Abstract
Diabetes mellitus (DM) has been associated with increased bone fracture rates, impaired bone regeneration, delayed bone healing, and depressed osteogenesis. However, the plausible pathogenic mechanisms remain incompletely understood. The aim of the present systematic review was to investigate whether oxidative stress (OS) plays a role in altered characteristics of diabetic bone under in vivo conditions. An electronic search of the MEDLINE (via PubMed) and Embase databases was performed. In vivo animal studies involving DM and providing information regarding assessment of OS markers combined with analyses of bone histology/histomorphometry parameters were selected. A descriptive analysis of selected articles was performed. Ten studies were included in the present review. Both bone formation and bone resorption parameters were significantly decreased in the diabetic groups of animals compared to the healthy groups. This finding was consistent regardless of different animal/bone models employed or different evaluation periods. A statistically significant increase in systemic and/or local OS status was also emphasised in the diabetic groups in comparison to the healthy ones. Markers of OS were associated with histological and/or histomorphometric parameters, including decreased trabecular bone and osteoid volumes, suppressed bone formation, defective bone mineralisation, and reduced osteoclastic activity, in diabetic animals. Additionally, insulin and antioxidative treatment proved to be efficient in reversing the deleterious effects of high glucose and associated OS. The present findings support the hypotheses that OS in the diabetic condition contributes at least partially to defective bone features, and that antioxidative supplementation can be a valuable adjunctive strategy in treating diabetic bone disease, accelerating bone healing, and improving osteointegration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
DM is one of the most prevalent diseases worldwide, defined as a group of metabolic disorders characterised by prolonged hyperglycaemia. The underlying pathophysiological mechanisms include impaired insulin secretion and/or action. The chronic hyperglycaemia of DM is associated with long-term damage, dysfunction, and failure of various organs and blood vessels, resulting in such well-known diabetic complications as microvascular (diabetic neuropathy, nephropathy, and retinopathy) and macrovascular (coronary artery disease, peripheral artery disease, and stroke) [1, 2]. However, in recent years, more and more attention has been drawn to a series of complications involving the skeletal system. Generally, diabetic bone has been associated with altered bone mineral density (BMD) and increased bone fracture rates [3,4,5]. Moreover, diminished bone formation and impaired bone regeneration potential, delayed healing of diabetic bone, and depressed osteogenesis have been reported [4, 6,7,8,9].
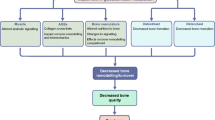
Nevertheless, the molecular and cellular mechanisms underlying the diabetic bone physiopathology remain incompletely understood. Diabetes-induced augmentation of OS has been suggested to play an important role in the development of diabetic complications including diabetic bone disorder [10, 11]. Increased formation of chemically reactive molecules containing oxygen or nitrogen (reactive oxygen species, ROS; reactive nitrogen species, RNS), together referred to as RONS [12], and the absence of the counterbalancing effects of endogenous antioxidants are known to induce cellular dysfunction in a wide variety of cell types, including osteoprogenitor and angioprogenitor cells [13, 14]. RONS can cause severe damage to cell structures such as proteins, lipids, and DNA, consequently leading to cell cycle arrest, reduction of cell proliferation, modulation of differentiation, and even apoptosis [15,16,17]. According to several in vitro studies, diabetic serum has led to excessive RONS production, both in mitochondria and cytoplasm [18, 19]. As a consequence, RONS support bone loss not only by directly promoting osteoclastogenesis, but also by inducing apoptosis, as well as decreased differentiation and activities of osteoblasts [20]. Therefore, it can be hypothesised that diabetes-induced RONS overproduction may play a critical role in the biological performance of diabetic bone. Since biomarkers of OS are commonly assessed under in vitro conditions, there is limited evidence regarding a correlation of OS status with the in vivo features of diabetic bone disease. Although in vitro findings alone may provide relevant information, there is a need to visualise the impact of these cellular and molecular malfunctions in in vivo models.
The overall objective of this systematic review was to critically evaluate the relationship between OS parameters and bone features in diabetic bone disease/remodelling under in vivo conditions. Moreover, the effect of systemic administration of insulin or antioxidants on bone parameters in the diabetic condition was assessed.
Materials and Methods
Focus Question(s)
The systematic review was performed according to PRISMA guidelines [21], and the focus questions were as follows:
-
Is OS status associated with diabetic bone features under in vivo conditions?
-
Does insulin or antioxidant treatment improve the diabetic bone features of DM subjects?
