Abstract
Optimal vitamin D concentrations for bone health have not been determined in the Korean population. The aim of this study was to define serum 25-hydroxyvitamin D (25[OH]D) concentrations that indicate insufficiency among older Korean adults as measured by serum intact parathyroid hormone (iPTH) concentrations and bone mineral density (BMD). We analyzed data from the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-3), which was conducted in Korea in 2009. We enrolled 1,451 men and 1,870 women aged 49 years and above. After adjusting for variables that could potentially affect serum 25(OH)D concentrations, we found that serum iPTH concentrations began to increase at serum 25(OH)D concentrations below 12.1 ng/mL (30.2 nmol/L). In addition, total-femur BMD increased until serum 25(OH)D concentrations dropped below 20.4 ng/mL (50.9 nmol/L); no significant changes were observed thereafter. Assuming that serum 25(OH)D concentrations below 12.1 and 20.4 ng/mL represent vitamin D insufficiency, the prevalences of vitamin D insufficiency were 8.7 and 50.4 % in men and 17.9 and 66.3 % in women, respectively. Serum 25(OH)D cutoff values of 12.1 ng/mL (OR = 1.26) and 20.4 ng/mL (OR = 1.54) were associated with osteoporosis (P < 0.01); osteoporosis was not associated with a 25(OH)D cutoff value of 30 ng/mL (75.0 nmol/L). In conclusion, serum 25(OH)D concentrations of 20 ng/mL might be sufficient for bone health in older Korean adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
A recent international epidemiologic study of postmenopausal osteoporotic women that was conducted in 18 countries demonstrated that the overall prevalence of vitamin D inadequacy, defined as a serum 25-hydroxyvitamin D (25[OH]D) concentration below 30 ng/mL, was 64 %. Mean 25(OH)D concentrations were lowest in South Korea (17.6 ng/mL), and 92 % of Korean subjects were categorized as having vitamin D inadequacy [1]. Although there is no consensus on the optimal serum concentration of 25(OH)D, vitamin D insufficiency has often been defined as a 25(OH)D concentration below 30 ng/mL [2–6]. The rationale for this definition is based on the finding that serum 25(OH)D concentrations are inversely associated with serum parathyroid hormone (PTH) concentrations. The PTH concentration remains stable until the serum 25(OH)D concentration falls below 30–40 ng/mL, at which point the PTH concentration begins to increase [6–8]. Even subtle changes in 25(OH)D concentrations are associated with increased serum PTH concentration, resulting in increased bone turnover and accelerated bone loss, which may result in increased risk of fracture [9]. In contrast to studies suggesting that vitamin D insufficiency occurs at serum 25(OH)D concentrations below 30 ng/mL, an Institute of Medicine (IOM) committee recently concluded that serum 25OHD concentrations of 20 ng/mL meet the requirements of almost the entire population of North America [10]. Thus, controversy still exists regarding the optimal serum 25(OH)D concentrations for bone health.
No previous studies have defined vitamin D insufficiency in relation to bone health among Koreans. Therefore, the aim of this study was to identify serum 25(OH)D concentrations that indicate insufficiency among Koreans based on serum PTH concentrations and bone mineral density (BMD) using a nationally representative sample of population data from the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV-3) conducted in 2009.
Materials and Methods
KNHANES IV
The KNHANES is a nationwide, population-based, cross-sectional health survey that was first performed in 1998. The KNHANES IV was conducted from July 2007 to December 2009. The subject population included all households recorded by the 2005 Population and Housing Census in Korea. The entire nation was divided into 29 units based upon administrative districts and housing types. Relevant households from 200 districts were randomly selected through stratified and multistage probability sampling. As rolling survey methods were used for sampling, the sample for each year was a probability sample representing all parts of the country, and each rolling sample had homogenous and independent characteristics.
The KNHANES IV included all members of the sampled households over 1 year of age. The KNHANES IV questionnaire was composed of a health interview, health examination, and nutrition survey. The health interview and health examination were conducted in adjacent public offices or in mobile examination centers, while the nutrition survey was conducted through house-to-house inquiries. Of a total of 31,705 KNHANES IV subjects selected to participate in the health interview and health examination survey, 23,632 (74.5 %) completed the surveys. Of a total of 27,050 subjects selected to participate in the nutrition survey, 22,137 (81.8 %) completed the survey. All participants provided written informed consent.
