI 0.209 to 2.159, p = 0.017) (Fig. 3).
Subgroup Analysis
For sclerostin, we carried out the subgroup analyses based on region, age, erythrocyte sedimentation rate (ESR), C-reaction protein (CRP), bath ankylosing spondylitis Disease Activity Index (BASDAI), Bath Ankylosing Spondylitis Functional Index (BASFI), modified stoke ankylosing spondylitis spinal score (mSASSS) and ELISA kit, and the results are shown in Table 2. In Europe and South America, AS patients had lower serum levels of sclerostin than controls (Fig. 4). Furthermore, in the subgroups of age ≥ 40 years, ESR ≤ 20 mm/h as well as BASFI < 4, serum sclerostin concentrations in AS group were significantly lower than that in control group. Additionally, in CRP ≤ 10 mg/L subgroup, marginally significant difference existed in serum sclerostin levels between AS patients and controls. In addition, serum levels of sclerostin were not different between AS group and control group when using kits from Biomedica, R&D Systems and BD Biosciences. However, when using kits from AUROGENE srl, ICL Lab Inc, TECOmedical, NovaTeinBio and from Rapidbio, serum sclerostin levels in AS patients were strikingly lower as compared with controls.
Regarding BMP-2, due to the limited number of studies and the lack of some data, subgroup analyses by country and CRP stratification were performed. The results showed that both Chinese and Korean patients with AS had significantly higher serum BMP-2 levels when compared to controls, and country may be a source of heterogeneity for the studies (Fig. 5). In addition, serum levels of BMP-2 in AS patients were higher than that in controls in the lower CRP subgroup rather than the higher CRP subgroup.
Random-Effects Meta-Regression Analyses
Meta-regression analyses were implemented to further detect the sources of heterogeneity for the studies on sclerostin. Publication year, total sample size and NOS score were respectively incorporated as covariates, however, none of them could explain for the heterogeneity of studies (Table 3).
Publication Bias and Sensitivity Analyses
Both egger’s and Begg’s tests showed no statistically significant publication bias whether across the overall studies or subgroup studies (all p > 0.05, Table 2). Sensitivity analyses suggested that the pooled results did not significantly alter when the data of any single study are deleted in turn, indicating the overall effect sizes of the included studies were robust (data not shown).
Discussion
Sclerostin emerges as a potent negative regulator of bone formation [23], while BMP-2 is an important promoting factor for osteogenesis and cartilage homeostasis [4]. Previous studies reported that sclerostin antibody administration to ovariectomized rats resulted in increased bone growth, bone mineral density and bone mass, and the increment of exogenous sclerostin suppressed differentiation and proliferation of human and mouse osteoblastic cells [38, 39]. Wildemann et al. demonstrated that BMP-2 had a powerful role in inducing the transformation of mesenchymal cells into osteoblasts [40]. Katagiri et al. held the view that implantation of BMP-2 into muscular tissues induced ectopic bone formation at the implantation site [41]. Literature evidence indicated that the cycles of bone resorption coupled with subsequent bone formation were typical processes during the development of AS [42]. Multiple studies have investigated the association of serum sclerostin or BMP-2 levels with AS, nevertheless, the results were discrepant. Hence, we conducted the present meta-analysis to probe into the role of serum levels of sclerostin and BMP-2 in AS development.
