Abstract
Elucidation of the molecular mechanisms that regulate the differentiation of adipose-derived mesenchymal stem cells into osteogenic cells may lead to new methods for bone tissue engineering. We examined the role of miR-26a-5p in the regulation of osteogenic differentiation of mouse adipose-derived mesenchymal stem cells (mADSCs) by using mimics and inhibitors of this microRNA. Our results showed that over-expression of miR-26a-5p inhibited osteogenesis and that suppression of endogenous miR-26a-5p promoted osteogenesis. Four bioinformatics algorithms indicated that the 3′UTR of Wnt5a was a potential target of miR-26a-5p. We confirmed this prediction by use of dual-luciferase reporter assay and GFP/RFP assay. We also examined the molecular mechanisms by which miR-26a-5p regulates osteogenesis. Fura-2AM and Western blot assays after transfection indicated that miR-26a-5p repressed WNT5A, inhibited calcium flux and protein kinase C, and suppressed osteogenic differentiation of mADSCs. By contrast, miR-26a-5p inhibition activated these signal proteins and promoted osteogenic differentiation. Taken together, our results suggest that up-regulation of miR-26a-5p inhibits osteogenic differentiation of mADSCs by directly targeting the 3′UTR of Wnt5a, thereby down-regulating the Wnt/Ca2+ signaling pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is an increasing focus on bone tissue engineering research because of the growing prevalence of bone-related diseases [1, 2]. Adipose-derived mesenchymal stem cells (ADSCs) are considered candidate seed cells for bone regeneration [3]. In recent years, there has also been increasing attention to the promotion of osteogenesis in adipose-derived stem cells. Osteogenic differentiation of mesenchymal stem cells (MSCs) is a complex process that involves many bioactive factors and signaling pathways, making study of this process more challenging [4–6].
MicroRNAs (miRNAs) are small non-coding endogenous RNAs, typically with 19–25 nucleotides, that bind to the 3′UTR of target mRNAs, alter mRNA stability or protein translation, and whereby regulate various cell signaling pathways [2, 7]. Although there is increasing interest in the effects of miRNAs on the osteogenesis of MSCs [8–11], research on the role of miR-26a in this process is limited. A review of the literature indicated there were approximately 274 studies of miR-26a, but only 8 of these studies examined its role in the osteogenic differentiation of MSCs [12–16]. Among these eight studies, Luzi et al. [12, 14] concluded that miR-26a was a negative regulator for terminal differentiation of hADSCs because it reduced the availability of active SMAD1. However, Trompeter et al. [13] found that miR-26a regulated osteo-inhibitory and osteo-promoting factors in parallel, but the osteo-inhibitory effects of CDK6 and HDAC4 likely outweighed the osteo-promoting effects of SMAD1. Thus, they concluded that miR-26a mainly promoted the osteogenic differentiation of unrestricted somatic stem cells (USSCs) [13]. Taken together, these results suggest the specific function and molecular mechanism of miR-26a are uncertain during the osteogenic differentiation of MSCs.
Previous studies have demonstrated that miR-26a can regulate osteogenic differentiation of MSCs through the BMP/SMAD1 signaling pathway. However, recent research indicated that miR-26a regulates osteogenic differentiation via inhibition of GSK-3β, a repressor of the Wnt canonical pathway [17]. The Wnt signaling pathways include the β-catenin-dependent canonical pathway and 2 β-catenin-independent non-canonical pathways (the planar cell polarity [PCP] pathway and the Wnt/calcium pathway) [18, 19]. There has been more focus on the canonical β-catenin-dependent pathway in osteogenetic differentiation [20, 21], but there is recent interest in the effect of non-canonical Wnt signaling on osteogenetic differentiation [22, 23]. Our previous study demonstrated that miR-154-5p regulated the Wnt non-canonical PCP pathway by targeting Wnt11 under tensile stress, and this led to suppression of osteogenesis [24]. WNT5A is another activator of non-canonical Wnt signaling. Santos et al. [25] confirmed that WNT5A activated non-canonical Wnt signaling via rho-associated kinase (ROCK) and thereby inducing osteogenic differentiation of human adipose stem cells. Furthermore, it is well known that WNT5A can activate the non-canonical Wnt/Ca2+ pathway by promoting calcium influx, and this signaling pathway may be related to osteogenesis [18]. Although Chakravorty et al. [26] reported that miR-26a is a potential regulator of the WNT5A gene during the differentiation of human osteoprogenitor cells, the specific mechanism is unclear and no available research supports this hypothesis at present. Based on our preliminary bioinformatics analysis, we predicted that Wnt5a is a potential target of miR-26a-5p. This finding and the research mentioned above provides the basis for our hypothesis that miR-26a-5p regulates osteogenic differentiation via non-canonical Wnt signaling.
In this study, we identified miR-26a-5p as a potential regulator of osteogenesis. By modulating miR-26a-5p activity, we demonstrated that over-expression of miR-26a-5p inhibited osteogenic differentiation, whereas suppression of miR-26a-5p promoted differentiation. The dual-luciferase reporter assay and our further gain- or loss-of-function experiments confirmed that miR-26a-5p interacted with the 3′UTR of Wnt5a and negatively modulated the expression of genes that have important roles in osteogenesis. Our findings suggest that an increased level of miR-26a-5p inhibits the expression of WNT5A, suppresses the Wnt/Ca2+ signaling pathway, and inhibits the differentiation of mADSCs into osteoblasts.