Search Strategy
An electronic search of the literature was performed using the following databases: PubMed/MEDLINE and Embase from their respective inception until June 1, 2016. No restrictions were imposed on the language or the date of publication. A detailed search strategy, as shown below, was developed for PubMed and then revised appropriately for each database used. The search terms included:
Population: “diabetes” OR “diabetic” OR “hyperglycemia” OR “advanced glycation end products” OR “AGE*”
Intervention: “diabetic bone disease” OR “DBD” OR “bone healing” OR “bone defect*” OR “bone fracture*” OR “bone remodel*” OR “bone regenerat*” OR “bone formation” OR “bone repair*” OR “bone augment*” OR “bone reconstruct*” OR “bone engineering” OR “bone graft*” OR “osteogenesis” OR “osseointegration” OR “osteointegration”
Control: not applicable
Outcome: “oxidative stress” OR “endoplasmic reticulum stress” OR “mitochondrial stress” OR “reactive oxygen species” OR “ROS” OR “oxygen free radical*” OR “free radical*” OR “oxidation product*”
The electronic search was followed by a manual search of the reference lists of all potentially eligible studies obtained as full reports to identify any further studies not retrieved by the electronic search. A grey literature search was not performed. The titles, abstracts, and full texts of all articles identified by the electronic and manual searches were screened independently by two reviewers and the level of agreement between the reviewers was calculated using kappa scores; in cases of disagreement, the inclusion or exclusion of a relevant article was determined through discussion and the assistance of a third reviewer.
The selection of the literature was set to include at least histological or histomorphometric bone analyses and their relation to OS exposure; thus, only those studies that met this specific requirement were assessed using a systematic approach.
Inclusion Criteria
-
In vivo animal studies
-
Studies involving DM (either type 1 or type 2)
-
A healthy control group
-
A bone model
-
Bone histology/histomorphometry analyses
-
Analyses of any OS parameter
Exclusion Criteria
-
In vitro studies
-
Literature reviews
-
Pilot studies
-
Preliminary reports
-
Letters to the editor
-
Short communications
Data Extraction
All selected papers were read carefully to identify the following data:
-
Author(s) and year of publication
-
Study design
-
Type of DM
-
Animals species
-
Animal characteristics (number, gender, age/months, and weight/kg)
-
Number and characteristics of groups
-
Animal experimental model
-
Bone experimental model
-
Length of observation period
-
Type and characteristics of analyses performed on bone samples
-
Type and characteristics of OS measurements
-
Results of analyses
Independent data extraction by two authors was performed and the data were registered in tables. When some information was not reported, the box was filled in with N/P (Not Provided).
Assessment of Study Quality
Each article was rated on its quality independently by two reviewers using the criteria outlined in the SYRCLE’s (SYstematic Review Centre for Laboratory animal Experimentation) RoB (Risk of Bias) tool. The tool contains 10 entries that are related to selection bias, performance bias, detection bias, attrition bias, reporting bias, and other biases [22].
Statistical Analysis
Each group comparison extracted from the selected articles that proved to be statistically significant was associated with a P value < 0.05. When available, P values were transcribed directly from the articles; otherwise they were calculated from data provided in tables and figures using the appropriate statistical test (Student’s t test or the Chi-squared test) whenever possible. Results were significant at the 5% critical level (P < 0.05). To combine several (say N) independent studies testing the same null hypothesis, the latter was rejected if at least K of them were “positive” (that is, had a P value < 0.05). The value of K was derived from the two-tailed “sign test”. For instance, for N = 6 studies, K should be equal to 6. Since no K-values are available for N ≤ 5, it was not possible to conclude in such situations. The degree of agreement between two readers was assessed by Cohen’s kappa coefficient.
Results
Search and Selection
The initial search yielded 606 potentially eligible articles. Following duplicate elimination, 439 articles were screened in a subsequent procedure. A total of 361 articles were excluded based on the screening of titles and abstracts. The remaining 78 articles were qualified for a full-text proofreading. Following this stage, 67 articles were excluded and only 11 articles met the defined inclusion criteria (inter-reviewer agreement, κ = 0.81). One additional article was excluded after quality assessment, leaving the final number of included articles at 10. The search strategy and retrieved publications are summarised in Fig. 1 and Table 1, respectively. The rejected articles are displayed in Appendix I.