Study Subjects
This study utilized data from the KNHANES IV-3, which was conducted in 2009. Of the 12,722 individuals who were sampled, 10,533 participated in the nutrition survey. Among those who participated in the survey, we enrolled 1,451 men and 1,870 women 49 years of age and older (mean age: men 63.5 ± 9.0, women 63.8 ± 9.3) for whom serum 25(OH)D and intact PTH (iPTH) concentration measurements were available.
Study Methods
Blood samples were collected from all subjects after at least 8 h of fasting. Specimens were immediately centrifuged, aliquoted, frozen at −70 °C, and moved to a central laboratory (NeoDIN Medical Institute, Seoul, South Korea), where they were analyzed within 24 h. Serum 25(OH)D concentrations were measured by radioimmunoassay (DiaSorin, Stillwater, MN) using a γ-counter (1470 Wizard; PerkinElmer, Turku, Finland); the reference range in adults was 9.0–37.6 ng/mL, with an interassay coefficient of variation (CV) of 3.5–6.2 %. Serum iPTH concentrations were measured using a chemiluminescence immunoassay (DiaSorin); the reference range in adults was 17.3–72.9 pg/mL, with an interassay CV of 2.4–11.5 %.
Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. The time of health examination was divided into four subgroups: (1) spring (March–May), (2) summer (June–August), (3) autumn (September–November), and (4) winter (December–February). The physical activity of the subjects was assessed by categorizing their recreational physical activity for the last 1 week into (1) none, no activity; (2) mild, >30 min of walking more than 5 days/week; (3) moderate, >30 min of moderate physical activity in which the subject was tired compared to an ordinary level or breathing slightly hard more than 5 days/week; and (4) vigorous physical activity, >20 min of vigorous physical activity in which the subject was exhausted compared to an ordinary level or breathing hard more than 3 days/week. Current smokers were defined as those who had smoked more than five packs of cigarettes during their lifetimes and were smoking currently. The remaining subjects were defined as nonsmokers. Regular alcohol drinkers were defined as subjects who currently drank alcohol more than two to four times per month, while all other subjects were defined as nondrinkers. Daily dietary calcium intake was assessed using the 24-hour dietary recall method. Data from the health interviews were used to obtain information about previous diagnoses of osteoporosis, osteoporotic fracture, treatment of osteoporosis, estrogen replacement, and other diseases including renal failure and rheumatoid arthritis.
Osteoporosis is defined by the World Health Organization (WHO) as BMD 2.5 SDs or more below the mean peak bone mass (average of young, healthy adults), measured at any section of the lumbar spine, total femur, or femoral neck, with dual-energy X-ray absorptiometry (Lunar, Madison, WI) [11].
Statistical Analysis
All data are presented as means ± SD or as proportions, except for skewed variables, which are presented as medians (interquartile range 25–75 %). To determine the relationship between serum 25(OH)D and iPTH concentrations while adjusting for confounders that could affect serum 25(OH)D concentrations (i.e., age, gender, body weight, calcium intake, physical activity, and season of year), we considered two linear regression models, one for subjects below a certain concentration of serum 25(OH)D and the other for subjects above that concentration. To determine the specific cutoffs, we fitted the two linear regression models described above and calculated the sums of squares of residuals (=observed PTH − estimated PTH) from the two models for each concentration of serum 25(OH)D. The models with the lowest residual sums of squares were our best models, and the corresponding concentrations of serum 25(OH)D were defined as the optimal cutoff values. We followed an identical approach to determine the relationship between serum 25(OH)D and BMD. We performed logistic regression analysis to validate the association between these newly developed serum 25(OH)D cutoff values and osteoporosis. Analyses were performed with R version 2.9.2 (http://www.r-project.org). P < 0.05 was considered statistically significant.
