Abstract
Intermittent and low-dose parathyroid hormone (PTH) injection to stimulate bone formation has been used in the treatment of osteoporosis. The N-terminal fragment 1–34 of PTH is quite similar in structure and function to N-terminal PTH-related protein (PTHrP). PTH(1–34) and PTHrP also share a coreceptor, the PTH/PTHrP receptor. Therefore, some studies have suggested that PTHrP could effectively stimulate bone formation, similar to PTH. We used an ovariectomized (OVX) rat model of osteoporosis to study the effects of PTHrP(1–34) on bone metabolism by measuring bone mineral density (BMD), bone histomorphometrics, and biomechanical parameters. We found that subcutaneous injection of PTHrP(1–34) (40 or 80 μg/kg body weight every day) in OVX rats increased lumbar and femoral BMD, improved bone biomechanical properties, enhanced bone strength, and promoted bone formation. We selected 40 μg/kg as the preferred therapeutic dose of PTHrP(1–34) and investigated the effects of frequency of treatment (per 1, 2, 3, or 7 days) on bone metabolism in OVX rats. We found that injection of PTHrP(1–34) once per day or every other day significantly improved the BMD and strength of OVX rats. Serum calcium and phosphate levels in all treated rats did not vary significantly from control rats. Based on our results, intermittent low-dose PTHrP(1–34) injection promoted bone formation in OVX rats, suggesting a high potential for therapeutic use in osteoporosis patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osteoporosis is highly prevalent worldwide—an estimated 200 million people suffer from this disease [1]. Postmenopausal osteoporosis is the most common type, and approximately 30 % of all postmenopausal women have osteoporosis in the United States and Europe [2]. Osteoporosis is treated with agents that diminish osteoclastic bone resorption or increase osteoblastic bone formation or both. Although agents that are antiresorptive incrementally increase bone mineral density (BMD) and reduce fractures, the effects are not enough to restore BMD to premenopausal levels, and many women remain sensitive to skeletal fractures. Thus, new agents are required that can increase BMD and reduce fractures beyond the levels achievable using antiresorptives.
The results of increasing numbers of preclinical animal studies and clinical trials have shown that intermittent injection of low-dose parathyroid hormone (PTH) can effectively stimulate bone formation, thereby increasing BMD and reducing the incidence of fractures [3–8]. These trials led to the approval in 2002 by the US Food and Drug Administration [9] of the N-terminal fragment 1–34 of PTH as a drug for the treatment of osteoporosis. Furthermore, recombinant human (rh) PTH(1–84) (PreosTM) and rhPTH(1–31)NH2 (Ostabolin-CTM), which are the two kinds of amino acid peptides of rhPTH, are currently being evaluated in phase II/III clinical trials [10]. In particular, rhPTH(1–31)NH2, an enterically coated oral tablet, has been proposed to increase BMD of the lumbar spine without inducing bone resorption in postmenopausal women with osteoporosis [11].
PTH-related protein (PTHrP) is so named because its 36 N-terminal amino acids are quite similar to PTH(1–34) in structure and function. PTHrP and PTH(1–34) not only are homologous in primary sequences and tertiary structures but also bind to a shared coreceptor, PTH/PTHrP. Stewart and colleagues [12] treated ovariectomized (OVX) rats with PTH(1–34), PTHrP(1–36), or SDZ-PTH 893 for 6 months. They then assessed bone mass, bone histomorphometry, and bone biomechanics and found that all three of these treatments promoted bone formation [12]. In a subsequent randomized, double-blind trial, 16 postmenopausal women were administered an increased dosage of PTHrP(1–36) (6.56 μg/kg/day, about 400 μg/day). After 3 months of treatment, vertebral BMD increased 4.7 % with no hypercalcemia or postural hypotension side effects, although the dose used was ten times higher than that of PTH (40 μg/day) [13]. Therefore, the authors proposed that the N-terminal PTHrP fragment could effectively promote bone formation, similar to PTH(1–34). Unlike PTH, PTHrP(1–36), even at the larger dose, does not stimulate bone absorption or elevate the blood calcium level. Therefore, PTHrP(1–36) can be considered more effective than PTH at selectively stimulating bone formation. However, to confirm the effectiveness of PTHrP(1–36), both the safety and frequency of its application require further research using larger sample populations.