Seventeen studies as for sclerostin were included in this meta-analysis. The pooled results revealed that serum sclerostin levels in AS patients were not significant different with that in healthy controls. This result is similar to the result previously reported by Shi et al. who conducted a meta-analysis synthesized seven studies, but they only retrieved English articles from limited databases, and did not implement further subgroup analyses [43]. The current study that included more studies had a large sample size and high statistical power. Subgroup analysis indicated that in Europe and South America, AS patients had lower serum sclerostin levels than controls, but there were no difference in serum levels of sclerostin between AS patients and controls in Asia, suggesting that region may have influence on the serum sclerostin levels. Indeed, populations from different regions have different physical qualities, genetic and environmental characteristics, and all of these may be associated with serum levels of sclerostin. Furthermore, AS is a complex autoimmune disorder involving a series of complicated pathological processes which include but are not limited to bone resorption and formation, and sclerostin mainly regulates the processes of bone remodeling [44]. In addition, one pivotal point should be noted that although sclerostin plays an important regulatory role in the osteogenesis process mediated by the Wnt/β-catenin signaling pathway, it is only one of many Wnt inhibitors. Dickkopf-1 (Dkk-1), another potent antagonist of Wnt signaling, has been proven to be implicated in the pathogenesis of AS [45]. Multiple lines of evidence have shown that the low levels of DKK-1 could lead to the overexpression of Wnt, thus inducing the new bone formation in AS [46, 47]. Diarra et al. [48] observed that by inhibiting DKK-1, the bone-destructive pattern of a mouse model of rheumatoid arthritis could be reversed to the bone-forming pattern of osteoarthritis, which suggested the effect of DKK-1 on regulating bone remodeling. Sclerostin cooperates with other inhibitors of Wnt signaling to involve in AS progression, and the amount of sclerostin in serum may be influenced by Dkk-1 [26]. Based on the above-mentioned issues, no significant difference in serum sclerostin levels between AS patients and healthy controls might be observed.
Analysis stratified by age showed that in the subgroup of age ≥ 40, serum levels of sclerostin in AS cases were lower than that in controls, suggesting that age may be associated with serum sclerostin of AS patients. Sakellariou et al. and Lu et al. have demonstrated a negative correlation between serum sclerostin levels with age in patients with AS [16, 25]. Interestingly, serum sclerostin levels have been revealed to significantly increase with age in healthy individuals [49]. However, age only accounted for a very small fraction of inter-individual variation in serum sclerostin [49]. Luchetti et al. [50] presented that the duration of articular symptoms was negatively associated with sclerostin. Hence, It can be inferred that a long-standing disease may have an effect on the sclerostin levels which is likely to be greater than the effect of age on sclerostin. In addition, stratification analyses manifested that AS patients with normal ESR (ESR ≤ 20 mm/h), lower CRP (CRP ≤ 10 mg/L) as well as lower BASFI (BASFI < 4) had lower serum concentrations of sclerostin when compared with healthy controls, revealing that ESR, CRP and BASFI may be correlated with serum sclerostin levels. ESR and CRP are widely used to evaluate systemic inflammation. Multiple studies have reported the association between sclerostin and inflammation. Plasma/serum concentrations of sclerostin were reported to be positively correlated with tumor necrosis factor (TNF)-α [51, 52], a pro-inflammatory cytokine associated with AS [53]. A recent study showed that TNF-α could increase the protein expression of SOST gene via regulating NF-κB signaling pathway [54]. BASFI is the admitted parameter to assess functional ability for AS patients [55]. Numerous studies have reported that anti-inflammatory therapy can give rise to a remarkable improvement on BASFI [56, 57]. In addition, Muntean et al. [58] revealed a positive correlation between serum sclerostin levels and BASFI values. Therefore, we speculated that serum sclerostin levels have a potential role in assessing inflammation and functional status in AS. Additionally, the results differed when different ELISA kits were used, suggesting that different immunoassay kits can impact the measuring values of serum sclerostin. Durozier et al. [59] indicated that in the same study, sclerostin levels were remarkably different according to the immunoassay kits used. Piec et al. [60] also observed that sclerostin levels in serum obtained from healthy subjects were significantly higher when using the Biomedica assay than R&D Systems and TECOmedical, and TECOmedical were more accurate than Biomedica assay and R&D Systems. In our study, we found that serum sclerostin levels were lower in AS patients than that in controls when using kits not only from TECOmedical but also from AUROGENE srl, ICL Lab Inc, NovaTeinBio and from Rapidbio. However, no study has evaluated the accuracy of these four kits in measuring serum sclerostin levels. In addition, the number of studies using these kits to measure sclerostin levels is limited, further researches are therefore warranted.