Results
Table 1 depicts the clinical characteristics of the study sample. Mean age was 63.6 years, and 56.3 % of the subjects were women. Mean serum 25(OH)D concentrations were 21.2 ± 7.1 (5.9–60.4 ng/mL) in men and 18.4 ± 6.8 (2.0–67.0 ng/mL) in women, while median (interquartile range) iPTH concentrations were 64.8 (50.9–79.5 pg/mL) in men and 65.0 (51.0–83.2 pg/mL) in women. There was an inverse correlation between serum 25(OH)D and iPTH concentrations (r = –0.104, P < 0.001). Subjects with normal BMD, osteopenia, and osteoporosis were 940 (28.3 %), 1,532 (46.1 %), and 793 (23.9 %), respectively; and serum 25(OH)D concentrations decreased as BMD decreased (P < 0.001, Fig. 1a). In addition, serum 25(OH)D concentrations varied in relation to the season of the year; serum 25(OH)D concentrations were highest in the summer (22.9 ± 7.7 ng/mL) and lowest in the spring (16.9 ± 6.2 ng/mL) (P < 0.001, Fig. 1b).
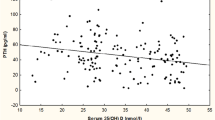
To determine the relationship between serum 25(OH)D and iPTH concentrations, we developed two linear regression models and found the serum 25(OH)D cutoffs for vitamin D sufficiency, after adjusting for variables that could affect serum 25(OH)D concentrations, including age, gender, body weight, calcium intake, physical activity, and season of the year. Regression coefficients for iPTH were −3.4537 (P = 0.008) in subjects with serum 25(OH)D concentrations below 12.1 ng/mL and −0.3742 in subjects with serum 25(OH)D concentrations above 12.1 ng/mL (P < 0.001) (Fig. 2a). We used the same approach to determine the relationship between serum 25(OH)D concentrations and BMD measured at the lumbar spine, total femur, and femoral neck. After adjusting for potential confounders, the BMD for the total femur increased until serum 25(OH)D concentrations dropped below 20.4 ng/mL; no significant changes in serum 25(OH)D concentrations were observed thereafter (Fig. 2b). No serum 25(OH)D cutoffs were found for BMD measured at the lumbar spine or femoral neck (data not shown).
Next, we determined the relationship between the risk for osteoporosis and three possible cutoff values for vitamin D insufficiency: the cutoff value of 30 ng/mL suggested by previous studies and our new cutoffs of 12.1 and 20.4 ng/mL. There was no relationship between vitamin D insufficiency and osteoporosis at serum 25(OH)D concentrations below 30 ng/mL. In contrast, the serum 25(OH)D concentration cutoffs of 12.1 ng/mL (OR = 1.26) and 20.4 ng/mL (OR = 1.54) were both associated with osteoporosis (Table 2).
On the basis of these data, we redefined the serum 25(OH)D concentration cutoffs for vitamin D insufficiency as 12.1 or 20.4 ng/mL, and we reassessed the prevalence of vitamin D insufficiency in the older Korean population. At the 12.1 ng/mL serum 25(OH)D concentration cutoff, 8.7 % (126/1,451) of older Korean men and 17.9 % (334/1,870) of older Korean women were vitamin D–insufficient. When subjects (49–95 years of age) were divided into four groups according to age (49–59, 60–69, 70–79, and ≥80 years), the age-specific prevalences of vitamin D insufficiency were 8.3, 8.3, 10.1, and 7.9 % for men and 17.0, 18.8, 16.5, and 24.4 % for women, respectively (Fig. 3a). At the 20.4 ng/mL serum 25(OH)D concentration cutoff, 50.4 % (731/1,451) of older Korean men and 66.3 % (1240/1,870) of older Korean women were vitamin D–insufficient. The age-stratified prevalences of vitamin D insufficiency were 53.2 %, 50.4 %, 46.5 %, and 46.0 % in men and 70.7, 64.9, 60.6, and 71.1 % in women, respectively (Fig. 3b).