Estrogen inhibits bone absorption and conversion and therefore prevents bone loss. Clinical research has shown that estrogen replacement therapy is effective for preventing postmenopausal osteoporosis since it can prevent bone loss and reduce the risk of both vertebral and nonvertebral fractures. In the present study, we hypothesized that PTHrP might be an effective skeletal anabolic agent in OVX rats and investigated the most appropriate dose and frequency of administration. We used estradiol (E2) as a reference in our experiments during a 3-month randomized, placebo-controlled pilot study, in which PTHrP at different dosages was compared with placebo [normal saline (NS)]–treated controls.
Materials and Methods
Experimental Design
Sixty healthy female nonpregnant Wistar rats, 4 months old, were housed at the Experimental Animal Center of Shandong University. The average weight of the animals was 269.1 ± 34 g. Each plastic cage housed five rats. The temperature was kept at 22 ± 2 °C in a 12-h light/dark cycle. Rats were fed ad libitum with standard solid particle food (1.13 % Ca and 0.66 % P).
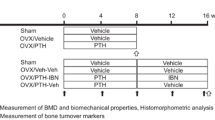
Rats were randomly assigned to six groups of ten rats: a sham-operated group (in which only a piece of fat around the ovary was ablated) and five groups of OVX rats, differentially treated (as described below). OVX rats underwent surgery in accordance with the method of Kalu [1].
All rats were treated every day for 12 consecutive weeks from the fifth week after surgery, by subcutaneous (SC) injection. The total dose per treatment was adjusted weekly according to body weight. The sham-operated group was given 0.2 mL NS per treatment. The placebo (OVX + NS) group received 0.2 mL NS per treatment. The OVX + E2 group was given 40 μg/kg estradiol benzoate (Shanghai GM Pharmaceutical, Shanghai, China ) SC per treatment. The OVX + PTHrP-20 (GL Biochem, Shanghai, China), OVX + PTHrP-40, and OVX + PTHrP-80 groups were given 20, 40, or 80 μg/kg, respectively, of PTHrP(1–34) per treatment.
Based on the results of the above experiment, we selected PTHrP at 40 μg/kg as an effective dose for a second set of experiments that evaluated dosage frequency. Seventy 4-month-old healthy female nonpregnant Wistar rats with a mean weight of 256.3 ± 40 g were then randomly assigned to seven groups of ten rats. The sham-operated, OVX + NS, and OVX + E2 groups were treated as in the first experiment. The groups designated PTHrP-1d, PTHrP-2d, PTHrP-3d, and PTHrP-7d were rats that were subjected to ovariectomy and then treated with SC injections of 40 μg/kg PTHrP once per day, every other day, every 3 days, or every 7 days, respectively, beginning from the fifth week after surgery for 12 consecutive weeks.
Sample Collection and Treatment
Blood samples were collected from the jugular veins of all animals before they were weighed and killed by bleeding. The serum was isolated and kept at −20 °C for calcium and phosphate measurements. The lumbar (L3–L4) and left femur were completely disarticulated, wrapped with NS-soaked gauze, and kept at −20 °C for BMD measurements and three-point bending and lumbar compression tests. The proximal tibia was soaked in 70 % ethanol and stored at 4 °C for bone morphometric analysis.
BMD Measurements
BMD measurements of the femur and lumbar spine (L3–L4) were made via peripheral dual-energy X-ray absorptiometry from Norland (Fort Atkinson, WI), with small-animal scanning software. Indices were measured three times, and the results are presented as the average of those measurements.