With regard to serum BMP-2 levels in AS, we observed that serum levels of BMP-2 in AS group were materially higher than that in control group. There is evidence that serum BMP-2 levels were closely associated with osteoarthritis and degenerative joint disease, and may act as an alternative biological indicator to estimate disease severity of primary osteoarthritis [61]. Results of subgroup analysis indicated that country may be a source of heterogeneity across the five studies. Both China and Korea subgroup showed a consistent result that serum BMP-2 levels were higher in AS patients when compared to controls. However, the SMD of Korea subgroup was dramatically higher than that of China subgroup, which meant that the serum BMP-2 difference between AS patients and healthy controls in Korea was greater than that in China. When interpreting this result, we took the following issues into consideration. First, although Chinese and Korean have little difference in appearance, their living habits, genetic and environmental background are different, which may explain why serum BMP-2 levels in Korean were different with that in Chinese population. Second, the Korean subgroup only contained one study in which AS patients had much higher disease activity (mean BASDAI = 7.3) than the patients in Chinese studies (all mean BASDAI no more than 4.5). Park et al. [36] observed a positive correlation between BMP-2 and BASDAI in AS patients. Therefore, it may be the discrepant disease activity that accounts for the different result between the countries. Additionally, serum levels of BMP-2 were associated with CRP in AS patients, suggesting a probable role of BMP-2 in inflammation. Indeed, BMP-2 can inhibit the expression of interleukin-34, a proinflammatory cytokine involved in rheumatoid arthritis (RA), thereby contributing to restrain inflammation and bone erosions in RA [62].
As a recommended indicator of assessing the severity of radiographic damage in AS, mSASSS is good for reflect the degree of erosions, sclerosis and syndesmophytes of the cervical and lumbar spine in AS patients [63]. Sun et al. [21] and Chen et al. [37] held the view that sclerostin was negatively related to mSASSS. While Klingberg et al. [28] revealed a positive association between sclerostin and mSASSS. Meanwhile, several other studies did not find the correlation of sclerostin and mSASSS [3, 16]. In the present meta-analysis, the synthetical results showed no association between sclerostin and mSASSS. The reasons may be as follows. On the one hand, although Wnt signaling pathway plays a crucial role in syndesmophytes formation, sclerostin is one of many inhibitors of Wnt signal. The another Wnt antagonist–DKK-1 have been reported to be negatively related to mSASSS in a recent meta-analysis [47]. On the other hand, mSASSS of AS patients is influenced by many factors, such as sex [64], occupation [65] and smoking [66]. But due to the limited information, it was difficult to conduct further stratification analysis. Hence, further longitudinal studies with high-quality data are required to determine their real relationship. Nevertheless, it was clearer and more consistent for the association between BMP-2 and radiographic damage, despite that no subgroup analysis was performed due to the limited number of studies. Serum BMP-2 levels were reported to be elevated in AS patients with spinal fusion whose mSASSS and Bath Ankylosing Spondylitis Radiology Index (BASRI) were significantly higher than those of patients without spinal fusion [35]. The positive relationships of mSASSS and BASRI with BMP-2 were also observed by many researchers [35, 37], which suggesting that BMP-2 is likely to exert a significant impact on the pathogenesis of spinal ankylosis in AS patients. Therefore, the serum levels of BMP-2 may reflect radiographic progression of AS.
In this study, several limitations should be taken into account. First, we could not understand the true source of heterogeneity among the studies about sclerostin by analyzing the limited factors due to the lack of available data. Other factors, like sex, BMI and drug use, might influence serum sclerostin levels in AS and partially generate heterogeneity. Second, the association of serum levels of BMP-2 with AS needs to be further validated considering the limited number of researches.
In conclusion, serum sclerostin levels are not significantly different between AS patients and healthy controls, and serum sclerostin levels in AS are associated with region, age, ESR, CRP, BASFI and ELISA kit. Serum BMP-2 levels in AS patients are higher than that in healthy controls, and are influenced by CRP.