Materials and Methods
Culture and Harvest of mADSCs
Six-week-old male C57BL/6 mice obtained from the Sichuan University Animal Experimental Center were used as the source of mADSCs, and all procedures were in accordance with the guidelines of the Animal Research Committee of Sichuan University. The inguinal fat pads of the mice were isolated as previously described [24], soaked in PBS with 1 % penicillin/streptomycin, and transferred into a sterile clean bench. The tissue was washed 3 times with PBS, minced as small as possible, and then digested by 0.2 % collagenase type I in a constant-temperature water bath (37 °C) on a shaker for 50 min. Following centrifugation at 1000 rpm for 8 min, mADSCs were resuspended in 3 mL of regular growth medium consisting of α-MEM (HyClone Laboratories Inc., Logan, UT), 10 % FBS (Gibco BRL, Grand Island, NY), penicillin (100 U/mL), and streptomycin (100 µg/mL) (Sigma-Aldrich, St. Louis, MO), and cultured in a 50 mL flask at 37 °C in a humidified incubator with 5 % CO2. The medium was refreshed every 2 days, and cells in the third passage were used for experiments. Our previous flow cytometry analysis of cells obtained by this method showed that the isolated mADSCs had high purity based on positivity for CD29, Sca-1, and CD105, and negativity for hematopoietic stem cell markers (CD45 and CD31) [24].
Induction of Osteogenic Differentiation In Vitro
To induce osteogenic differentiation, the medium was replaced with osteoblast-inducing conditional α-MEM consisting of 10 % FBS, 10 mM β-glycerophosphate, 1 × 10−8 M dexamethasone, 50 μg/mL l-ascorbic acid (Sigma-Aldrich, St. Louis, MO), 0.01 µM 1,25-dihydroxyvitamin D3, 100 U/mL penicillin, and 100 µg/mL streptomycin. The extent of differentiation was assayed by alkaline phosphatase (ALP) staining at 7 days after induction and the mineralization of the matrix was evaluated by Alizarin Red staining at 14 days after induction. The expressions of osteoblast marker genes (Alp and Ocn) were also monitored by qRT-PCR at 0, 3, 7, and 14 days after induction. Cells were visualized using bright-field optics on a microscope and the images were captured using a camera.
Construction of Lentivirus Vectors and Transfection
GFP-labeled lentivirus vectors containing miR-26a-5p, miR-NC, antimiR-26a-5p, and antimiR-NC were obtained from Saier Biotechnology (Tianjin, China). The primers are listed in Table 1. Cells were seeded in 6-well plates (4 × 105 cells/well) before transfection. Transfection was conducted in Opti-MEM with 8 mg/mL of polybrene for 24 h. Each lentivirus was added to reach a multiplicity of infection (MOI) of 50. After transfection, the medium was changed into induction medium for the study of differentiation or into regular growth medium for other experiments.
Bioinformatics Analysis
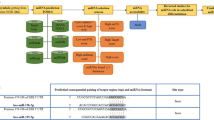
A search for predicted target mRNAs was performed using four databases: TargetScan (http://www.targetscan.org), microRNA.org (http://www.microrna.org), RNA22 (https://cm.jefferson.edu/rna22v2/), and DIANAMicroT (http://diana.cslab.ece.ntua.gr/microT/).
Luciferase Reporter and Luciferase Assays
Reporter constructs which contain a miR-26a-5p-binding site (pmirGLO-Wnt5a-WT) or a mismatch sequence (pmirGLO-Wnt5a-Mu) in the 3′UTR of Wnt5a mRNA were synthesized by Saier Biotechnology (Tianjin, China). Table 1 shows the primer sequences for Wnt5a 3′UTR-wild type (WT) and Wnt5a 3′UTR-mutation (Mu).
Each recombinational vector, with 100 ng pmirGLO and 200 nmol/L miR-26a-5p mimics, miR-26a-5p inhibitors or their negative controls, was transfected into 293T cells using the Lipofectamine 2000 reagent (Invitrogen, USA) as previously reported [24]. Cells were harvested at 24 h after transfection and assayed for Renilla and Firefly luciferase activity using the Dual-Luciferase Reporter Gene Assay Kit (Beyotime, Shanghai, China).
GFP/RFP Reporter Assay
Cells were inoculated onto 24-well plates with growth medium 1 day before transfection. When they were at 60–80 % confluence, the medium was replaced with 5 μg/mL polybrene. GFP/RFP/Wnt5a-UTR (GFP: internal reference, RFP: reporter) was co-transfected with miR-26a-5p, antimiR-26a-5p, or their negative controls at an MOI of 10. After 72 h, the medium was again switched to complete medium without polybrene and cells were incubated for another 72 h at 37 °C in an atmosphere of 5 % CO2. The expression of GFP and RFP were observed by fluorescence microscopy. The constructed sequences of Wnt5a 3′UTR are listed in Table 1.
Quantitative Real-Time PCR (qRT-PCR) Analysis
The expression of miR-26a-5p and the mRNA levels of Wnt5a, Ocn, Opn, Col1, Runx2, Alp, and Osx were determined by qRT-PCR. For these experiments, total RNA was extracted from cells with Trizol (Invitrogen, Carlsbad, CA), cDNA was prepared, and qPCR was performed in triplicate as previously described [19]. The relative expression of mRNA or miRNA was evaluated by 2−△△Ct method and normalized to that of GAPDH or U6. Table 2 shows the primers that were used.
Western Blotting Analysis
The protein levels of WNT5A, CaMKII, COL1, OPN, ALP, and β-catenin were determined by Western blotting. Protein lysates were generated using a radio-immunoprecipitation assay lysis buffer and the protein concentration was determined using the BCA protein assay kit (Beyotime, Jiangsu, China). Protein (32 μl) from each sample was subjected to 10 % SDS–PAGE, after which proteins were transferred to a PVDF membrane (Millipore, USA). The membrane was blocked with a BLOTTO solution and then incubated with anti-WNT5A, anti-OPN, anti-COL1, anti-CaMKII, anti-ALP, or anti-β-catenin at 4 °C overnight. The membrane was then re-probed with appropriate secondary antibodies conjugated with horseradish peroxidase for 2 h (Santa Cruz Biotechnology Inc., Santa Cruz, CA). Blots were processed using Western Lightning®-ECL, enhanced chemiluminescence substrate (PerkinElmer, USA), and exposed to film. The results were analyzed using the Quantity One system (Bio-Rad, Hercules, CA).