Production of Undecalcified Bone Sections of Rats and Bone Morphometric Analysis
For the first fluorescent label, all animals were given an intraperitoneal (IP) injection of 30 mg/kg 1 % tetracycline (Sigma, St. Louis, MO) once daily for two consecutive days, beginning 14 days before the animals were killed. For the second fluorescent label, 5 mg/kg 1 % calcein (Sigma) was injected IP once daily for two consecutive days starting on the fourth day prior to death. Under the fluorescence microscope, tetracycline deposited at the bone surface is visible in yellow and calcein is visible in green. Images of trabecular bone under epiphyseal plates within 1 mm of all bone sections were randomly selected and compiled in the computer from inner, middle, and outer points.
Each sample was analyzed by histology with three methods (von Kossa staining, Giemsa staining, and fluorescence). Images were processed and analyzed (MPIAS-500 image analyzer and Qingping Imaging Software from Tongji Medical University, Wuhan, China) to obtain the following indices: trabecular bone volume (TBV)/total tissue volume (TTV), i.e., the percentage of bone tissue volume occupied by trabecular bone; osteoblast surface (ObS); osteoclast surface (OcS); percentage of tetracycline single-labeled surface of the total trabecular bone surface (STS); percentage of tetracycline double-labeled surface of the total trabecular bone surface (DTS); and mineral apposition rate (MAR, micrometers per day).
Bone Biomechanical Tests
Femur Three-Point Bending Test
Femoral specimens were slowly thawed and kept moist, then freely placed on the brackets with the curved side down. The midpoint of the femur was placed in alignment with the loading point (AG-1S universal testing instrument; Shimadzu, Kyoto, Japan). Specimens were uniformly loaded until broken, and load-displacement curves were obtained digitally. The accuracy of the load cell was 0.01 N, the position measurement accuracy was 0.001 mm, the loading speed was 2 mm/min, and the span was 20 mm. The inner diameter and wall thickness of the broken bones were measured in the long and short axes by vernier calipers, and the experimental data were used to calculate the mechanical properties of each specimen. The parameters measured were maximal loading (L), maximal bending stress (σ), and bending modulus of elasticity (E f).
Lumbar Compression Test
Before the test, bone specimens (fifth lumbar) were thawed at room temperature, while keeping them moist. Soft tissues, spine processes, transverse processes, and intervertebral discs were carefully removed, keeping intact the cortex of the vertebral bodies. Vertebral bodies were shaped as cylinders with two parallel planes and approximately 5.5–7 mm in height. The heights of the vertebral bodies were measured by vernier calipers, and the vertebral cross-sectional area was calculated. Specimens were placed in the universal tester to perform compression tests with a loading speed of 2 mm/min before load-displacement curves and experimental data were recorded. Other conditions of the test were similar to those used for the femur three-point bending test. The parameters measured were maximal loading (L) and compression modulus (E c).
Serum Ca/P Determination
Serum Ca/P was determined by colorimetry with an automatic biochemical analyzer.
Statistical Analysis
Statistical data were analyzed with SPSS10.0 statistical software (SPSS, Inc., Chicago, IL, USA) and reported as mean ± standard deviation. The independent samples t test was used for comparisons with control groups. Comparisons among PTHrP treatment groups were performed using one-way ANOVA. The critical significance level was P ≤ 0.05 (two-tailed).
Results
Lumbar (3–6) and Femoral BMD
In the different dose groups, the lumbar and femoral BMDs of the sham-operated and OVX + E2 groups were higher than those of the OVX + NS group (P < 0.01, Fig. 1), and the BMDs of the OVX + E2 and sham groups were comparable (P > 0.05). The lumbar and femoral BMDs of the OVX + PTHrP-20 group were mildly elevated compared with the OVX + NS group, though differences were not significant (P > 0.05), and still lower than that of the sham group. The BMDs of the OVX + PTHrP-40 and OVX + PTHrP-80 groups markedly exceeded that of the OVX + NS group (P < 0.01), with an upward tendency that was dose-dependent; but differences between the OVX + PTHrP-40 and OVX + PTHrP-80 groups were not statistically significant (P > 0.05), nor were the differences in BMDs significant when compared to the sham or OVX + E2 group (P > 0.05; Fig. 1; Table 1). Therefore, we selected 40 μg/kg as the effective dose.