References
Braun J, Sieper J (2007) Ankylosing spondylitis. Lancet 369(9570):1379–1390. https://doi.org/10.1016/s0140-6736(07)60635-7
Machado P, Landewe R, Braun J, Hermann KG, Baker D, van der Heijde D (2010) Both structural damage and inflammation of the spine contribute to impairment of spinal mobility in patients with ankylosing spondylitis. Ann Rheumat Dis 69(8):1465–1470. https://doi.org/10.1136/ard.2009.124206
Perrotta FM, Ceccarelli F (2018) Serum sclerostin as a possible biomarker in ankylosing spondylitis: a case-control study. J Immunol Res. https://doi.org/10.1155/2018/9101964
Liao HT, Lin YF, Tsai CY, Chou TC (2018) Bone morphogenetic proteins and Dickkopf-1 in ankylosing spondylitis. Scandin J Rheumatol 47(1):56–61. https://doi.org/10.1080/03009742.2017.1287305
Moester MJ, Papapoulos SE, Lowik CW, van Bezooijen RL (2010) Sclerostin: current knowledge and future perspectives. Calcif Tissue Int 87(2):99–107. https://doi.org/10.1007/s00223-010-9372-1
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148(6):2635–2643. https://doi.org/10.1210/en.2007-0270
Zhou Y, Wang T, Hamilton JL, Chen D (2017) Wnt/beta-catenin signaling in osteoarthritis and in other forms of arthritis. Curr Rheumatol Rep 19(9):53. https://doi.org/10.1007/s11926-017-0679-z
Lories RJ, Luyten FP (2007) Bone morphogenetic proteins in destructive and remodeling arthritis. Arthrit Res Therapy 9(2):207. https://doi.org/10.1186/ar2135
Hsu WK, Wang JC (2008) The use of bone morphogenetic protein in spine fusion. Spine J 8(3):419–425. https://doi.org/10.1016/j.spinee.2008.01.008
Liu T, Gao Y, Sakamoto K, Minamizato T, Furukawa K, Tsukazaki T, Shibata Y, Bessho K, Komori T, Yamaguchi A (2007) BMP-2 promotes differentiation of osteoblasts and chondroblasts in Runx2-deficient cell lines. J Cell Physiol 211(3):728–735. https://doi.org/10.1002/jcp.20988
Nakashima K, de Crombrugghe B (2003) Transcriptional mechanisms in osteoblast differentiation and bone formation. Trends Genet TIG 19(8):458–466. https://doi.org/10.1016/s0168-9525(03)00176-8
Rawadi G, Vayssiere B, Dunn F, Baron R, Roman-Roman S (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Mineral Res 18(10):1842–1853. https://doi.org/10.1359/jbmr.2003.18.10.1842
Fukuda T, Kokabu S, Ohte S, Sasanuma H, Kanomata K, Yoneyama K, Kato H, Akita M, Oda H, Katagiri T (2010) Canonical Wnts and BMPs cooperatively induce osteoblastic differentiation through a GSK3beta-dependent and beta-catenin-independent mechanism. Differentiation 80(1):46–52. https://doi.org/10.1016/j.diff.2010.05.002
Zhang R, Oyajobi BO, Harris SE, Chen D, Tsao C, Deng HW, Zhao M (2013) Wnt/beta-catenin signaling activates bone morphogenetic protein 2 expression in osteoblasts. Bone 52(1):145–156. https://doi.org/10.1016/j.bone.2012.09.029
Genre F, Rueda-Gotor J, Remuzgo-Martinez S, Corrales A, Ubilla B, Mijares V, Fernandez-Diaz C, Portilla V, Blanco R, Hernandez JL, Llorca J, Lopez-Mejias R, Gonzalez-Gay MA (2018) Implication of osteoprotegerin and sclerostin in axial spondyloarthritis cardiovascular disease: study of 163 Spanish patients. Clin Exp Rheumatol 36(2):302–309. https://doi.org/10.3899/jrheum.170833
Sakellariou GT, Iliopoulos A, Konsta M, Kenanidis E, Potoupnis M, Tsiridis E, Gavana E, Sayegh FE (2017) Serum levels of Dkk-1, sclerostin and VEGF in patients with ankylosing spondylitis and their association with smoking, and clinical, inflammatory and radiographic parameters. Joint Bone Spine 84(3):309–315. https://doi.org/10.1016/j.jbspin.2016.05.008
