Abstract
A case series of six women with postmenopausal osteoporosis who had received continuous denosumab for 7 years and were then given a single infusion of zoledronate (5 mg) is reported. During denosumab treatment, bone mineral density (BMD) in the spine increased 18.5% (P = 0.006), and total hip BMD by 6.9% (P = 0.03). Post-zoledronate BMDs were measured 18–23 months after treatment, and there were significant declines at each site (P spine = 0.043, P hip = 0.005). Spine BMD remained significantly above the pre-denosumab baseline (+9.3%, P = 0.003), but hip BMD was not significantly different from baseline (−2.9%). At the time of post-zoledronate BMD measurements, serum PINP levels were between 39 and 60 μg/L (mean 52 μg/L), suggesting that the zoledronate treatment had not adequately inhibited bone turnover. It is concluded that this regimen of zoledronate administration is not adequate to preserve the BMD gains that result from long-term denosumab treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Denosumab is a monoclonal antibody directed against the protein RANK-L, the principal regulator of osteoclast development. Thus, it acts as a potent anti-resorptive agent and is now widely used in the treatment of osteoporosis. Circulating denosumab levels fall rapidly following treatment discontinuation, and this is followed by substantial increases in bone turnover markers to well above baseline levels, bone resorption reaching twice baseline levels for about 6 months [1, 2]. Over the first 12 months off therapy, all the bone density gained on treatment is lost [1], and bone mineral densities (BMDs) significantly below pre-treatment values have been reported a year after discontinuation of long-term denosumab [3]. Recent case reports have suggested that, in some patients, this rapid bone loss is associated with the occurrence of multiple vertebral fractures [4,5,6,7,8].
To counter this rapid offset of anti-fracture efficacy, transitioning patients from denosumab to other anti-resorptives has been suggested, and alendronate has shown some benefits in this context [9]. The present report addresses whether zoledronate might also be effective, by reporting BMD changes in patients who had been on long-term denosumab and were transitioned to zoledronate at the time of denosumab discontinuation.
Methods
This is a case series of patients involved in the FREEDOM study [10], the phase 3 trial which led to the registration of denosumab for osteoporosis treatment. At the end of the 3-year trial, all patients were offered open-label denosumab, and this was continued in some until 10 years from study initiation. Our center recruited 28 patients into FREEDOM at baseline. 26 completed the core study, and 19 continued on open-label denosumab. Twelve patients took denosumab until study conclusion, two who received active drug during the core trial and the rest from the original placebo group. The latter patients had, thus, received 7 years of denosumab treatment. At the close-out of the FREEDOM extension, 6 months after the last injection of denosumab, each patient met with a physician and a decision was made regarding ongoing treatment. Eight patients decided to have an infusion of zoledronate at this time, and this report presents follow-up data on six of these women. Follow-up bone density measurements were not available on the other two: one died, and a second developed dementia, so post-zoledronate scans were not carried out in those individuals. The women described here were treatment-naïve at the time of entry to FREEDOM.
BMD measurements of the lumbar spine and left total hip used a Prodigy dual-energy, X-ray absorptiometer (GE-Lunar, Madison, WI, USA). The same densitometer was used throughout the entire study period. Procollagen-I N-terminal propeptide (PINP) was measured using an electrochemiluminescence method (E170, Roche Diagnostics, Mannheim, Germany). The inter-assay coefficient of variation was 5%.
Data (absolute and percent change from baseline) were analyzed using a mixed models approach to repeated measures with maximum likelihood estimation assuming an unstructured covariance matrix. Least squares means and confidence intervals are shown. For convenience, results from the percent change from baseline analysis are shown. P < 0.05 was considered significant, protected in post hoc comparisons using Tukey’s method. SAS v 9.4 (Cary, NC, USA) was used.
Results
The six patients presented here were all randomized to placebo in the core FREEDOM study, so had received 7 years of continuous denosumab at the time they received a single infusion of zoledronate 5 mg. Their clinical characteristics are shown in Table 1. One patient was taking tamoxifen at the time of this assessment (for management of breast cancer, started 6 years into her denosumab treatment course) but they were otherwise not receiving bone-active medications nor suffering from major systemic illnesses other than osteoporosis.