Assays for Protein Kinase C Activation and Quantitative ALP
Measurement of protein kinase C (PKC) activation was performed using the PepTag® Non-Radioactive PKC Assay kit (Promega, USA) and spectrophotometry was used to quantitate kinase activity. To measure intracellular ALP, washed cells (1 × 105) were homogenized in the assay buffer and centrifuged at 13,000×g for 3 min to remove insoluble material. Then, 10 μL was added into each well of a 96-well plate and the total volume was brought to 80 μL by addition of assay buffer. ALP was then measured using the Alkaline Phosphatase Assay Kit (Biovision, Milpitas, CA).
Alkaline Phosphatase (ALP) Staining and Alizarin Red Staining (ARS)
After induction, transfected control and transfected mADSCs were prepared for ARS/ALP staining. ALP staining was performed with the BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, Shanghai, China). For Alizarin Red staining, the solution was prepared with Tris–HCl at pH 4.2. The mADSCs were first washed with PBS and fixed in 4 % paraformaldehyde for 30 min. Then, fixed cells were incubated with the staining solution for 6–10 min. Finally, the cells were washed thoroughly with distilled water before visualization.
Fura-2AM Assay
After washing cells with D-Hanks balanced salt solution (D-HBSS), cells were incubated for 45 min in a Fura-2AM working solution in darkness. Then, the Fura-2AM working solution was removed and D-HBSS was used to wash cells. Cells were incubated for another 20 min in D-HBSS to assure thorough de-esterification of the AM group. Fluorescence was measured at 380 nm (Fura-2) and 340 nm (Ca-Fura-2).
Statistical Analysis
All data are expressed as means ± SDs and all experiments were performed at least three times. Statistical analysis was performed by SPSS Statistics 20.0 software, using Student’s two-tailed t test or a one-way ANOVA. Statistical values were illustrated using GraphPad PRISM, version 6.05. A p value of 0.05 was considered statistically significant.
Results
Osteogenic Differentiation of mADSCs
Our initial experiments demonstrated that mADSCs turned to osteogenic differentiation when cultured in the differentiation-inducing medium, as indicated by cell morphology (Fig. 1a) and ALP and Alizarin Red staining (Fig. 1b, c). Moreover, during differentiation, we measured the expression of two osteoblast marker genes (Alp and Ocn) at different times after induction. The results show that relative to day 0, Alp expression was elevated at days 3, 7, and 14 (Fig. 1d) and Ocn expression was elevated at days 7 and 14 (p < 0.05 for all comparisons) (Fig. 1e).
Changes in cell morphology and gene expression during osteogenic differentiation of mADSCs. a Morphology of undifferentiated mADSCs (left) and differentiated mADSCs (right) induced by osteo-differentiation-inducing medium. b Osteogenic differentiation was determined by ALP staining (top) at day 7 and Alizarin Red staining (bottom) at day 14. c Calcium nodes in Alizarin Red-stained cells after osteogenic induction. d, e qRT-PCR analysis showed increased expression of osteogenesis-specific genes (Alp and Ocn) during osteogenic differentiation. Expression was normalized to GAPDH and presented as means ± SDs (n = 3; *p < 0.05 compared with day 0)
Expression of miR-26a-5p and Wnt5a During Osteogenic Differentiation of mADSCs
Our qPCR experiments showed that endogenous miR-26a-5p decreased gradually during cell differentiation; the level was significantly lower at day 7 and continued to decrease with as differentiation progressed. Compared with the undifferentiated control group, the expression of miR-26a-5p was more than 50 % lower at day 14 (p < 0.05) (Fig. 2a). The expression of Wnt5a during differentiation had the opposite pattern. In particular, the level of Wnt5a was significantly higher at day 7, and this elevation remained at days 10 and 14 (Fig. 2b). The expression of WNT5A protein (Fig. 2c) was similar to that of Wnt5a mRNA.
Changes in expression of miR-26a-5p and WNT5A during osteogenic differentiation of mADSCs. a The endogenous expression of miR-26a-5p was measured by qRT-PCR at 0, 3, 7, 10, and 14 days after induction of osteogenesis. Expression was normalized to U6 and presented as means ± SDs (n = 3; *p < 0.05 compared with day 0). b Expression of Wnt5a mRNA was measured by qRT-PCR at various times after induction of osteogenesis. Expression was normalized to GAPDH and presented as means ± SDs (n = 3; *p < 0.05 compared with day 0). c Western blotting of WNT5A during osteogenesis and quantifications of these results. Expression was normalized to GAPDH and presented as means ± SDs (n = 3; *p < 0.05 compared with day 0). d Bioinformatics analyses of the complementarity of the miR-26a-5p seed sequence to the 3′UTR of Wnt5a and conservation of the putative-binding site in vertebrates
We examined the relationship between miR-26a-5p and Wnt5a by using four different miRNA target prediction databases to assess their potential interaction. All four of the computational algorithms indicated that miRNA-26a-5p targets the 3′UTR of Wnt5a. Moreover, the miR-26a-5p target site in the 3′UTR of Wnt5a is highly conserved among vertebrates (Fig. 2d). These results indicate that miR-26a-5p and WNT5A play important roles in regulating osteogenic differentiation of mADSCs and that there is a direct relationship between these molecules.