Characteristics of the Selected Publications
Rats were used in 5 out of 10 included studies; 3 studies were conducted on mice, 1 on rabbits, and 1 on sheep. Bone experimental models included diabetic bone disease (7 studies), bone defect healing (1 study), and osteointegration (2 studies) (Table 2; column I). Spontaneously diabetic Torii (SDT) rats were used in 3 studies, while 7 studies reported use of a chemically induced model of DM by means of streptozotocin (STZ) in 6 studies and alloxan (ALX) in 1 study. All selected studies had at least 1 control (healthy) and 1 DM group. Four studies considered DM combined with insulin treatment and 7 articles investigated the effects of several types of antioxidative agents. Regarding animal gender and age, all studies that reported this information used males with skeletal maturity. To confirm diabetes, serum glucose levels (GLs) and haemoglobin A1c were used in 7 and 3 studies, respectively. Animals with GLs of over 200 or 300 mg/dL were considered diabetic. Most studies recorded animal weight weekly. Analyses were performed after an observation period ranging from 10 days to 16 weeks (Table 1). Considering the heterogeneity of the data, a meta-analysis could not be performed. Study results presented in Tables 2 and 3 were analysed using the sign test as described above.
Diabetic Bone Features
Histology/Histomorphometry
Regarding bone formation, 7 articles reported data on the bone formation rate per bone surface (BFR/BS) and the mineral apposition rate (MAR) of diabetic bone (Table 2; columns II and III). Histomorphometric analyses in all 7 studies emphasised a significant reduction (P < 0.05) of both parameters in the diabetic groups compared to the control groups, reflecting the decrease in the number and/or function of osteoblasts in diabetic animals; hence, according to the sign test, it can be considered as statistically significant regardless of the experimental model.
Further, bone formation parameters, such as osteoid volume per bone volume (OV/BV), osteoblast surface per bone surface (Ob.S/BS), osteoid thickness (O.Th), mineral surface per bone surface (MS/BS), and bone volume per total volume (BV/TV) were analysed in some studies. Even though all the studies displayed significantly better bone formation parameters in healthy animals compared to diabetic animals, no formal improvement could be evidenced because of the limited number of studies (N ≤ 4) evaluating these parameters (Table 2; columns IV and V).
Noteworthy, the eroded surface per bone surface (ES/BS) was the most common parameter to evaluate bone resorption in the selected studies (N = 6). ES/BS was found to be lower (P < 0.05) in the DM groups than in the control groups for all 6 studies, regardless of the type of DM (Table 2; column VI). This finding was therefore considered statistically significant.
X-ray/micro-CT
All 6 studies reporting results on bone mineral density (BMD), based on x-ray absorptiometry, showed significantly lower BMD in diabetic animals compared to healthy ones, and severe osteopenia was described in 4 studies (Table 2; column VII). Based on 4 studies that employed micro-CT, bone volume fraction (BV/TV) or new bone formation (BV/PV) was also significantly reduced in diabetic compared to non-diabetic bones (Table 2; column VIII).
Molecular Analyses: OS Markers
All selected studies evaluated OS status in diabetic versus healthy tissues. Most of the studies (6 out of 10) investigated systemic levels of OS using ELISA to determine the urinary excretion of 8-hydroxydeoxyguanosine (8-OHdG), a sensitive indicator of oxidative DNA damage, and all of them showed significantly higher rates of urinary 8-OHdG in the diabetic animals compared to the healthy ones (Table 3; column I). A similar finding was reported for the plasma levels of advanced oxidation protein products (AOPP) and a lipid peroxidation marker, malondialdehyde (MDA), in 1 study (Table 3; columns III and IV).
Furthermore, in 4 studies, significantly higher local levels of OS (8-OHdG-positive staining of bone cells) were found in the diabetic bone compared to the control bone samples (Table 3; column II).
Additionally, 2 studies analysed markers of OS in osteoblasts cultured in diabetic serum acquired from diabetic animals in comparison to the normal serum, reporting a significantly higher intracellular ROS production, including elevated MDA (TBARS) and H2O2 levels under diabetic condition (Table 3; columns III, V, and VI).
Considering antioxidative defence mechanisms, total serum antioxidant capacity (TAC) was measured in 1 study showing significantly lower values in diabetic versus non-diabetic rats. Additionally, the levels of several antioxidative enzymes, superoxide dismutase (SOD), glutathione peroxidase (GPx), xanthine oxidase (XO), and catalase (CAT), were considered in some studies (Table 3; columns VII, VIII, IX, X, and XI). The levels of SOD, also including protein expression of manganese superoxide dismutase (MnSOD), and GPx levels were significantly decreased in diabetes. In contrast, 1 study that compared diabetic with non-diabetic ageing mice showed a slight increase in XO that occurred in the diabetic mice, while the levels of SOD and CAT were not significantly different between the groups. Given the small number of studies, no formal general conclusions could be drawn from the observations above.