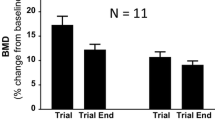
BMD changes from the time that denosumab was initiated are shown in Fig. 1. Spine BMD increased 18.5% after 7 years on denosumab (P = 0.006), and total hip by 6.9% (P = 0.03). Post-zoledronate BMDs were measured 18–23 months after treatment (all shown as “9 years” in Fig. 1), and there were significant declines at each site (P spine = 0.043, P hip = 0.005). Spine BMD remained significantly above the pre-denosumab baseline (P = 0.003), but hip did not. At the time of post-zoledronate BMD measurements, serum PINP levels were between 39 and 60 μg/L (mean 52 μg/L).
Effects of denosumab followed by zoledronate on BMD in postmenopausal women with osteoporosis. Changes in BMD from the time that denosumab was initiated are shown as mean and 95% confidence intervals. Denosumab was administered, 60 mg every 6 months, from 0 to 7 years, then a single infusion of zoledronate 5 mg, given at year 7. BMDs were measured 18–23 months after zoledronate treatment. No osteoporosis treatments were given between years 7 and 9
Discussion
These patients demonstrate the substantial gains in BMD that follow the long-term use of denosumab, which increase by 18.5 and 6.9% at the spine and hip, respectively, after 7 years treatment. These changes are very similar to those observed after this duration of denosumab use in the extension of the FREEDOM study [11]. The present data also demonstrate the rapid loss of BMD that occurs after denosumab discontinuation, and indicate that this loss is not prevented by a single dose of zoledronate.
Bone et al. have reported follow-up in 256 osteopenic, postmenopausal women who were treated with denosumab 60 mg every 6 months for 24 months, and then followed off-treatment for a further 24 months [1]. During treatment, spine BMD increased more than 6%, but all of this gain was lost in 12 months off-treatment. In the total hip, the increase was 3½% on treatment, again completely lost during the following 12 months. Popp reported rapid bone loss in nine women coming off therapy after 10 years of denosumab use. During denosumab, hip BMD increased 8.2%, but declined by 12.5% in the first year off-treatment, resulting in hip BMDs 5.4% below baseline [3]. Thus, there is a contrast in the outcome in the present study at the spine, where only half of the gain resolved over almost 2 years of follow-up, but loss in the hip appears to be unaffected by this zoledronate dosing regimen.
Whether more intensive dosing with zoledronate (e.g., annually) would be more effective remains to be explored. We chose not to repeat the zoledronate dose at 12 months because of evidence that bone resorption is suppressed by zoledronate for much more than 1 year, indeed for at least 5 years [12, 13]. However, following denosumab withdrawal, the anti-resorptive efficacy of zoledronate appears to be less adequate, judging from the serum PINP levels measured in the present patients. In treatment-naive osteopenic postmenopausal women, PINP levels 18–24 months after a single infusion of zoledronate are about half the values found in these in the present patients (mean 27 μg/L [14], compared with 52 μg/L in the present patients).
In contrast to the failure of zoledronate to maintain the BMD increases produced by 7 years of denosumab in the present study, alendronate did completely prevent post-denosumab bone loss after 1 year’s treatment with denosumab [9]. This might be attributable to the shorter duration of denosumab treatment in that study, resulting in smaller increases in BMD, or it might result from differences between oral and intravenous bisphosphonates. Following denosumab treatment, bone turnover is substantially reduced, so it would be expected that bisphosphonate uptake to bone surfaces would also be low at that time. Therefore, a single dose of intravenous bisphosphonate might be much less effective than it would be in treatment-naive patients. In contrast, oral bisphosphonates are administered every week, so while the initial uptake of bisphosphonate might be low, as the denosumab-induced reduction in bone turnover diminishes over time there will be a resultant increase in skeletal uptake of oral bisphosphonates leading to control of bone turnover and stabilization of BMD. These considerations suggest that oral agents might be preferable in this context, or that if intravenous agents are to be used, their administration should be delayed until bone turnover markers have risen into the normal range. PINP was not measured at the time of zoledronate administration, but is highly likely to have been suppressed, based on published data at month 6 after the last denosumab injection in long-term users [11].