Xie Z, Wang P, Li Y, Deng W, Zhang X, Su H, Li D, Wu Y, Shen H (2016) Imbalance between bone morphogenetic protein 2 and noggin induces abnormal osteogenic differentiation of mesenchymal stem cells in ankylosing spondylitis. Arthrit Rheumatol 68(2):430–440. https://doi.org/10.1002/art.39433
Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB (2000) Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA 283(15):2008–2012
Wan X, Wang W, Liu J, Tong T (2014) Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 14:135. https://doi.org/10.1186/1471-2288-14-135
Luo D, Wan X, Liu J, Tong T (2018) Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res 27(6):1785–1805. https://doi.org/10.1177/0962280216669183
Sun W, Tian L, Jiang L, Zhang S, Zhou M, Zhu J, Xue J (2018) Sclerostin rather than Dickkopf-1 is associated with mSASSS but not with disease activity score in patients with ankylosing spondylitis. Clin Rheumatol. https://doi.org/10.1007/s10067-018-4356-z
Luchetti MM, Ciccia F, Avellini C, Benfaremo D, Guggino G, Farinelli A, Ciferri M, Rossini M, Svegliati S, Spadoni T, Bolognini L, Fava G, Mosca P, Gesuita R, Skrami E, Triolo G, Gabrielli A (2018) Sclerostin and anti sclerostin antibody serum levels predict the presence of axial spondyloarthritis in patients with inflammatory bowel disease. J Immunol Res 45(5):630–637. https://doi.org/10.1155/2018/910196410.3899/jrheum.170833
Niu CC, Lin SS, Yuan LJ, Chen LH, Yang CY, Chung AN, Lu ML, Tsai TT, Lai PL, Chen WJ (2017) Correlation of blood bone turnover biomarkers and Wnt signaling antagonists with AS, DISH, OPLL, and OYL. BMC musculoskeletal disorders 18(1):61. https://doi.org/10.1186/s12891-017-1425-4
Tian L, Zhou M, Zhang S, Xue J (2017) Correlation analysis of serum osteotin level and bone imaging changes in patients with ankylosing spondylitis. Zhejiang clinical medicine 19(8):1394–1396
Lu Z, Guan Z (2017) Serum levels of Dickkopf-1, sclerostin and vascular endothelial growth factor A and their correlation with ankylosing spondylitis progression. Chin J Tissue Eng Res 21(32):5085–5090
Rossini M, Viapiana O, Idolazzi L, Ghellere F, Fracassi E, Troplini S, Povino MR, Kunnathully V, Adami S, Gatti D (2016) Higher level of Dickkopf-1 is associated with low bone mineral density and higher prevalence of vertebral fractures in patients with ankylosing spondylitis. Calcif Tissue Int 98(5):438–445. https://doi.org/10.1007/s00223-015-0093-3
Xie J, Yu X (2015) [Correlation between sclerostin level and radiographic changes in patients with ankylosing spondylitis]. Zhonghua yi xue za zhi 95(17):1300–1304
Klingberg E, Nurkkala M, Carlsten H, Forsblad-d’Elia H (2014) Biomarkers of bone metabolism in ankylosing spondylitis in relation to osteoproliferation and osteoporosis. J Rheumatol 41(7):1349–1356. https://doi.org/10.3899/jrheum.131199
Tuylu T, Sari I, Solmaz D, Kozaci DL, Akar S, Gunay N, Onen F, Akkoc N (2014) Fetuin-A is related to syndesmophytes in patients with ankylosing spondylitis: a case control study. Clinics (Sao Paulo Brazil) 69(10):688–693. https://doi.org/10.6061/clinics/2014(10)07
Ustun N, Tok F, Kalyoncu U, Motor S, Yuksel R, Yagiz AE, Guler H, Turhanoglu AD (2014) Sclerostin and Dkk-1 in patients with ankylosing spondylitis. Acta Reumatol port 39(2):146–151
Korkosz M, Gasowski J, Leszczynski P, Pawlak-Bus K, Jeka S, Kucharska E, Grodzicki T (2013) High disease activity in ankylosing spondylitis is associated with increased serum sclerostin level and decreased wingless protein-3a signaling but is not linked with greater structural damage. BMC Musculoskelet Disord 14:99. https://doi.org/10.1186/1471-2474-14-99