In the different dose frequency groups, the lumbar and femoral BMDs of the OVX + PTHrP-1d and OVX + PTHrP-2d groups were markedly raised compared with the OVX + NS group (P < 0.01) and remarkably higher than that of the OVX + PTHrP-7d group (P < 0.05). The difference between the OVX + PTHrP-1d and OVX + PTHrP-2d groups was not statistically significant. Lumbar and femoral BMDs of the OVX + PTHrP-3d and OVX + PTHrP-7d groups did not show an obvious change when compared with the OVX + NS group (P > 0.05; Fig. 2; Table 2).
Biomechanical Indices
In the different dose groups, all the biomechanical indices measured (maximal loading, maximal bending stress, and elastic modulus in the femur three-point bending test and maximal loading and elastic modulus in the lumbar compression test) were higher in the sham-operated and OVX + E2 groups than in the OVX + NS group (Fig. 3, Table 3). All the indices of the OVX + PTHrP-40 and OVX + PTHrP-80 groups remarkably exceeded those of the OVX + NS group (P < 0.05, Fig. 3). The indices between the OVX + PTHrP-20 and placebo groups were not significantly different (P > 0.05). All biomechanical indices tended to increase with dose.
In the different dose frequency groups, the biomechanical indices of both the OVX + PTHrP-1d and OVX + PTHrP-2d groups significantly exceeded those of the OVX + NS group (Fig. 4; Table 4). With the increase in frequency of injection, all biomechanical indices tended to increase but were similar between the OVX + PTHrP-1d and OVX + PTHrP-2d groups. There were no obvious differences between the OVX + PTHrP-3d and OVX + PTHrP-7d groups compared to the OVX + NS group.
Bone Histomorphometric Parameters
In the different dose groups, the TBV/TTV of the OVX + NS group was markedly lower than that of the sham group but ObS, OcS, STS, and DTS were significantly higher. After estrogen (E2) replacement therapy, the TBV/TTV of the OVX + E2 group was elevated compared with the OVX + NS group (P < 0.05), although it was still lower than that of the sham group. There was no significant difference between the OVX + E2 and sham groups (P > 0.05). ObS, OcS, STS, and DTS were significantly reduced in the OVX + E2 group compared with the placebo group (P < 0.01). The MAR of the OVX + E2 group was comparable to both the OVX + NS and sham groups (P > 0.05, Figs. 5, 6; Table 5).
After 12 weeks of treatment with PTHrP(1–34), the TBV/TTV of each PTHrP group was significantly higher than that of the OVX + NS group (P < 0.05 or < 0.01) and especially marked in the OVX + PTHrP-40 and OVX + PTHrP-80 groups (P < 0.01; Figs. 5, 6; Table 5). However, when compared with the sham group, the differences were not statistically significant. The ObS, OcS, STS, DTS, and MAR of each PTHrP(1–34) treatment group were significantly higher than those of the sham group (P < 0.05 or < 0.01; Figs. 5, 6; Table 5).
In the different dose frequency groups, the TBV/TTV of the OVX + PTHrP-1d and OVX + PTHrP-2d groups increased compared with the OVX + NS group (Fig. 7;Table 6). Their ObS, OcS, STS, DTS, and MAR were higher than those of the sham group. The TBV/TTV of the PTHrP-7d group was not significantly different from that of the OVX + NS group (Fig. 7; Table 6).