It is concluded that a single infusion of zoledronate 6 months after the last dose of denosumab is not adequate to preserve BMD gains over the first 2 years following discontinuation of long-term denosumab treatment.
References
Bone HG, Bolognese MA, Yuen CK et al (2011) Effects of denosumab treatment and discontinuation on bone mineral density and bone turnover markers in postmenopausal women with low bone mass. J Clin Endocrinol Metab 96:972–980
Miller PD, Bolognese MA, Lewiecki EM et al (2008) Effect of denosumab on bone density and turnover in postmenopausal women with low bone mass after long-term continued, discontinued, and restarting of therapy: a randomized blinded phase 2 clinical trial. Bone 43:222–229
Popp AW, Buffat H, Senn C et al (2016) Rebound-associated bone loss after non-renewal of long-term denosumab treatment offsets 10-year gains at the total hip within 12 months. J Bone Miner Res 31(suppl):S408
Popp AW, Zysset PK, Lippuner K (2016) Rebound-associated vertebral fractures after discontinuation of denosumab—from clinic and biomechanics. Osteoporos Int 27:1917–1921
Aubry-Rozier B, Gonzalez-Rodriguez E, Stoll D et al (2016) Severe spontaneous vertebral fractures after denosumab discontinuation: three case reports. Osteoporos Int 27:1923–1925
Anastasilakis AD, Polyzos SA, Makras P et al (2017) Clinical features of 24 patients with rebound-associated vertebral fractures following denosumab discontinuation: systematic review and additional cases. J Bone Miner Res. doi:10.1002/jbmr.3110
Anastasilakis AD, Makras P (2016) Multiple clinical vertebral fractures following denosumab discontinuation. Osteoporos Int 27:1929–1930
Lamy O, Gonzalez-Rodriguez E, Stoll D et al (2017) Severe rebound-associated vertebral fractures after denosumab discontinuation: 9 clinical cases report. J Clin Endocrinol Metab 102:354–358
Freemantle N, Satram-Hoang S, Tang ET et al (2012) Final results of the DAPS (denosumab adherence preference satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int 23:317–326
Cummings SR, San Martin J, McClung MR et al (2009) Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 361:756–765
Papapoulos S, Lippuner K, Roux C et al (2015) The effect of 8 or 5 years of denosumab treatment in postmenopausal women with osteoporosis: results from the FREEDOM Extension study. Osteoporos Int 26:2773–2783
Grey A, Bolland MJ, Horne A et al (2012) Five years of anti-resorptive activity after a single dose of zoledronate—results from a randomized double-blind placebo-controlled trial. Bone 50:1389–1393
McClung M, Miller P, Recknor C et al (2009) Zoledronic acid for the prevention of bone loss in postmenopausal women with low bone mass a randomized controlled trial. Obstet Gynecol 114:999–1007
Grey A, Bolland M, Wattie D et al (2010) Prolonged antiresorptive activity of zoledronate: a randomized, controlled trial. J Bone Miner Res 25:2251–2255
Acknowledgement
This study was supported by the Health Research Council of New Zealand.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Ian R. Reid has received research grants and honoraria from Amgen, Novartis, Merck, and Lilly. Anne M. Horne, Borislav Mihov, and Gregory D.Gambie have nothing to declare.
Human and Animal Rights and Informed Consent
The FREEDOM study and its extensions were approved by our regional ethics committee.
Rights and permissions
About this article
Cite this article
Reid, I.R., Horne, A.M., Mihov, B. et al. Bone Loss After Denosumab: Only Partial Protection with Zoledronate. Calcif Tissue Int 101, 371–374 (2017). https://doi.org/10.1007/s00223-017-0288-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0288-x