Effect of miR-26a-5p on Osteoblast Differentiation of mADSCs
We transfected mADSCs with four different lentiviruses (miR-NC, miR-26a-5p, antimiR-NC, and antimiR-26a-5p) to over-express or inhibit miR-26a-5p, and then measured the expression of GFP by fluorescence microscopy to evaluate the efficiency of transfection. The results indicate successful transfection of 80–90 % of cells (Fig. 3a). We also measured the expression of miR-26a-5p in lentivirus-modified mADSCs by qRT-PCR during differentiation. Mature miR-26a-5p levels were elevated ∼4.5-fold in the miR-26a-5p group compared with the miR-NC group (p < 0.05), and remained over-expressed up to day 12 of differentiation. By comparison, the level of miR-26a-5p in the antimiR-26a-5p group was reduced by ∼fivefold relative to the negative control (p < 0.05), and this inhibition continued over time (Fig. 3b). Thus, miR-26a-5p had a sustained and stable over-expression in the miR-26a-5p group during differentiation, but the expression of miR-26a-5p was repressed at all times in the antimiR-26a-5p group.
Effect of up-regulation and down-regulation of miR-26a-5p on osteogenic differentiation of mADSCs. a Transfection efficiency of miRNA was estimated as 80–90 % based on GFP expression in mADSCs observed by fluorescence microscopy. b Expression of miR-26a-5p in mADSCs infected with miR-26a-5p (left) or antimiR-26a-5p (right) was assayed by qRT-PCR at 0, 5, and 12 days after induction. Expression was normalized to U6 and presented as means ± SDs (n = 3; *p < 0.05 compared with the negative control). c Expression of osteoblast marker genes (Ocn, Opn, Col1, Runx2, Alp, and Osx) determined by qRT-PCR at 10 days after transfection of mADSCs with miR-26a-5p or antimiR-26a-5p. Expression was normalized to GAPDH and presented as means ± SDs (n = 3; *p < 0.05 compared with the negative control). d Western blotting of ALP and OPN after transfection with miR-26a-5p mimics or an inhibitor. Expression was normalized to GAPDH, and one of three independent experiments is shown. e Histological staining for ALP (top) at day 7 and Alizarin Red (bottom) at day 14 after transfection with miR-26a-5p mimics or inhibitor
We also used qRT-PCR to monitor the expression of osteogenesis-specific genes (Ocn, Opn, Col1, Runx2, Alp, and Osx) in mADSCs transfected with miR-NC, miR-26a-5p, antimiR-NC, or antimiR-26a-5p. Relative to the negative controls, transfection with miR-26a-5p reduced the expression of Ocn, Opn, Col1, Runx2, Alp, and Osx, whereas transfection with antimiR-26a-5p increased the expression of these genes (Fig. 3c). Western blot analysis showed similar changes in ALP and OPN expression (Fig. 3d). ALP and ARS staining showed significantly less cell differentiation in the miR-26a-5p group than the miR-NC group, but more differentiation in the antimiR-26a-5p group (Fig. 3e). These results show that over-expression of miR-26a-5p inhibited osteoblast differentiation, and therefore indicate that miR-26a-5p plays a negative role in the osteogenesis of mADSCs.
Direct Association of miRNA-26a-5p with Wnt5a 3′UTR
We examined the specific selectivity of miRNA-26a-5p for Wnt5a mRNA by use of a dual-luciferase reporter assay in 293T cells. The results show that the Renilla luciferase activity of pmirGLO-Wnt5a-WT transfected cells decreased by more than 60 % in miR-26a-5p co-transfected cells compared with miR-NC co-transfected cells or blank controls; however, expression increased more than 2.2-fold in antimiR-26a-5p co-transfected cells compared with negative or blank controls. In addition, co-transfection of lentiviruses and the mutant 3′UTR-binding site (pmirGLO-Wnt5a-Mu) had no effect on luciferase levels (Fig. 4a). These results indicated that the 3′UTR of Wnt5a was a target of miR-26a-5p.
Interaction of miR-26a-5p with Wnt5a 3′UTR and effect on expression of Wnt5a mRNA and protein. a pmirGLO-Wnt5a-WT or pmirGLO-Wnt5a-Mu reporter constructs were co-transfected with miR-26a-5p, miR-NC, antimiR-26a-5p, or antimiR-NC into 293T cells. Renilla luciferase activity was normalized to firefly luciferase. Data are presented as means ± SDs (n = 3; *p < 0.05 compared with negative control and blank control). b, d The GFP/RFP/Wnt5a-UTR reporter vector was co-transfected with miR-26a-5p or antimiR-26a-5p into mADSCs. GFP and RFP levels were measured by a fluorometer and RFP values were normalized to GFP. Data are presented as means ± SDs (n = 3; *p < 0.05 compared with separate negative controls). c Expression of Wnt5a mRNA (top, qRT-PCR) and WNT5A protein (bottom, Western blotting) after transfection with miR-26a-5p or antimiR-26a-5p. The expression was normalized to GAPDH and presented as means ± SDs (n = 3; *p < 0.05 compared with separate negative controls)
These results are in agreement with experiments used to detect whether miR-26a-5p binds to Wnt5a. In these experiments, we co-transfected GFP/RFP/Wnt5a-UTR with miR-26a-5p, antimiR-26a-5p, or their negative controls; GFP was the internal control and RFP was the reporter. Fluorescence microscopy indicated that red fluorescence was notably attenuated compared with green fluorescence in the group co-transfected with miR-26a-5p and GFP/RFP/Wnt5a-UTR, but red fluorescence was stronger in antimiR-26a-5p group. There were no obvious differences between the red and green fluorescence in the negative control groups after co-transfection. Further analysis showed that co-transfection with miR-26a-5p and GFP/RFP/Wnt5a-UTR decreased luciferase expression compared with the miR-NC group. Moreover, there was an increase of red fluorescence in the antimiR-26a-5p group compared with its negative control (Fig. 4b, d). These data further verify that miR-26a-5p binds to Wnt5a.
We also harvested protein and mRNA samples of mADSCs after transfection with miR-26a-5p mimics or inhibitors. The qRT-PCR results indicate a significant decrease in Wnt5a mRNA in cells transfected with miR-26a-5p compared with the negative control, but an increase in Wnt5a mRNA in the antimiR-26a-5p group. On the other hand, the Western blotting results show that WNT5A protein level was lower in miR-26a-5p group than in the miR-NC group, and higher in the antimiR-26a-5p group than in the negative control (Fig. 4c).