Role of Insulin and Antioxidants
Impaired bone features observed in diabetic groups were ameliorated by insulin in all 4 studies that examined its therapeutic effects, regardless of the animal and bone experimental models or the type of DM. The bone formation and resorption parameters (BFR/BS, MAR, OV/BV, and ES/BS), BMD, as well as the trabecular number and trabecular/cortical thickness were found to be significantly higher in insulin-treated diabetic groups compared to the untreated animals (Table 2).
Five studies assessed the effects of antioxidant (AO) treatment on bone features in diabetic animals. All of them showed better bone formation, enhanced trabecular bone volumes, as well as increased new bone formation in osteointegration models, in diabetic animals treated with antioxidative agents such as N-acetyl-cysteine (AO1), carvedilol (AO2), quercetin at 30 and 50 mg/kg (AO3), and 22-oxacalcitriol (a vitamin D receptor activator) compared to the untreated diabetic animals (Table 2). Based on a single study, the administration of melatonin seemed also to exhibit similar antioxidative properties as shown to be able to emphasise new mineralised tissue formation. Finally, insulin and AO treatment also significantly reduced systemic and local OS caused by DM (Table 3).
Discussion
Correlation Between OS and Diabetic Bone Features
To the best of our knowledge, this is the first systematic review linking diabetic bone features and OS. Despite the limited number of included studies and their heterogeneity, all displayed a high association between histological and/or morphometric diabetic bone features and local and/or systemic biomarkers of OS.
The studies showed a significant difference in both qualitative and quantitative bone characteristics between diabetic bone and the healthy control. Histological and histomorphometric analyses demonstrated decreased trabecular bone and osteoid volumes, suppressed bone formation, defective bone mineralisation, and reduced osteoclastic activity in diabetic animals. These findings were consistent regardless of various animal models or different evaluation periods and are in accordance with a recent review [23]. In all models, the impaired bone features were associated with local and systemic marker levels of OS. These observations are consistent with the conclusions of Hamada et al. reporting on the role of OS in diabetic bone disorder [10].
Intracellular ROS regulate several cellular signalling pathways affecting various processes, including cell metabolism, proliferation, differentiation, and survival, as well as antioxidant, anti-inflammatory, and DNA damage response [24]. In terms of the sources of augmented generation of OS under diabetic condition, multiple non-enzymatic, enzymatic, and mitochondrial pathways have been described [25]. Besides the findings that ROS overproduction in the hyperglycaemic condition of diabetes could lead to impaired bone formation through inhibition of bone marrow stromal cell differentiation into osteoblasts, as well as osteoblast proliferation and function [17, 26,27,28,29], several in vitro studies have shown that OS may even provoke osteoblast insults and apoptosis [30, 31]. Specifically, it has been previously demonstrated that ROS overproduction could antagonise cell survival-related signalling and promote pro-apoptotic action in a ROS-dependent manner [32]. Weinberg et al. have shown that the bone marrow of hyperglycaemic rats contained fewer osteoprogenitor cells when compared to the healthy controls [33]. Furthermore, ROS seem to stimulate osteoclast bone resorption and osteoclast differentiation [34, 35]. Mody et al. reported that ROS released by osteoclasts may be involved in degrading the bone matrix, resulting in the promotion of bone resorption [14]. However, the number of osteoclasts in several studies in this review appeared to be lower in diabetic than in control animals, which could have resulted in low bone turnover. Fujii and colleagues [36] suggested that osteoblast function was more inhibited than osteoclast function, which in turn led to diabetic osteopenia, even though, clinically, low bone turnover has been linked to increased bone mass. Indeed, while the results of human studies evaluating the effects of diabetes on markers of bone resorption have been mixed, the consistent data regarding impaired osteoblast differentiation and function suggests that osteoblasts are the main culprits underlying bone fragility in diabetes [37]. Besides OS, a number of other cellular and molecular mechanisms have been postulated to mediate defects in osteoblast activity. Sclerostin, the negative regulator of Wnt signalling pathway essential for osteoblast differentiation, secreted by osteocytes, has been suggested to have a role in shaping bone quality in diabetes [37]. In DM type 1, several genetic players, such as runt domain factor-2 (Runx-2) gene which encodes proteins associated with osteoblast differentiation, have also been identified. Additionally, DM is associated with increased systemic inflammation, especially during disease onset, and several inflammatory factors are known to effectively suppress anabolic functions, including osteoblast maturation and bone formation [38, 39]. It is also worth noting that 7 of the 10 included studies represented type 1 DM, and the remaining 3 studies used an SDT rat model, which is a model of non-obese type 2 DM. While type 1 diabetes has been regularly associated with decreased BMD, the effect of type 2 DM on BMD in humans remains controversial [40]. In this review, decreased BMD was a consistently reported finding in both type 1 and non-obese type 2 diabetic animals. Indeed, animal models are limited in their ability to recapitulate all of the features of diabetic bone disease in humans [41]. The similarity in physiologic and pathophysiologic responses between the human and rodent skeleton have made it a valuable model in bone-related research. However, several differences have been noted; for instance, rodents continue to model their skeleton throughout their life cycle and growth plates remain open throughout adulthood, but there is a lack of Haversian remodelling [42]. Moreover, the impact of diabetes on bone depends on the age of onset—before or after the animals reach skeletal maturity—affecting bone mass acquisition and development or skeletal maintenance activities [41]. Animals with skeletal maturity were used in the selected studies included in this review.