Changes in Serum Calcium and Phosphate
Serum calcium and phosphate levels were similar between the OVX + NS and sham groups (P > 0.05). Twelve weeks after PTHrP(1–34) treatments with different doses and frequencies, serum calcium and phosphate in each group showed no remarkable changes.
Discussion
We tested different doses of PTHrP(1–34) to treat OVX rats for 12 weeks. Since we observed no differences between the animals of the OVX + PTHrP-40 and OVX + PTHrP-80 groups, we selected 40 μg/kg to further investigate the most appropriate frequency of administration.
In 1987 Burtis et al. [14] isolated a cytokine from malignant tumors that was associated with hypercalcemia. The cytokine was quite similar to PTH in gene structure and sequence and was subsequently named “parathyroid hormone-related protein.” The N terminus of PTHrP is strikingly similar to that of PTH as its 1–13 amino acids are 70 % homologous with PTH in primary sequence. However, there was no homologous sequence between amino acids 14 and 36. PTH and PTHrP also share a coreceptor, the PTH/PTHrP receptor. Thus, it is thought that the similar role of PTHrP and PTH is mainly due to the 1–13 amino acid sequence; prior studies have shown that both PTHrP(1–34) and PTHrP(1–36) promote bone formation [14–18]. Some preclinical animal studies and clinical trials have shown that the N terminus of PTHrP promoted bone formation similarly to PTH(1–34).
Animal experiments have already proved that PTHrP can promote bone formation in OVX rats [17]. Hock et al. [19] found that when PTH(1–34) or PTHrP(1–34) was injected into male Sprague-Dawley rats, the bone mass of the trabeculae and the surface involved in bone formation were significantly increased at high doses of PTHrP(1–34) (16–32 μg/100 g) but the promotion of bone formation was less so with PTH(1–34). Further clinical trials have found that lumbar BMD in postmenopausal women increased after intermittent SC PTHrP(1–36) injections [13]. Furthermore, osteocalcin, a biochemical index of bone formation, was notably elevated by this treatment. However, there was no change in urinary deoxypyridinoline, an index of bone resorption; and this is quite different from PTH.
Some authors [13] have proposed that PTHrP only selectively stimulates bone formation and does not interfere with bone resorption. If this hypothesis is correct, this treatment would be superior to PTH in accelerating bone formation. Further in-depth studies are needed to test this possibility. Those studies also found that a high dose of N-terminal PTHrP (10–20 times higher than that of PTH) had no adverse effects on mineral stability in the environment and showed no obvious adverse effects that could be due to the differences in the pharmacokinetics of these two peptides. Serum PTH(1–34) reaches a maximum concentration 30–45 min after injection, but only 5–10 min were required for the N-terminal PTHrP fragment. Consequently, the absorption, peak serum concentration, and clearance of PTHrP in rats are faster than those of PTH, which is probably due to the higher dosage. PTHrP does not cause malignant hypercalcemia [19–22].
Our results indicate that PTHrP(1–34) administered in a 40-μg/kg dose either once per day (PTHrP-1d) or once every other day (PTHrP-2d) for 12 weeks can induce statistically and biologically important incremental increases in femoral and lumbar BMD in the OVX rat model of osteoporosis. Maximum load and elastic modulus measured in lumbar compression tests of OVX rats treated with 40 μg/kg of PTHrP(1–34) every day, every other day, or every 3 days were significantly higher than for OVX rats administered only NS. Similarly, each femoral biomechanical index for PTHrP-treated OVX rats in these groups notably exceeded that of the OVX + NS group. Regarding the bone histomorphometric parameters, the TBV/TTV in all PTHrP-treated OVX rats was markedly higher than that of OVX rats in the NS group, particularly those treated with 40 μg/kg PTHrP(1–34) every day, every other day, or every 3 days and OVX rats treated with 80 μg/kg every 3 days. The ObS, OcS, STS, DTS, and MAR of each PTHrP-treated group were significantly higher with reference to the sham-operated group. After being treated with E2 for 12 weeks, the femoral and lumbar BMD and the biomechanical indices also exceeded the values obtained for the placebo group. Unlike PTHrP(1–34), the ObS, OcS, STS, and DTS of the OVX + E2 group were significantly reduced compared with those of the placebo group. These results allow us to conclude that bone resorption was inhibited by the treatment.