Taken together, these results suggest that miR-26a-5p underwent direct binding to the 3′UTR of Wnt5a and thereby affecting the expression of Wnt5a mRNA and WNT5A protein during the course of osteogenic differentiation.
miR-26a-5p Regulates Osteogenesis via Wnt5a/Ca2+ the Signaling Pathway
The results described above suggest that miR-26a-5p depresses osteogenic differentiation by directly inhibiting the expression of Wnt5a. WNT5A is a ligand that can activate the Wnt signaling pathway by binding to receptors on the cell surface. We examined the signaling pathway of osteogenesis that is regulated by miR-26a-5p by measuring changes of pivotal proteins in this signaling cascade. The results show that over-expression of miR-26a-5p down-regulated WNT5A protein compared with the negative control, and that this decrease accompanied a decrease in CaMKII protein (a crucial factor in the Wnt5a/Ca2+ signaling pathway) and COL1 protein (an osteogenesis-related marker protein). Interestingly, relative to controls, cells transfected with miR-26a-5p also had an increased level of β-catenin, which is associated with the canonical Wnt pathway (Fig. 5a).
Effect of miR-26a-5p on WNT5A and the non-canonical Wnt/Ca2+ pathway during osteogenic differentiation of mADSCs. a Western blotting of key proteins in the Wnt signaling pathway (WNT5A, CaMKII, and β-catenin) and a marker of osteogenesis (COL1) at 10 days after transfection with miR-26a-5p or antimiR-26a-5p. b, c Level of phosphorylated PKC and total PKC at 10 days after transfection with miR-26a-5p or antimiR-26a-5p. The level of phosphorylated PKC was normalized to total PKC and data are presented as means ± SDs (n = 3; *p < 0.05 compared with separate negative controls). d Alkaline phosphatase activity at 10 days after transfection with miR-26a-5p or antimiR-26a-5p. Activities are presented as means ± SDs (n = 3; *p < 0.05 compared with each negative control). e Calcium levels (measured by the Fura-2AM assay) at 10 days after transfection with miR-26a-5p or antimiR-26a-5p. The levels are presented as means ± SDs (n = 3; *p < 0.05 compared with each negative control)
An assay for PKC, another key factor in Wnt5a/Ca2+ signaling, indicated a 58 % reduction in the miR-26a-5p group and an increase in the antimiR-26a-5p group (Fig. 5b, c). A quantitative ALP assay showed that ALP activity declined by 33 % after transfection with miR-26a-5p, but increased by 1.3-fold after transfection with antimiR-26a-5p (Fig. 5d). Even more remarkable was that the levels of intracellular calcium, an important second messenger, decreased in the miR-26a-5p group but increased in the antimiR-26a-5p group (Fig. 5e). Thus, miR-26a-5p suppressed osteogenic differentiation of mADSCs via the Wnt5a/Ca2+ signaling pathway by reducing the expression of Wnt5a. In addition, suppression of Wnt5a/Ca2+ pathway was associated with an increase of β-catenin expression.
Discussion
ADSCs are a type of mesenchymal stem cells that are considered candidates for the generation of new bone tissue. Various physical, chemical, and biological factors can regulate the osteogenic differentiation potency of ADSCs [20, 27–29]. The results of the present study confirmed that our osteoblast-inducing medium can induce the osteogenic differentiation of mADSCs. Moreover, we assessed the exact function of miR-26a-5p during osteogenic differentiation of mADSCs by use of transfection experiments. These results showed that inhibition of miR-26a-5p function enhanced the differentiation of mADSCs, whereas miR-26a-5p over-expression inhibited the differentiation of mADSCs. Thus, miR-26a-5p is a negative regulator of osteogenic differentiation. This result is consistent with the results of Luzi et al. [12] but in conflict with those of Trompeter et al. [13]. The discrepancy may be due to the use of cells from different tissues and species, different conditions for induction of differentiation, different methods of gene transfection, and measurement of responses at different time points [17].
Our bioinformatics analysis indicated that the 3′UTR of Wnt5a had a perfect 7-nt match to the miR-26a-5p seed region. We also used dual-luciferase reporter assay and GFP/RFP assay to verify the predictions of the bioinformatics analysis and to study the molecular mechanism by which miR-26-5p regulates osteogenic differentiation of mADSCs. The results of these experiments strongly confirmed that the 3′UTR of Wnt5a was a direct target of miR-26-5p. There are two major non-canonical Wnt signaling pathways downstream of WNT5A: the PCP pathway and the Wnt/calcium pathway [18, 19]. Previous research indicated that WNT5A has a role in activation of the PCP pathway via the phosphorylation of Dsh and activation of RhoA, ROCK, and JNK [18, 30, 31]. Intracellular calcium is a pivotal second messenger for Wnt/calcium signaling, and WNT5A can influence calcium flux and whereby regulate PKC and CaMKII, key downstream effectors of calcium signaling [19, 32–34]. Recent studies have also implicated an important role for Wnt/calcium signaling in osteogenesis [22, 23, 35].
Although WNT5A plays a crucial role in activation of the non-canonical Wnt pathways [19, 23], the specific molecular mechanisms by which miR-26-5p regulates osteogenic differentiation through WNT5A was heretofore unclear. Thus, we examined the effects of transfection of mADSCs with miR-26-5p mimics or inhibitors to investigate the miR-26-5p signaling pathway. After transfection, we incubated mADSCs in osteoblast-inducing medium, and measured the concentrations of calcium and key signaling proteins. The data showed that over-expression of miR-26a-5p reduced the protein content of WNT5A, leading to a decrease of intracellular calcium, an important second messenger. In response, the phosphorylation of PKC and CaMKII was suppressed, leading to an attenuation of cell differentiation. The presence of reduced levels of osteoblast markers (COL1, OPN, and ALP) supports this interpretation.