Both excessive ROS production and inadequate antioxidant defence mechanisms operating in cells can cause the disturbance of oxidative balance. Although several studies in the present review assessed antioxidant capacity under diabetic conditions, the data were too limited to draw general conclusions. However, the antioxidant enzymes were found to be decreased in diabetes, except in 1 study where an additional ageing condition may have contributed to a different result. On the other hand, clinical studies have revealed inconsistent findings, with both reduction [43, 44] and increase [45] in endogenous antioxidant enzyme activity in DM patients compared to healthy subjects being reported.
Benefits of Antioxidant and Insulin Therapy
Scavenging ROS with exogenous antioxidant therapies was shown to significantly decrease the levels of OS parameters and reversed the deleterious effects of high glucose. Therefore, OS appears to be a potential therapeutic target according to several studies included in this review. Feng et al. have demonstrated that scavenging ROS with N-acetyl-cysteine markedly attenuated cell apoptosis and osteoblast dysfunction and improved bone ingrowth within titanium scaffolds.
In addition, the use of insulin to control diabetes was also considered in some of the selected articles. Severe uncontrolled DM has been shown to exert a direct influence on bone healing by inhibiting bone remodelling and mineralisation [46] as well as impairing osteogenesis within laminar titanium implants [47]. Insulin treatment restored reduced BMD and improved bone turnover in diabetic groups. Fuji et al. have shown that insulin improved the number and function of both osteoblasts and osteoclasts and recovered BMD in animals with type 2 DM [36].
However, only 1 article included in the present review considered local treatment of OS; chitosan, which has been shown to exhibit antioxidant activity, was used as surface coating for titanium implants in an osteointegration model under diabetic conditions [48]. Chitosan coating exerted supportive function on osteoblastic biological behaviour, ameliorating osteoblast morphology, adhesion, proliferation, and differentiation, while alleviating apoptosis. This was associated with markedly suppressed diabetes-mediated ROS overproduction in osteoblasts, which ultimately led to improved osteointegration. Other authors have tempted to treat OS locally in order to improve bone healing and regeneration outcomes in diabetes. Koerdt et al. showed that, in diabetic animals, a combination of autogenous bone and deproteinised bovine bone graft covered by a membrane, in a guided bone regeneration model, led to less bone resorption, higher osteoblast activity, osteoclast inhibition, and less RONS expression in comparison to autogenous bone alone. The authors considered the RONS reduction as a determinant for the stability of autogenous bone grafts [49]. Pradeep et al. demonstrated that locally delivered antioxidant (simvastatin) as an adjunct to standard dental therapy for the treatment of intrabony periodontal defect in type 2 DM patients improved defect healing [50]. This finding supports recent reports that statins may have significant antioxidant properties [51] and consequently could promote bone formation. Hence, adjunctive therapeutic strategies to reduce OS locally may be beneficial in treating diabetic bone disorder and further investigations leading to more targeted OS control under diabetic conditions on both systemic and local levels are needed. Despite clear correlation evidence between OS and diabetic bone features, the present systematic review suffers from some limitations. The data gathered in this review did not allow a meta-analysis because of the great heterogeneity of the study designs. Different animal species and experimental models were combined together in the review, which did not allow us to draw quantitatively strong conclusions, and this needs to be taken into account when interpreting the results. Moreover, the quality of data reporting differed among the studies, and we failed to extract some additional numerical data. In addition, the risk of bias is possibly present due to the fact that 6 studies included in the present review were conducted by almost the same group of authors. This may also compromise the assumption of independence of observations inherent to the sign test. Thus, the present findings need to be interpreted cautiously.