The ultimate objective of osteoporosis treatment is the prevention of fractures. The main purpose for using animal models of osteoporosis is to determine whether treatments can improve bone biomechanical properties and effectively reduce the incidence of fractures. Although BMD is highly correlated with bone strength, large numbers of animal experiments and clinical trials have shown that bone quality does not necessarily improve with increased BMD but sometimes even declines after some treatments. Therefore, tests of bone biomechanical properties are not only useful as a direct evaluation of bone quality but also among the best methods for evaluating the efficacy of therapies against bone loss.
In the present study, the dosages that correlated with a significantly favorable response in biomechanical properties were the same as those that were associated with increased BMD; 40 μg/kg PTHrP administered once per day or every other day to OVX rats showed good therapeutic effects. The maximum load and elastic modulus were significantly higher in these groups than in the OVX rats that received only NS and exceeded those receiving 40 μg/kg PTHrP once per week. Therefore, administration once per day or once every other day effectively improved bone strength and bone quality of the lumbar spine in the OVX rat model of osteoporosis and may prevent the occurrence of osteoporotic fractures.
Quantitative analysis using bone histomorphometry of bone structure and morphology dynamics has been internationally recognized as the most reliable method to measure therapeutic effects in the treatment of osteoporosis. Unlike measurements of BMD, bone histomorphometry is able to evaluate the effect of drugs early and with high sensitivity. Because bone remodeling in adult rats requires more than a 30-day cycle, the treatment period should exceed 30 days. In this study, we administrated treatment to OVX rats for 12 weeks, starting from the fifth week after ovariectomy, before bone histomorphometric analysis was performed.
As shown in the micrographs of bone histomorphometry (Figs. 5, 6, 7), trabeculae under the epiphyseal plate of the tibia were fewer and thinner in OVX control rats. On the edges of the trabeculae, bone resorption increased and tetracycline labeling could be seen by fluorescence microscopy. This result shows that OVX rats displayed high-turnover osteoporosis. The increase in bone resorption surpassed bone formation and resulted in a negative balance of the bone turnover. However, changes in bone mineralization were subtle and caused bone mass reduction and trabecular BMD decrease.
Previous studies have shown that estrogen replacement therapy slowed the rate of bone turnover, inhibiting bone resorption and correcting a negative balance in bone turnover in OVX rats [23, 24]. In the present study, compared to NS, PTHrP(1–34) at 40 or 80 μg/kg delivered SC to OVX rats every day or every other day for 12 weeks increased the width and conjunction of trabeculae. The tetracycline labeling also significantly increased. TBV/TTV, ObS, OcS, STS, and DTS were significantly higher than those of the OVX + NS group. This suggests that PTHrP(1–34) increased bone mass by promoting bone formation. Thus, PTHrP(1–34) may be involved in activating bone remodeling and increasing the rate of bone turnover and bone formation, in addition to its dose-dependent effect.
Since the peptide PTHrP was discovered during investigations of malignancies associated with hypercalcemia, this may raise the concern that using PTHrP in osteoporosis treatment may cause patients to develop hypercalcemia and hypophosphatemia. The present study found that serum calcium and phosphate in all groups treated with PTHrP were no different from those of the sham-operated and OVX + NS groups. This result suggests that the doses and dose frequencies used in this study had no marked effects on serum calcium and phosphate, with a safe therapeutic effect. We thus conclude that PTHrP is potentially valuable as a bone formation accelerator for osteoporosis treatment in the clinic. Its effect in bone formation is consistent with hPTH but also reduces bone resorption; and being purely a promoter of bone formation, it could be superior to hPTH.