The opposite outcome occurred when the cells were transfected with antimiR-26a-5p. Furthermore, a comparison of the effects of miR-26a-5p and antimiR-26a-5p on osteogenic differentiation indicated that the osteo-inhibitory effects of miR-26a-5p were much stronger than the osteo-promoting effects of antimiR-26a-5p. This may be attributed to the decreased expression of endogenous miR-26a-5p during the osteogenic differentiation of mADSCs.
Previous studies have shown that WNT5A-mediated activation of different pathways is associated with binding to distinct receptors on the cell surface. The binding of WNT5A to frizzled receptor 3 (Fz3) and the tyrosine kinase-like orphan receptor 2 (Ror2) leads to the activation of the non-canonical Wnt pathways [36]. Moreover, Ror2, by itself or in combination with Frizzled protein through its Frizzled-like cysteine-rich domain [37], mediates diverse Wnt5a signaling by activating the non-canonical Wnt pathway [38] and inhibiting the β-catenin-T cell factor/lymphoid enhancer factor pathway [39]. β-catenin is an important component of the canonical Wnt pathway, and recent research showed that Wnt5a suppressed canonical Wnt signaling during cell differentiation [36]. Moreover, Liu et al. [22] demonstrated that down-regulation of β-catenin leads to activation of the non-canonical Wnt/Ca2+ pathway, resulting in the promotion of osteogenic differentiation in periodontal stem cells from chronic periodontitis patients (P-PDLSCs). Consistent with these findings, our study also indicated down-regulation of β-catenin during enhanced Wnt/Ca2+ pathway signaling during the inhibition of miR-26a-5p and osteogenic differentiation of mADSCs. The opposite effect occurred in the miR-26a-5p group. This may indicate an interaction between the non-canonical and canonical Wnt pathways.
Based on previous studies, we can hypothesize that the activation of the non-canonical Wnt pathway could restrain the canonical Wnt pathway by several possible mechanisms [19, 22]. Further study is required to determine whether the Wnt canonical and non-canonical pathways interact by one of these mechanisms or by some other mechanism. Moreover, the nature of the interactions between the two Wnt non-canonical pathways should also be investigated. Based on the foundation provided by the present study and our previous findings, we will examine the cross-talk between the Wnt/Ca2+ pathway, which is regulated by miR-26a-5p, and the Wnt/PCP pathway, which is regulated by miR-154-5p, during the osteogenic differentiation of ADSCs.
Conclusion
Previous research indicated that miR-26a, WNT5A, and the Wnt/Ca2+ pathway have roles in the osteogenic differentiation of ADSCs. The present study is the first to determine the nature of the inter-relationships of these molecules during osteogenesis (Fig. 6). Reducing the endogenous level of miR-26a-5p led to up-regulation of WNT5A, and thereby increasing the level of intracellular calcium, PKC, and CaMKII, leading to the enhancement of osteogenic differentiation. Suppression of endogenous miR-26a-5p reduced the level of β-catenin. It indicates an interaction between the canonical and non-canonical Wnt signaling pathways. Our analysis of the miR-26a-5p/Wnt5a/Ca2+ signaling pathway elucidates the mechanism of osteogenesis of mADSCs and suggests that certain experimental methods can be used to promote new bone formation. Such methods may have potential use for the development of therapies for bone-related diseases.
Proposed regulation of gene expression in the non-canonical Wnt pathway during osteogenic differentiation of mADSCs. miR-26a-5p inhibits the expression of WNT5A by targeting Wnt5a 3′UTR. WNT5A activates a G-protein after binding with its receptors on the cell membrane (Fz and Ror2), leading to calcium influx. The increase of intracellular calcium stimulates activation of the calcium-sensitive kinases PKC and CAMKII, implicating the Wnt/calcium pathway. Enhancement of the Wnt/calcium pathway leads to suppression of β-catenin. The Wnt/calcium pathway and the Wnt/PCP pathways (Wnt/JNK and Wnt/ROCK signaling) promote the differentiation of mADSCs into osteoblasts by alteration of osteogenic marker genes expression. Further studies are needed to determine whether there are interactions between the Wnt/calcium pathway and Wnt/PCP pathways
Abbreviations
- ADSCs:
-
Adipose-derived mesenchymal stem cells
- mADSCs:
-
Mouse adipose-derived mesenchymal stem cells
- hADSCs:
-
Human adipose-derived mesenchymal stem cells
- MSCs:
-
Mesenchymal stem cells
- miRNAs:
-
MicroRNAs
- miR-26a:
-
MicroRNA-26a
- miR-NC:
-
MicroRNA-26a-5p negative control
- UTR:
-
Untranslated regions
- CDK6:
-
Cyclin-dependent kinase 6
- HDAC4:
-
Histone deacetylase 4
- USSCs:
-
Unrestricted somatic stem cells
- BMP:
-
Bone morphogenetic protein
- GSK-3β:
-
Glycogen synthase kinase -3β
- PCP:
-
Planar cell polarity
- ROCK:
-
Rho-associated kinase
- PBS:
-
Phosphate buffer saline
- FBS:
-
Fetal calf serum
- qRT-PCR:
-
Quantitative real-time polymerase chain reaction
- GFP:
-
Green fluorescent protein