Discussion
Previously, the WHO defined vitamin D insufficiency as a serum 25(OH)D concentration below 20 ng/mL, and concentrations below 10 ng/mL were considered vitamin D–deficient [12]. However, many observational studies suggest that the optimal serum 25(OH)D concentration for bone health is greater than 30 ng/mL and that concentrations between 30 and 20 ng/mL are considered vitamin D–insufficient. Concentrations below 20 ng/mL are considered to represent vitamin D deficiency [2–6]. There are two rationales for defining the optimal serum 25(OH)D concentration to be greater than 30 ng/mL. First, concentrations of iPTH rise when concentrations of 25(OH)D fall below 30 ng/mL, as observed in the present study [2]; second, active calcium absorption is optimal when the concentration of 25(OH)D is 30 ng/mL [7, 13]. It is well known that serum 25(OH)D concentrations are affected by a number of factors, including season of the year, age, body weight, and exercise levels, as well as factors that influence PTH, including dietary calcium intake and body weight [14, 15]. In this study serum 25(OH)D concentrations varied in relation to the season of the year (P < 0.001), being highest in the summer (22.9 ± 7.7 ng/mL) and lowest in the spring (16.9 ± 6.2 ng/mL); they also varied in relation to the degree of physical activity (P = 0.001). In addition, serum 25(OH)D levels were significantly correlated with dietary calcium intake (r = 0.065, P < 0.001) and showed an increasing tendency in subjects with exercise compared to those without exercise (P < 0.0001) (data not shown). Therefore, we determined the serum 25(OH)D concentration for vitamin D adequacy using its relationship with iPTH after adjusting for potential confounders, including age, gender, body weight, calcium intake, physical activity, and season of the year. Our results indicate that the optimal 25(OH)D concentrations for vitamin D adequacy were at least 12.1 ng/mL.
We also determined the optimal 25(OH)D concentrations for bone health using its relationship with BMD. We found that BMD for the total femur increased until serum 25(OH)D concentration dropped below 20.4 ng/mL; thereafter, no significant changes in serum 25(OH)D concentration were observed. To determine the clinical relevance of the new cutoffs, we assessed the risk for osteoporosis at three possible cutoff values for vitamin D insufficiency: 12.1, 20.4, and 30 ng/mL. The serum 25(OH)D concentration cutoffs of 12.1 ng/mL (OR = 1.26) and 20.4 ng/mL (OR = 1.54) were both associated with the presence of osteoporosis as defined by the WHO criteria. In contrast, the 30 ng/mL cutoff was not associated with osteoporosis. Previous studies have found that the Korean population has the lowest absolute concentration of serum 25(OH)D (17.6 ng/mL) compared to populations in other countries from a wide variety of latitudes (range 64°N–38°S) and that vitamin D inadequacy, defined as serum 25(OH)D concentrations below 30 ng/mL, was highest in Korea (92 %) [1]. Nonetheless, the 10 year probability of hip fracture in the Korean population is categorized as low compared to 29 other countries [16]. Thus, the prevalence of vitamin D insufficiency may not be as common as previously suggested. Optimal serum 25(OH)D concentrations for bone health may be lower than 30 ng/mL, and concentrations of 20 ng/mL might be sufficient.
In agreement with our observations, accumulating evidence suggests that adequate serum 25(OH)D for bone health is lower than previously suggested [10, 17, 18]. Among older, community-dwelling men in the United States, those with 25(OH)D concentrations below 20 ng/mL had higher rates of hip bone loss but rates of loss were similar among men with 25(OH)D concentrations above 20 ng/mL [19]. In addition, Nakamura et al. [20] investigated the threshold value for serum 25(OH)D concentration in relation to elevated serum PTH concentrations in 582 elderly community-dwelling Japanese women. The authors concluded that serum 25(OH)D for ambulant elderly Japanese women should be maintained at 16.0 ng/mL or higher. A histomorphometric study conducted in Germany found that 96.5 % of osteomalacia cases occurred at serum 25(OH)D concentrations below 20 ng/mL [21]. In addition, the IOM committee recently suggested that the recommended dietary allowance for vitamin D should be set at 600 IU/day for ages 1–70 and 800 IU/day for ages 71 and older. This would correspond to a serum 25(OH)D concentration of at least 20 ng/mL and would include at least 97.5 % of the population [10]. Sai et al. [17] also suggested that defining vitamin D insufficiency as a serum 25(OH)D concentration below 30 ng/mL based on serum PTH suppression is not supported by a literature review of 70 studies, and they concluded that vitamin D insufficiency should be redefined as a serum 25(OH)D concentration below 20 ng/mL, at least with regard to bone health.