Conclusions
The present systematic review, within its limits, supports the hypothesis that markers of OS are associated with the histomorphometric and morphologic parameters of diabetic bone. The findings indicate that OS in diabetic condition may contribute at least partially to defective bone features and that antioxidative treatments and insulin therapy may present a valuable adjunctive strategy in treating diabetic bone disease and accelerating bone healing and osteointegration.
References
The Expert Committee on the Diagnosis and Classification of Diabetes Mellitus (1997) Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 20(7):1183–1197
Fowler MJ (2008) Microvascular and macrovascular complications of diabetes. Clin Diabetes 26(2):77–82. doi:10.2337/diaclin.26.2.77
De Leeuw I, Abs R (1977) Bone mass and bone density in maturity-type diabetics measured by the 125I photon-absorption technique. Diabetes 26(12):1130–1135
Cozen L (1972) Does diabetes delay fracture healing? Clin Orthop Relat Res 82:134–140
Strotmeyer ES, Cauley JA (2007) Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes 14(6):429–435. doi:10.1097/MED.0b013e3282f1cba3
Goodman WG, Hori MT (1984) Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes 33(9):825–831
Follak N, Kloting I, Ganzer D, Merk H (2003) Scanning electron microscopic examinations on retarded bone defect healing in spontaneously diabetic BB/O(ttawa)K(arlsburg) rats. Histol Histopathol 18(1):111–120
von Wilmowsky C, Stockmann P, Harsch I, Amann K, Metzler P, Lutz R, Moest T, Neukam FW, Schlegel KA (2011) Diabetes mellitus negatively affects peri-implant bone formation in the diabetic domestic pig. J Clin Periodontol 38(8):771–779. doi:10.1111/j.1600-051X.2011.01746.x
Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, Leone CW, Morgan EF, Gerstenfeld LC, Einhorn TA, Graves DT (2007) Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J Bone Miner Res 22(4):560–568. doi:10.1359/jbmr.070115
Hamada Y, Fujii H, Fukagawa M (2009) Role of oxidative stress in diabetic bone disorder. Bone 45(Suppl 1):S35–S38. doi:10.1016/j.bone.2009.02.004
Giacco F, Brownlee M (2010) Oxidative stress and diabetic complications. Circ Res 107(9):1058–1070. doi:10.1161/CIRCRESAHA.110.223545
Weidinger A, Kozlov AV (2015) Biological activities of reactive oxygen and nitrogen species: oxidative stress versus signal transduction. Biomolecules 5(2):472–484. doi:10.3390/biom5020472
Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39(1):44–84. doi:10.1016/j.biocel.2006.07.001
Mody N, Parhami F, Sarafian TA, Demer LL (2001) Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31(4):509–519
Zhang Y, Du Y, Le W, Wang K, Kieffer N, Zhang J (2011) Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal 15(11):2867–2908. doi:10.1089/ars.2010.3685
Grishko VI, Ho R, Wilson GL, Pearsall AW (2009) Diminished mitochondrial DNA integrity and repair capacity in OA chondrocytes. Osteoarthr Cartil 17(1):107–113. doi:10.1016/j.joca.2008.05.009
Bai XC, Lu D, Bai J, Zheng H, Ke ZY, Li XM, Luo SQ (2004) Oxidative stress inhibits osteoblastic differentiation of bone cells by ERK and NF-kappaB. Biochem Biophys Res Commun 314(1):197–207
Bansal S, Siddarth M, Chawla D, Banerjee BD, Madhu SV, Tripathi AK (2012) Advanced glycation end products enhance reactive oxygen and nitrogen species generation in neutrophils in vitro. Mol Cell Biochem 361(1–2):289–296. doi:10.1007/s11010-011-1114-9
Wang X, Yu S, Wang CY, Wang Y, Liu HX, Cui Y, Zhang LD (2015) Advanced glycation end products induce oxidative stress and mitochondrial dysfunction in SH-SY5Y cells. In Vitro Cell Dev Biol Anim 51(2):204–209. doi:10.1007/s11626-014-9823-5
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15(10):468–477. doi:10.1016/j.molmed.2009.08.004
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 339:b2700. doi:10.1136/bmj.b2700