Taylan A, Sari I, Akinci B, Bilge S, Kozaci D, Akar S, Colak A, Yalcin H, Gunay N, Akkoc N (2012) Biomarkers and cytokines of bone turnover: extensive evaluation in a cohort of patients with ankylosing spondylitis. BMC Musculoskelet Disord 13:191. https://doi.org/10.1186/1471-2474-13-191
Saad CG, Ribeiro AC, Moraes JC, Takayama L, Goncalves CR, Rodrigues MB, de Oliveira RM, Silva CA, Bonfa E, Pereira RM (2012) Low sclerostin levels: a predictive marker of persistent inflammation in ankylosing spondylitis during anti-tumor necrosis factor therapy? Arthritis Res Therapy 14(5):R216. https://doi.org/10.1186/ar4055
Appel H, Ruiz-Heiland G, Listing J, Zwerina J, Herrmann M, Mueller R, Haibel H, Baraliakos X, Hempfing A, Rudwaleit M, Sieper J, Schett G (2009) Altered skeletal expression of sclerostin and its link to radiographic progression in ankylosing spondylitis. Arthritis Rheum 60(11):3257–3262. https://doi.org/10.1002/art.24888
Chen HA, Chen CH, Lin YJ, Chen PC, Chen WS, Lu CL, Chou CT (2010) Association of bone morphogenetic proteins with spinal fusion in ankylosing spondylitis. J Rheumatol 37(10):2126–2132. https://doi.org/10.3899/jrheum.100200
Park MC, Park YB, Lee SK (2008) Relationship of bone morphogenetic proteins to disease activity and radiographic damage in patients with ankylosing spondylitis. Scand J Rheumatol 37(3):200–204. https://doi.org/10.1080/03009740701774941
Chen Z, Wang S, Zhu G (2015) The relationship between serum ossification and mSASSS score in ankylosing spondylitis patients. World Latest Med Inf 15(83):1–3
Ke HZ, Richards WG, Li X, Ominsky MS (2012) Sclerostin and Dickkopf-1 as therapeutic targets in bone diseases. Endocr Rev 33(5):747–783. https://doi.org/10.1210/er.2011-1060
Stolina M, Dwyer D, Niu QT, Villasenor KS, Kurimoto P, Grisanti M, Han CY, Liu M, Li X, Ominsky MS, Ke HZ, Kostenuik PJ (2014) Temporal changes in systemic and local expression of bone turnover markers during six months of sclerostin antibody administration to ovariectomized rats. Bone 67:305–313. https://doi.org/10.1016/j.bone.2014.07.031
Wildemann B, Burkhardt N, Luebberstedt M, Vordemvenne T, Schmidmaier G (2007) Proliferating and differentiating effects of three different growth factors on pluripotent mesenchymal cells and osteoblast like cells. J Orthop Surg Res 2:27. https://doi.org/10.1186/1749-799x-2-27
Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T (1994) Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol 127(6 Pt 1):1755–1766
Cortes A, Maksymowych WP, Wordsworth BP, Inman RD, Danoy P, Rahman P, Stone MA, Corr M, Gensler LS, Gladman D, Morgan A, Marzo-Ortega H, Ward MM, Learch TJ, Reveille JD, Brown MA, Weisman MH (2015) Association study of genes related to bone formation and resorption and the extent of radiographic change in ankylosing spondylitis. Ann Rheum Dis 74(7):1387–1393. https://doi.org/10.1136/annrheumdis-2013-204835
Shi J, Ying H, Du J, Shen B (2017) Serum sclerostin levels in patients with ankylosing spondylitis and rheumatoid arthritis: a systematic review and meta-analysis. Biomed Res Int 2017:9295313. https://doi.org/10.1155/2017/9295313
Koide M, Kobayashi Y (2018) Regulatory mechanisms of sclerostin expression during bone remodeling. J Bone Miner Metab. https://doi.org/10.1007/s00774-018-0971-7
Zhang L, Ouyang H, Xie Z, Liang ZH, Wu XW (2016) Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Exp Mol Med 48:e228. https://doi.org/10.1038/emm.2016.12