There are still several issues to settle before it will be possible to consider the clinical application of PTHrP. PTHrP may cause adverse effects if it is systemically distributed in the human body during the treatment of osteoporosis. The optimal dose and frequency of treatment to achieve the best therapeutic effects with minimal side effects need to be established. The possible combination with other bone resorption inhibitors may be investigated, and the efficacy of different PTHrP fragments should be evaluated. In the near future, we need to continue to develop new oral preparations like rhPTH(1–31)NH2 (Ostabolin-C).
In summary, SC injection of PTHrP(1–34) at 40 μg/kg daily or on alternate days promoted bone formation and significantly increased BMD and the biomechanical properties of the femur and lumbar spine in OVX rats. The treatments did not affect the serum levels of calcium and phosphate. PTHrP(1–34) acted as a bone formation accelerator and may have wide clinical application in the treatment of osteoporosis in the future. However, further basic research, long-term clinical observation, and larger sample populations are required to ensure the safety of its use.
Abbreviations
- BMD:
-
Bone mineral density
- DTS:
-
Double-labeled surface in total trabecular bone surface
- MAR:
-
Mineral apposition rate
- ObS:
-
Osteoblast surface
- OcS:
-
Osteoclast surface
- OVX:
-
Ovariectomized
- PTH:
-
Parathyroid hormone
- PTHrP:
-
Parathyroid hormone-related protein
- STS:
-
Single-labeled surface in total trabecular bone surface
- TBV/TTV:
-
Total bone volume/total tissue volume
References
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15:175–191
Nguyen TV, Center JR, Eisman JA (2000) Association between breast cancer and bone mineral density: the Dubbo Osteoporosis Epidemiology study. Maturitas 36(1):27–34
Rosen CJ, Bilezikian JP (2001) Anabolic therapy for osteoporosis. J Clin Endocrinol Metab 86:957–964
Cosman F, Nieves J, Woelfert L, Formica C, Gordon S, Shen V, Lindsay R (2001) Parathyroid hormone added to established hormone therapy: effects on vertebral fracture and maintenance of bone mass after parathyroid hormone withdrawal. J Bone Miner Metab 16:925–931
Kurland ES, Cosman F, McMahon DJ, Rosen CJ, Lindsay R, Bilezikian JP (2000) Parathyroid hormone as a therapy for idiopathic osteoporosis in men: effects on bone mineral density and bone markers. J Clin Endocrinol Metab 85:3069–3076
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mitlak BH (2001) Effect of parathyroid hormone (1–34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Dempster DW, Cosman F, Kurland ES, Zhou H, Nieves J, Woelfert L, Shane E, Plavetić K, Müller R, Bilezikian J, Lindsay R (2001) Effects of daily treatment with parathyroid hormone on bone microarchitecture and turnover in patients with osteoporosis: a paired biopsy study. J Bone Miner Res 16:1846–1853
Rittmaster RS, Bolognese M, Ettinger MP, Hanley DA, Hodsman AB, Kendler DL, Rosen CJ (2000) Enhancement of bone mass in osteoporotic women with parathyroid hormone followed by alendronate. J Clin Endocrinol Metab 85:2129–2134
Poynton AR, Lane JM (2002) Safety profile for the clinical use of bone morphogenetic proteins in the spine. Spine 27(16 suppl 1):s40–s48
Whitfield JF (2006) Parathyroid hormone: a novel tool for treating bone marrow depletion in cancer patients caused by chemotherapeutic drugs and ionizing radiation. Cancer Lett 244:8–15