In addition to vitamin D deficiency, a variety of factors are associated with an increased risk of developing osteoporosis and related fracture. For example, advanced age, female sex, Caucasian or Asian ethnicity, family history of fracture or osteoporosis, short stature, and premature menopause are important non-modifiable risk factors for osteoporotic fracture. In addition, various nutritional and lifestyle factors, including low calcium intake, smoking, alcohol, and inactivity, and drugs or diseases are closely related to BMD [18, 22, 23]. To determine the effect of serum 25(OH)D on the hip BMD, we performed linear regression analysis. Although serum 25(OH)D levels were associated with hip BMD (P < 0.0001), the calculated R 2 was only 0.02; thus, the contribution of 25(OH)D to hip BMD might be minimal. In addition, no difference in serum 25(OH)D levels was observed between subjects with any fracture (19.7 ± 6.9 ng/mL) and those without any fracture (19.6 ± 7.1 ng/mL) (data not shown). Furthermore, although age, female sex, low calcium intake, low BMI, and BMD at any site were significantly associated with the presence of any fracture, serum 25(OH)D level, physical activity, season, and smoking were not associated with the presence of any fracture in a univariate logistic regression analysis. Only age and BMD were independently associated with any fracture in multiple logistic regression analysis (data not shown).
Our study has some limitations. First, the use of serum iPTH concentrations alone in determining vitamin D insufficiency may be problematic [10, 17]. For example, our results may have been stronger if we had also determined the relationship between serum 25(OH)D concentrations and maximal intestinal calcium absorption. Likewise, an overall assessment of the optimal 25(OH)D concentrations for bone health should include additional factors, such as bone turnover markers and fractures. Second, the previous history of fractures was determined by a health interview and was not confirmed by any objective measures, such as radiologic examination. Therefore, recall bias may exist and underestimation of prevalence of fractures is possible. As a result, the prevalence of osteoporotic fractures (1.45 %) was low in our study sample; and thus, we were unable to determine whether our newly defined serum 25(OH)D cutoffs could more effectively identify subjects with fractures compared to the previous serum 25(OH)D cutoff of 30 ng/mL. Regarding the low prevalence of fractures, another explanation is possible. For example, our study population was older (mean age 63.6 years), and low activity in elderly people may decrease the risk of falls and possibly contributed to the low prevalence of fractures. Third, a small number of subjects with secondary hyperparathyroidism, especially due to decreased renal function, was included in this study. After calculating the estimated glomerular filtration ratio (GFR) from the Modification of Diet in Renal Disease formula, a total of 166 from 3,321 study subjects (5.0 %) were diagnosed with chronic kidney disease (CKD). Among the 166 subjects with CKD, stages 3, 4, and 5, there were 154 (4.6 %), 7 (0.2 %), and 5 (0.2 %), respectively; that is, most of the study subjects with CKD had estimated GFR levels of more than 60 mL/min · 1.73 m−2. Therefore, the effects of decreased renal function on serum 25(OH)D and iPTH levels might be minimal in our study population. Forth, we did not have data regarding medications and supplementations that affect bone metabolism, such as vitamin D supplementation. Despite these limitations, our study was designed to investigate the optimal serum 25(OH)D concentrations for bone health by assessing both serum iPTH concentrations and BMD, and our study sample was relatively large and broadly representative of the older Korean population.
In summary, the relationship we observed between serum 25(OH)D concentration and iPTH concentration suggests that optimal serum 25(OH)D concentrations are greater than 12.1 ng/mL and that the BMD for the total femur began to decrease at serum 25(OH)D concentrations below 20.4 ng/mL. In addition, the risk for osteoporosis was higher at serum 25(OH)D concentrations below 20.4 ng/mL. Therefore, the minimum serum 25(OH)D concentrations for bone health appear to be approximately 20 ng/mL in older adult Koreans.