Zou YC, Yang XW, Yuan SG, Zhang P, Ye YL, Li YK (2016) Downregulation of dickkopf-1 enhances the proliferation and osteogenic potential of fibroblasts isolated from ankylosing spondylitis patients via the Wnt/beta-catenin signaling pathway in vitro. Connect Tissue Res 57(3):200–211. https://doi.org/10.3109/03008207.2015.1127916
Wu M, Chen M, Ma Y, Yang J, Han R, Yuan Y, Hu X, Wang M, Zhang X, Xu S, Liu R, Jiang G, Xu J, Shuai Z, Zou Y, Pan G, Pan F (2018) Dickkopf-1 in ankylosing spondylitis: Review and meta-analysis. Clin Chim Acta 481:177–183. https://doi.org/10.1016/j.cca.2018.03.010
Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G (2007) Dickkopf-1 is a master regulator of joint remodeling. Nat Med 13(2):156–163. https://doi.org/10.1038/nm1538
Kuipers AL, Zhang Y, Yu S, Kammerer CM, Nestlerode CS, Chu Y, Bunker CH, Patrick AL, Wheeler VW, Miljkovic I, Zmuda JM (2014) Relative influence of heritability, environment and genetics on serum sclerostin. Osteoporosis international: a journal established as result of cooperation between the European Foundation for Osteoporosis and the National Osteoporosis Foundation of the USA. Osteoporos Int 25 (3):905–912. https://doi.org/10.1007/s00198-013-2517-0
Luchetti MM, Ciccia F, Avellini C, Benfaremo D, Guggino G, Farinelli A, Ciferri M, Rossini M, Svegliati S, Spadoni T, Bolognini L, Fava G, Mosca P, Gesuita R, Skrami E, Triolo G, Gabrielli A (2018) Sclerostin and antisclerostin antibody serum levels predict the presence of axial spondyloarthritis in patients with inflammatory bowel disease. J Rheumatol 45(5):630–637. https://doi.org/10.3899/jrheum.170833
Pietrzyk B, Wyskida K, Ficek J, Kolonko A, Ficek R, Wiecek A, Olszanecka-Glinianowicz M, Chudek J (2018) Relationship between plasma levels of sclerostin, calcium-phosphate disturbances, established markers of bone turnover, and inflammation in haemodialysis patients. Int Urol Nephrol. https://doi.org/10.1007/s11255-018-2050-3
Almroth G, Lonn J, Uhlin F, Brudin L, Andersson B, Hahn-Zoric M (2016) Sclerostin, TNF-alpha and Interleukin-18 correlate and are together with Klotho related to other growth factors and cytokines in haemodialysis patients. Scand J Immunol 83(1):58–63. https://doi.org/10.1111/sji.12392
Croft M, Siegel RM (2017) Beyond TNF: TNF superfamily cytokines as targets for the treatment of rheumatic diseases. Nat Rev Rheumatol 13(4):217–233. https://doi.org/10.1038/nrrheum.2017.22
Li SS (2017) Mechanisms underlying the post-transcriptional regulation of murine sclerostin by TNF-alpha. Thesis of master, Shandong university
Calin A, Garrett S, Whitelock H, Kennedy LG, O’Hea J, Mallorie P, Jenkinson T (1994) A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol 21(12):2281–2285
Li Q, Li L, Bi L, Xiao C, Lin Z, Cao S, Liao Z, Gu J (2016) Kunxian capsules in the treatment of patients with ankylosing spondylitis: a randomized placebo-controlled clinical trial. Trials 17(1):337. https://doi.org/10.1186/s13063-016-1438-6
Zong HX, Xu SQ, Tong H, Wang XR, Pan MJ, Teng YZ (2018) Effect of anti-tumor necrosis factor alpha treatment on radiographic progression in patient with ankylosing spondylitis: a systematic review and meta-analysis. Mod Rheumatol. https://doi.org/10.1080/14397595.2018.1525017
Muntean L, Lungu A, Gheorghe SR, Valeanu M, Craciun AM, Felea I, Petcu A, Filipescu I, Simon SP, Rednic S (2016) Elevated serum levels of sclerostin are associated with high disease activity and functional impairment in patients with axial spondyloarthritis. Clin Lab 62(4):589–597