Hooijmans CR, Rovers MM, de Vries RB, Leenaars M, Ritskes-Hoitinga M, Langendam MW (2014) SYRCLE’s risk of bias tool for animal studies. BMC Med Res Methodol 14:43. doi:10.1186/1471-2288-14-43
Retzepi M, Donos N (2010) The effect of diabetes mellitus on osseous healing. Clin Oral Implants Res 21(7):673–681. doi:10.1111/j.1600-0501.2010.01923.x
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24(5):981–990. doi:10.1016/j.cellsig.2012.01.008
Johansen JS, Harris AK, Rychly DJ, Ergul A (2005) Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol 4:5. doi:10.1186/1475-2840-4-5
Bouillon R, Bex M, Van Herck E, Laureys J, Dooms L, Lesaffre E, Ravussin E (1995) Influence of age, sex, and insulin on osteoblast function: osteoblast dysfunction in diabetes mellitus. J Clin Endocrinol Metab 80(4):1194–1202. doi:10.1210/jcem.80.4.7714089
Colombo JS, Balani D, Sloan AJ, Crean SJ, Okazaki J, Waddington RJ (2011) Delayed osteoblast differentiation and altered inflammatory response around implants placed in incisor sockets of type 2 diabetic rats. Clin Oral Implants Res 22(6):578–586. doi:10.1111/j.1600-0501.2010.01992.x
Guan CC, Yan M, Jiang XQ, Zhang P, Zhang XL, Li J, Ye DX, Zhang FQ (2009) Sonic hedgehog alleviates the inhibitory effects of high glucose on the osteoblastic differentiation of bone marrow stromal cells. Bone 45(6):1146–1152. doi:10.1016/j.bone.2009.08.009
Lu H, Kraut D, Gerstenfeld LC, Graves DT (2003) Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144(1):346–352. doi:10.1210/en.2002-220072
Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, Pischon N, Trackman PC, Gerstenfeld L, Graves DT (2007) Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 40(2):345–353. doi:10.1016/j.bone.2006.09.011
Suh KS, Rhee SY, Jung WW, Kim NJ, Jang YP, Kim HJ, Kim MK, Choi YK, Kim YS (2013) Chrysanthemum zawadskii extract protects osteoblastic cells from highly reducing sugar-induced oxidative damage. Int J Mol Med 32(1):241–250. doi:10.3892/ijmm.2013.1371
Ma Y, Wang H (2012) PI3 K/Akt/FoxO: a novel participant in signal transduction in bone cells under mechanical stimulation. Cell Biol Int 36(10):923–926. doi:10.1042/CBI20120078
Weinberg E, Maymon T, Moses O, Weinreb M (2014) Streptozotocin-induced diabetes in rats diminishes the size of the osteoprogenitor pool in bone marrow. Diabetes Res Clin Pract 103(1):35–41. doi:10.1016/j.diabres.2013.11.015
Garrett IR, Boyce BF, Oreffo RO, Bonewald L, Poser J, Mundy GR (1990) Oxygen-derived free radicals stimulate osteoclastic bone resorption in rodent bone in vitro and in vivo. J Clin Investig 85(3):632–639. doi:10.1172/JCI114485
Fraser JH, Helfrich MH, Wallace HM, Ralston SH (1996) Hydrogen peroxide, but not superoxide, stimulates bone resorption in mouse calvariae. Bone 19(3):223–226
Fujii H, Hamada Y, Fukagawa M (2008) Bone formation in spontaneously diabetic Torii-newly established model of non-obese type 2 diabetes rats. Bone 42(2):372–379. doi:10.1016/j.bone.2007.10.007
Khan TS, Fraser L-A (2015) Type 1 diabetes and osteoporosis: from molecular pathways to bone phenotype. J Osteoporos 2015:174186. doi:10.1155/2015/174186
McCabe LR (2009) Switching fat from the periphery to bone marrow: why in Type I diabetes? Exp Rev Endocrinol Metab 4(3):203–207
Christopher MJ, Link DC (2008) Granulocyte colony-stimulating factor induces osteoblast apoptosis and inhibits osteoblast differentiation. J Bone Miner Res 23(11):1765–1774. doi:10.1359/JBMR.080612
Adil C, Aydin T, Taspinar O, Kiziltan H, Eris AH, Hocaoglu IT, Posul S, Kepekci M, Denizli E, Guler M (2015) Bone mineral density evaluation of patients with type 2 diabetes mellitus. J Phys Ther Sci 27(1):179–182. doi:10.1589/jpts.27.179
Fajardo RJ, Karim L, Calley VI, Bouxsein ML (2014) A review of rodent models of Type 2 diabetic skeletal fragility. J Bone Miner Res 29(5):1025–1040. doi:10.1002/jbmr.2210