References
Lips P, Hosking D, Lippuner K, Norquist JM, Wehren L, Maalouf G, Ragi-Eis S, Chandler J (2006) The prevalence of vitamin D inadequacy amongst women with osteoporosis: an international epidemiological investigation. J Int Med 260:245–254
Holick MF (2007) Vitamin D deficiency. N Engl J Med 357:266–281
Holick MF (2006) High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc 81:353–373
Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B (2006) Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes. Am J Clin Nutr 84:18–28
Malabanan A, Veronikis IE, Holick MF (1998) Redefining vitamin D insufficiency. Lancet 351:805–806
Thomas MK, Lloyd-Jones DM, Thadhani RI, Shaw AC, Deraska DJ, Kitch BT, Vamvakas EC, Dick IM, Prince RL et al (1998) Hypovitaminosis D in medical inpatients. N Engl J Med 338:777–783
Chapuy MC, Preziosi P, Maamer M, Arnaud S, Galan P, Hercberg S, Meunier PJ (1997) Prevalence of vitamin D insufficiency in an adult normal population. Osteoporos Int 7:439–443
Holick MF, Siris ES, Binkley N, Beard MK, Khan A, Katzer JT, Petruschke RA, Chen E, de Papp AE (2005) Prevalence of vitamin D inadequacy among postmenopausal North American women receiving osteoporosis therapy. J Clin Endocrinol Metab 90:3215–3224
Lips P (2001) Vitamin D deficiency and secondary hyperparathyroidism in the elderly: consequences for bone loss and fractures and therapeutic implications. Endocr Rev 22:477–501
Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL et al (2011) The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58
Kanis JA (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: synopsis of a WHO report. WHO Study Group. Osteoporos Int 4:368–381
Institute of Medicine (1997) Dietary reference intakes for calcium, phosphorus, magnesium, vitamin D, and fluoride. National Academies Press, Washington DC
Heaney RP, Dowell MS, Hale CA, Bendich A (2003) Calcium absorption varies within the reference range for serum 25-hydroxyvitamin D. J Am Coll Nutr 22:142–146
Bolland MJ, Grey AB, Ames RW, Mason BH, Horne AM, Gamble GD, Reid IR (2007) The effects of seasonal variation of 25-hydroxyvitamin D and fat mass on a diagnosis of vitamin D sufficiency. Am J Clin Nutr 86:959–964
Steingrimsdottir L, Gunnarsson O, Indridason OS, Franzson L, Sigurdsson G (2005) Relationship between serum parathyroid hormone levels, vitamin D sufficiency, and calcium intake. JAMA 294:2336–2341
Kanis JA, Johnell O, De Laet C, Jonsson B, Oden A, Ogelsby AK (2002) International variations in hip fracture probabilities: implications for risk assessment. J Bone Miner Res 17:1237–1244
Sai AJ, Walters RW, Fang X, Gallagher JC (2011) Relationship between vitamin D, parathyroid hormone, and bone health. J Clin Endocrinol Metab 96:E436–E446
Rosen CJ (2011) Clinical practice. Vitamin D insufficiency. N Engl J Med 364:248–254
Ensrud KE, Taylor BC, Paudel ML, Cauley JA, Cawthon PM, Cummings SR, Fink HA, Barrett-Connor E, Zmuda JM et al (2009) Serum 25-hydroxyvitamin D levels and rate of hip bone loss in older men. J Clin Endocrinol Metab 94:2773–2780
Nakamura K, Nashimoto M, Tsuchiya Y, Saito T, Nishiwaki T, Ueno K, Okuda Y, Oshiki R, Yamamoto M (2006) Threshold value of serum 25-hydroxyvitamin D concentration in relation to elevated serum parathyroid hormone concentrations in elderly Japanese women. J Bone Miner Metab 24:395–400
Priemel M, von Domarus C, Klatte TO, Kessler S, Schlie J, Meier S, Proksch N, Pastor F, Netter C et al (2010) Bone mineralization defects and vitamin D deficiency: histomorphometric analysis of iliac crest bone biopsies and circulating 25-hydroxyvitamin D in 675 patients. J Bone Miner Res 25:305–312
Poole KE, Compston JE (2006) Osteoporosis and its management. BMJ 333:1251–1256
Raso AA, Navarra SV, Li-Yu J, Torralba TP (2009) Survey of vitamin D levels among post-menopausal Filipino women with osteoporosis. Int J Rheum Dis 12:225–229
Acknowledgments
This study was performed using raw data from the Fourth Korea National Health and Nutrition Examination Survey (KNHANES IV), which was conducted by the Korean Centers for Disease Control and Prevention. This study was supported in part by a grant from the Clinical Research Institute, Kyung Hee University Hospital at Gangdong, in 2010 (KHNMCCRI-2010-001).
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hwang, YC., Ahn, HY., Jeong, IK. et al. Optimal Serum Concentration of 25-Hydroxyvitamin D for Bone Health in Older Korean Adults. Calcif Tissue Int 92, 68–74 (2013). https://doi.org/10.1007/s00223-012-9669-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-012-9669-3