Durosier C, van Lierop A, Ferrari S, Chevalley T, Papapoulos S, Rizzoli R (2013) Association of circulating sclerostin with bone mineral mass, microstructure, and turnover biochemical markers in healthy elderly men and women. J Clin Endocrinol Metab 98(9):3873–3883. https://doi.org/10.1210/jc.2013-2113
Piec I, Washbourne C, Tang J, Fisher E, Greeves J, Jackson S, Fraser WD (2016) How accurate is your sclerostin measurement? Comparison between three commercially available sclerostin ELISA kits. Calcif Tissue Int 98(6):546–555. https://doi.org/10.1007/s00223-015-0105-3
Liu Y, Hou R, Yin R, Yin W (2015) Correlation of bone morphogenetic protein-2 levels in serum and synovial fluid with disease severity of knee osteoarthritis. Med Sci Monit. 21:363–370. https://doi.org/10.12659/msm.892160
Chemel M, Brion R, Segaliny AI, Lamora A, Charrier C, Brulin B, Maugars Y, Le Goff B, Heymann D, Verrecchia F (2017) Bone morphogenetic protein 2 and transforming growth factor beta1 inhibit the expression of the proinflammatory cytokine IL-34 in rheumatoid arthritis synovial fibroblasts. Am J Pathol 187(1):156–162. https://doi.org/10.1016/j.ajpath.2016.09.015
Creemers MC, Franssen MJ, van’t Hof MA, Gribnau FW, van de Putte LB, van Riel PL (2005) Assessment of outcome in ankylosing spondylitis: an extended radiographic scoring system. Ann Rheum Dis 64(1):127–129. https://doi.org/10.1136/ard.2004.020503
Deminger A, Klingberg E, Geijer M, Gothlin J, Hedberg M, Rehnberg E, Carlsten H, Jacobsson LT, Forsblad-d’Elia H (2018) A five-year prospective study of spinal radiographic progression and its predictors in men and women with ankylosing spondylitis. Arthritis Res Therapy 20(1):162. https://doi.org/10.1186/s13075-018-1665-1
Ramiro S, Landewe R, van Tubergen A, Boonen A, Stolwijk C, Dougados M, van den Bosch F, van der Heijde D (2015) Lifestyle factors may modify the effect of disease activity on radiographic progression in patients with ankylosing spondylitis: a longitudinal analysis. RMD Open 1(1):e000153. https://doi.org/10.1136/rmdopen-2015-000153
Maas F, Arends S, Wink FR, Bos R, Bootsma H, Brouwer E, Spoorenberg A (2017) Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-alpha inhibitors. PloS ONE 12(6):e0177231. https://doi.org/10.1371/journal.pone.0177231
Acknowledgements
This study was supported by Grants from the National Natural Science Foundation of China (30972530, 81273169, 81573218 and 81773514).
Funding
This study was funded by grants from the National Natural Science Foundation of China (30972530, 81273169, 81573218 and 81773514).
Author information
Authors and Affiliations
Contributions
Corresponding author Faming Pan came up with the idea and he is guarantor. Authors #a and #b performed the literature search. Author #c was responsible for statistical analysis. Author Jiajia Yang wrote the first draft of the article. Author #d and #e modified the manuscript. All authors reviewed the paper and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
Jiajia Yang, Shanshan Xu, Mengya Chen, Yaping Yuan, Xu Zhang, Yubo Ma, Meng Wu, Renfang Han, Xingxing Hu, Rui Liu, Jixiang Deng, Shiyang Guan, Xing Gao, Meijuan Pan, Shengqian Xu, Zongwen Shuai, Shanqun Jiang, Shihe Guan, Liwen Chen, and Faming Pan declare they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
For this type of study formal consent is not required.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yang, J., Xu, S., Chen, M. et al. Serum Sclerostin and Bone Morphogenetic Protein-2 Levels in Patients with Ankylosing Spondylitis: A Meta-Analysis. Calcif Tissue Int 105, 37–50 (2019). https://doi.org/10.1007/s00223-019-00542-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00542-z