Gomes PS, Fernandes MH (2011) Rodent models in bone-related research: the relevance of calvarial defects in the assessment of bone regeneration strategies. Lab Anim 45(1):14–24. doi:10.1258/la.2010.010085
Kesavulu MM, Giri R, Kameswara Rao B, Apparao C (2000) Lipid peroxidation and antioxidant enzyme levels in type 2 diabetics with microvascular complications. Diabetes Metab 26(5):387–392
Kumawat M, Sharma TK, Singh I, Singh N, Ghalaut VS, Vardey SK, Shankar V (2013) Antioxidant enzymes and lipid peroxidation in type 2 diabetes mellitus patients with and without nephropathy. N Am J Med Sci 5(3):213–219. doi:10.4103/1947-2714.109193
Bandeira SDM, Guedes GDS, da Fonseca LJS, Pires AS, Gelain DP, Moreira JCF, Rebelo LA, Vasconcelos SML, Goulart MOF (2012) Characterization of blood oxidative stress in type 2 diabetes mellitus patients: increase in lipid peroxidation and SOD activity. Oxid Med Cell Longev 2012:13. doi:10.1155/2012/819310
Shyng YC, Devlin H, Sloan P (2001) The effect of streptozotocin-induced experimental diabetes mellitus on calvarial defect healing and bone turnover in the rat. Int J Oral Maxillofac Surg 30(1):70–74. doi:10.1054/ijom.2000.0004
Giglio MJ, Giannunzio G, Olmedo D, Guglielmotti MB (2000) Histomorphometric study of bone healing around laminar implants in experimental diabetes. Implant Dent 9(2):143–149
Li X, Ma XY, Feng YF, Ma ZS, Wang J, Ma TC, Qi W, Lei W, Wang L (2015) Osseointegration of chitosan coated porous titanium alloy implant by reactive oxygen species-mediated activation of the PI3 K/AKT pathway under diabetic conditions. Biomaterials 36:44–54. doi:10.1016/j.biomaterials.2014.09.012
Koerdt S, Siebers J, Bloch W, Ristow O, Kuebler AC, Reuther T (2014) Role of oxidative and nitrosative stress in autogenous bone grafts to the mandible using guided bone regeneration and a deproteinized bovine bone material. J Cranio Maxillo Fac Surg 42(5):560–567. doi:10.1016/j.jcms.2013.07.027
Pradeep AR, Rao NS, Bajaj P, Kumari M (2013) Efficacy of subgingivally delivered simvastatin in the treatment of patients with type 2 diabetes and chronic periodontitis: a randomized double-masked controlled clinical trial. J Periodontol 84(1):24–31. doi:10.1902/jop.2012.110721
Yilmaz MI, Baykal Y, Kilic M, Sonmez A, Bulucu F, Aydin A, Sayal A, Kocar IH (2004) Effects of statins on oxidative stress. Biol Trace Elem Res 98(2):119–127. doi:10.1385/BTER:98:2:119
Acknowledgements
The authors are grateful to Prof. Réne Rizzoli, Prof. Olivier Bruyère, and Dr. Véronique Rabenda for their insightful comments and helpful suggestions for improvement of the manuscript.
Author information
Authors and Affiliations
Contributions
MB, BB, and FL designed the study. FL is the guarantor. MB, BB, and FL performed the literature search and collected the data. MB, BB, AA, ER, RR, and FL analysed and interpreted the data. AA is responsible for statistical analysis of the data. MB and FL drafted this manuscript. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and ensure that any questions relating to the accuracy and integrity of the study are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflicts of interest
Miljana Bacevic, Bozidar Brkovic, Adelin Albert, Eric Rompen, Regis P. Radermecker, and France Lambert declare that they have no conflicts of interest. No external funding was available for this study, except for the internal support of the authors’ institution.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bacevic, M., Brkovic, B., Albert, A. et al. Does Oxidative Stress Play a Role in Altered Characteristics of Diabetic Bone? A Systematic Review. Calcif Tissue Int 101, 553–563 (2017). https://doi.org/10.1007/s00223-017-0327-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0327-7