- RFP:
-
Red fluorescent protein
- MOI:
-
Multiplicity of infection
- WT:
-
Wild type
- Mu:
-
Mutation
- PKC:
-
Protein kinase C
- ALP:
-
Alkaline phosphatase
- OCN:
-
Osteocalcin
- OPN:
-
Osteopontin
- COL1:
-
Collagen type 1
- Runx2:
-
Runt homology domain transcription factor 2
- Osx:
-
Osterix
- CaMKII:
-
Calmodulin-dependent protein kinase II
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- ARS:
-
Alizarin red staining
- D-HBSS:
-
D-Hanks balanced salt solution
- Dsh:
-
Dishevelled
- RhoA:
-
Ras homolog gene family, member A
- JNK:
-
c-Jun N-terminal kinase
- Fz3:
-
Frizzled receptor 3
- Ror2:
-
Receptor tyrosine kinase-like orphan receptor 2
- P-PDLSCs:
-
Periodontal ligament stem cells from chronic periodontitis patients
References
Lu CH, Chang YH, Lin SY, Li KC, Hu YC (2013) Recent progresses in gene delivery-based bone tissue engineering. Biotechnol Adv 31:1695–1706
Sriram M, Sainitya R, Kalyanaraman V, Dhivya S, Selvamurugan N (2015) Biomaterials mediated microRNA delivery for bone tissue engineering. Int J Biol Macromol 74:404–412
Konno M, Hamabe A, Hasegawa S, Ogawa H, Fukusumi T, Nishikawa S, Ohta K, Kano Y, Ozaki M, Noguchi Y, Sakai D, Kudoh T, Kawamoto K, Eguchi H, Satoh T, Tanemura M, Nagano H, Doki Y, Mori M, Ishii H (2013) Adipose-derived mesenchymal stem cells and regenerative medicine. Dev Growth Differ 55:309–318
Peppo GMD, Marolt D (2013) Modulating the biochemical and biophysical culture environment to enhance osteogenic differentiation and maturation of human pluripotent stem cell-derived mesenchymal progenitors. de Peppo Marolt Stem Cell Res Therapy 4:106
Grottkau BE, Lin Y (2013) Osteogenesis of adipose—derived stem cells. Bone Res 2:133–145
Zhang Z, Wang J, Lu X (2014) An integrated study of natural hydroxyapatite-induced osteogenic differentiation of mesenchymal stem cells using transcriptomics, proteomics and microRNA analyses. Biomed Mater 9:045005
Martin EC, Qureshi AT, Dasa V, Freitas MA, Gimble JM, Davis TA (2015) MicroRNA regulation of stem cell differentiation and diseases of the bone and adipose tissue: perspectives on miRNA biogenesis and cellular transcriptome. Biochimie. doi:10.1016/j.biochi.2015.02.012
Zhang J, Tu Q, Bonewald LF, He X, Stein G, Lian J, Chen J (2011) Effects of miR-335-5p in modulating osteogenic differentiation by specifically downregulating Wnt antagonist DKK1. J Bone Mineral Res 26:1953–1963
Huszar JM, Payne CJ (2014) MIR146A inhibits JMJD3 expression and osteogenic differentiation in human mesenchymal stem cells. FEBS Lett 588:1850–1856
Zeng Y, Qu X, Li H, Huang S, Wang S, Xu Q, Lin R, Han Q, Li J, Zhao RC (2012) MicroRNA-100 regulates osteogenic differentiation of human adipose-derived mesenchymal stem cells by targeting BMPR2. FEBS Lett 586:2375–2381
Li Z, Hassan MQ, Volinia S, van Wijnen AJ, Stein JL, Croce CM, Lian JB, Stein GS (2008) A microRNA signature for a BMP2-induced osteoblast lineage commitment program. Proc Natl Acad Sci USA 105:13906–13911
Luzi E, Marini F, Sala SC, Tognarini I, Galli G, Brandi ML (2008) Osteogenic differentiation of human adipose tissue-derived stem cells is modulated by the miR-26a targeting of the SMAD1 transcription factor. J Bone Mineral Res 23:287–295
Trompeter H-I, Dreesen J, Hermann E, Iwaniuk KM, Hafner M, Renwick N, Tuschl T, Wernet P (2013) MicroRNAsmiR-26a, miR-26b, and miR-29b accelerate osteogenic differentiation of unrestricted somatic stem cells from human cord blood. BMC Genomics 14:1471–2164
Luzi E, Marini F, Tognarini I, Galli G, Falchetti A, Brandi ML (2012) The regulatory network menin-microRNA 26a as a possible target for RNA-based therapy of bone diseases. Nucl Acid Ther 22:103–108
Li Y, Fan L, Hu J, Zhang L, Liao L, Liu S, Wu D, Yang P, Shen L, Chen J, Jin Y (2015) MiR-26a rescues bone regeneration deficiency of mesenchymal stem cells derived from osteoporotic mice. Mol Therapy J Am Soc Gene Therapy 23:1349–1357
Li Y, Fan L, Liu S, Liu W, Zhang H, Zhou T, Wu D, Yang P, Shen L, Chen J, Jin Y (2013) The promotion of bone regeneration through positive regulation of angiogenic-osteogenic coupling using microRNA-26a. Biomaterials 34:5048–5058
Su X, Liao L, Shuai Y, Jing H, Liu S, Zhou H, Liu Y, Jin Y (2015) MiR-26a functions oppositely in osteogenic differentiation of BMSCs and ADSCs depending on distinct activation and roles of Wnt and BMP signaling pathway. Cell Death Dis 6:e1851
Wang Y, Li Y-P, Paulson C, Shao J-Z, Zhang X, Wu M, Chen W (2014) Wnt and the Wnt signaling pathway in bone development and disease. Front Biosci (Landmark Ed) 19:379–407
Veeman MT, Axelrod JD, Moon RT (2003) A second canon: functions and mechanisms of β-catenin-independent Wnt signaling. Dev Cell 5:367–377
Lin SS, Ueng SW, Niu CC, Yuan LJ, Yang CY, Chen WJ, Lee MS, Chen JK (2014) Hyperbaric oxygen promotes osteogenic differentiation of bone marrow stromal cells by regulating Wnt3a/beta-catenin signaling–an in vitro and in vivo study. Stem Cell Rese 12:260–274