Henriksen K, Andersen JR, Riis BJ, Mehta N, Tavakkol R, Alexandersen P, Byrjalsen I, Valter I, Nedergaard BS, Teglbjaerg CS, Stern W, Sturmer A, Mitta S, Nino AJ, Fitzpatrick LA, Christiansen C, Karsdal MA (2013) Evaluation of the efficacy, safety and pharmacokinetic profile of oral recombinant human parathyroid hormone [rhPTH(1–31)NH(2)] in postmenopausal women with osteoporosis. Bone 53:160–166
Stewart AF, Cain RL, Burr DB, Jacob D, Turner CH, Hock JM (2000) Six-month daily administration of parathyroid hormone and parathyroid hormone-related protein peptides to adult ovariectomized rats markedly enhances bone mass and biomechanical properties: a comparison of human parathyroid hormone 1–34, parathyroid hormone-related protein 1-36, and SDZ-parathyroid hormone 893. J Bone Miner Res 15:1517–1525
Horwitz MJ, Tedesco MB, Gundberg C, Garcia-Ocana A, Stewart AF (2003) Short-term, high-dose parathyroid hormone-related protein as a skeletal anabolic agent for the treatment of postmenopausal osteoporosis. J Clin Endocrinol Metab 88:569–575
Burtis WJ, Wu T, Bunch C, Wysolmerski JJ, Insogna KL, Weir EC, Broadus AE, Stewart AF (1987) Identification of a novel 17,000-dalton parathyroid hormone-like adenylate cyclase—stimulating protein from a tumor associated with humoral hypercalcemia of malignancy. J Biol Chem 262:7151–7156
Dunbar ME, Wysolmerski JJ (1999) Parathyroid hormone–related protein: a developmental regulatory molecule necessary for mammary gland development. J Mammary Gland Biol Neoplasia 4:21–34
Kaiser SM, Sebag M, Rhim JS, Kremer R, Goltzman D (1994) Antisense-mediated inhibition of parathyroid hormone: related peptide (PTHrP) production in a keratinocyte cell line impedes differentiation. Mol Endocrinol 8:139–147
Datta NS, Abou-Samra AB (2009) PTH and PTHrP signaling in osteoblasts. Cell Signal 21(8):1245–1254
Lanske B, Kronenberg HM (1998) Parathyroid hormone: related peptide (PTHrP) and parathyroid hormone (PTH)/PTHrP receptor. Crit Rev Eukaryot Gene Expr 8(3–4):297–320
Hock JM, Fonseca J, Gunness-Hey M, Kemp BE, Martin TJ (1989) Comparison of the anabolic effects of synthetic parathyroid hormone–related protein (PTHrP) 1–34 and PTH(1–34) on bone in rats. Endocrinology 125:2022–2027
Weir EC, Terwilliger G, Sartori L, Insogna KL (1992) Synthetic parathyroid hormone-like protein (1–74) is anabolic for bone in vivo. Calcif Tissue Int 51:30–34
Horwitz MJ, Tedesco MB, Garcia-Ocaña A, Sereika SM, Prebehala L, Bisello A, Hollis BW, Gundberg CM, Stewart AF (2010) Parathyroid hormone: related protein for the treatment of postmenopausal osteoporosis: defining the maximal tolerable dose. J Clin Endocrinol Metab 95(3):1279–1287
Hildreth BE 3rd, Werbeck JL, Thudi NK, Deng X, Rosol TJ, Toribio RE (2010) PTHrP 1–141 and 1–86 increase in vitro bone formation. J Surg Res 162(2):e9–e17
Wronski TJ, Cintrón M, Doherty AL, Dann LM (1988) Estrogen treatment prevents osteoporosis and depresses bone turnover in ovariectomized rats. Endocrinology 123(2):681–686
Cruess RL, Hong KC (1979) The effect of long term estrogen administration on bone metabolism in the female rat. Endocrinology 104(4):1188–1193
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Xu, J., Rong, H., Ji, H. et al. Effects of Different Dosages of Parathyroid Hormone-Related Protein 1–34 on the Bone Metabolism of the Ovariectomized Rat Model of Osteoporosis. Calcif Tissue Int 93, 276–287 (2013). https://doi.org/10.1007/s00223-013-9755-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9755-1