Egea V, Zahler S, Rieth N, Neth P, Popp T, Kehe K, Jochum M, Ries C (2012) Tissue inhibitor of metalloproteinase-1 (TIMP-1) regulates mesenchymal stem cells through let-7f microRNA and Wnt/beta-catenin signaling. Proc Natl Acad Sci USA 109:E309–E316
Liu N, Shi S, Deng M, Tang L, Zhang G, Liu N, Ding B, Liu W, Liu Y, Shi H, Liu L, Jin Y (2011) High levels of beta-catenin signaling reduce osteogenic differentiation of stem cells in inflammatory microenvironments through inhibition of the noncanonical Wnt pathway. J Bone Mineral Res 26:2082–2095
Tu X, Joeng KS, Nakayama KI, Nakayama K, Rajagopal J, Carroll TJ, McMahon AP, Long F (2007) Noncanonical Wnt signaling through G protein-linked PKCdelta activation promotes bone formation. Dev Cell 12:113–127
Li J, Hu C, Han L, Liu L, Jing W, Tang W, Tian W, Long J (2015) MiR-154-5p regulates osteogenic differentiation of adipose-derived mesenchymal stem cells under tensile stress through the Wnt/PCP pathway by targeting Wnt11. Bone 78:130–141
Santos A, Bakker AD, de Blieck-Hogervorst JM, Klein-Nulend J (2010) WNT5A induces osteogenic differentiation of human adipose stem cells via rho-associated kinase ROCK. Cytotherapy 12:924–932
Chakravorty N, Ivanovski S, Prasadam I, Crawford R, Oloyede A, Xiao Y (2012) The microRNA expression signature on modified titanium implant surfaces influences genetic mechanisms leading to osteogenic differentiation. Acta Biomater 8:3516–3523
Lin YF, Jing W, Wu L, Li XY, Wu Y, Liu L, Tang W, Long J, Tian WD, Mo XM (2008) Identification of osteo-adipo progenitor cells in fat tissue. Cell Prolif 41:803–812
Yoshizawa S, Brown A, Barchowsky A, Sfeir C (2014) Magnesium ion stimulation of bone marrow stromal cells enhances osteogenic activity, simulating the effect of magnesium alloy degradation. Acta Biomater 10:2834–2842
Santos A, Bakker AD, Zandieh-Doulabi B, Semeins CM, Klein-Nulend J (2009) Pulsating fluid flow modulates gene expression of proteins involved in Wnt signaling pathways in osteocytes. J Orthop Res 27:1280–1287
Hagenmueller M, Riffel JH, Bernhold E, Fan J, Katus HA, Hardt SE (2014) Dapper-1 is essential for Wnt5a induced cardiomyocyte hypertrophy by regulating the Wnt/PCP pathway. FEBS Lett 588:2230–2237
Vivancos V, Chen P, Spassky N, Qian D, Dabdoub A, Kelley M, Studer M, Guthrie S (2009) Wnt activity guides facial branchiomotor neuron migration, and involves the PCP pathway and JNK and ROCK kinases. Neural Dev 4:7
Nitzki F, Zibat A, Konig S, Wijgerde M, Rosenberger A, Brembeck FH, Carstens PO, Frommhold A, Uhmann A, Klingler S, Reifenberger J, Pukrop T, Aberger F, Schulz-Schaeffer W, Hahn H (2010) Tumor stroma-derived Wnt5a induces differentiation of basal cell carcinoma of Ptch-mutant mice via CaMKII. Cancer Res 70:2739–2748
Hutchins BI, Li L, Kalil K (2011) Wnt/calcium signaling mediates axon growth and guidance in the developing corpus callosum. Dev Neurobiol 71:269–283
Dissanayake SK, Weeraratna AT (2008) Detecting PKC phosphorylation as part of the Wnt/calcium pathway in cutaneous melanoma. Methods Mol Biol 468:157–172
Olivares-Navarrete R, Hyzy SL, Hutton DL, Dunn GR, Appert C, Boyan BD, Schwartz Z (2011) Role of non-canonical Wnt signaling in osteoblast maturation on microstructured titanium surfaces. Acta Biomater 7:2740–2750
Baksh D, Boland GM, Tuan RS (2007) Cross-talk between Wnt signaling pathways in human mesenchymal stem cells leads to functional antagonism during osteogenic differentiation. J Cell Biochem 101:1109–1124
Green JL, Kuntz SG, Sternberg PW (2008) Ror receptor tyrosine kinases: orphans no more. Trends Cell Biol 18:536–544
Oishi I, Suzuki H, Onishi N, Takada R, Kani S, Ohkawara B, Koshida I, Suzuki K, Yamada G, Schwabe GC, Mundlos S, Shibuya H, Takada S, Minami Y (2003) The receptor tyrosine kinase Ror2 is involved in non-canonical Wnt5a/JNK signalling pathway. Genes Cells 8:645–654
Mikels AJ, Nusse R (2006) Purified Wnt5a protein activates or inhibits beta-catenin-TCF signaling depending on receptor context. PLoS Biol 4:e115
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 10502037, 31070833, and 31570950), the Science and Technology Foundation of Sichuan Province (Nos. 2010GZ0225, 2011GZ0335, and 2009SZ0139), and the Cooperation Science Foundation between Sichuan University and Luzhou city (No. 2013CDLZ-S19).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Shasha Li, Chen Hu, Jianwei Li, Lei Liu, Wei Jing, Wei Tang, Weidong Tian, and Jie Long have declared no conflict of interest.
Human and Animal Rights and Informed Consent
All experiments were approved by the Animal Research Committee of Sichuan University and were conducted in accordance with the guidelines for the management and handling of experimental animals.
Additional information
Shasha Li, Chen Hu, and Jianwei Li are co-first author and equally contributed to this work.
Rights and permissions
About this article
Cite this article
Li, S., Hu, C., Li, J. et al. Effect of miR-26a-5p on the Wnt/Ca2+ Pathway and Osteogenic Differentiation of Mouse Adipose-Derived Mesenchymal Stem Cells. Calcif Tissue Int 99, 174–186 (2016). https://doi.org/10.1007/s00223-016-0137-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0137-3