Abstract
The objective of the present study was to examine the effect of dairy products enriched with calcium, vitamin D3, and phylloquinone (vitamin K1) or menaquinone-7 (vitamin K2) on parameters of bone metabolism in postmenopausal women following a 12-month intervention. Postmenopausal women were divided into three intervention groups and a control group (CG). All three intervention groups attended biweekly sessions and received fortified dairy products providing daily 800 mg of calcium and 10 μg of vitamin D3 (CaD). Furthermore, in two of the three intervention groups the dairy products were also enriched with vitamin K, providing daily 100 μg of either phylloquinone (CaDK1) or menaquinone-7 (CaDK2). The increase observed for serum 25(OH)D levels in all intervention groups and the increase observed for serum IGF-I levels in the CaDK2 group differed significantly compared to the changes observed in CG (P = 0.010 and P = 0.028, respectively). Furthermore, both the CaDK1 and CaDK2 groups had a significantly lower mean serum undercarboxylated osteocalcin to osteocalcin ratio and urine deoxypyridinoline levels at follow-up compared to the CaD and CG groups (P = 0.001 and P = 0.047, respectively). Significant increases in total-body BMD were observed in all intervention groups compared to CG (P < 0.05), while significant increases in lumbar spine BMD were observed only for CaDK1 and CaDK2 compared to CG (P < 0.05) after controlling for changes in serum 25(OH)D levels and dietary calcium intake. In conclusion, the present study revealed more favorable changes in bone metabolism and bone mass indices for the two vitamin K-supplemented groups, mainly reflected in the suppression of serum levels of bone remodeling indices and in the more positive changes in lumbar spine BMD for these two study groups.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The skeleton is a metabolically active organ that undergoes continuous remodeling throughout life. The activities of bone-forming osteoblasts and bone-resorbing osteoclasts are controlled by a variety of hormones, mainly parathyroid hormone (PTH) and calcitriol [1]. During bone resorption, osteoclastic activity leads to the release of breakdown products including pyridinium cross-links, such as pyridinoline (Pyr) and deoxypyridinoline (D-Pyr) [2]. In healthy bone, the resorption cavity created by osteoclasts is completely filled with new osteoid material secreted by active osteoblasts, such as osteocalcin (OC) collagen and hydroxyapatite. Besides bone remodeling indices, the identification of the osteoclastogenesis inducer receptor activator of nuclear factor-kappaB ligand (RANKL), its cognate receptor RANK, and its decoy receptor osteoprotegerin (OPG) has also contributed enormously to our understanding of the molecular mechanisms involved in osteoclast differentiation and activity. RANKL binds to RANK on the osteoclastic precursors or mature osteoclasts and promotes osteoclastogenesis and bone resorption, while OPG strongly inhibits bone resorption by binding to its ligand, RANKL, and thereby blocking the interaction between RANKL and RANK [3, 4]. Prevention of pathological bone loss therefore depends on an appreciation of the mechanisms by which osteoclasts differentiate from their precursors and degrade the skeleton [5].
Disorders of skeletal insufficiency, such as osteoporosis, typically represent enhanced osteoclastic bone resorption relative to bone formation. As life expectancy in developed countries is increasing, osteoporosis is becoming a major public health problem of great concern, particularly for susceptible population groups such as postmenopausal women [6]. Women have an increased rate of bone loss during and after menopause, which has been shown to slow down later [7, 8]. Some of the main risk factors for the progression and development of bone loss in these population groups are related to lifestyle and particularly to inadequate dietary intake of certain essential for bone health micronutrients (i.e., calcium and vitamin D) [6]. In addition to calcium and vitamin D, recent evidence suggests that supplementation with menaquinone-7 seems to have a favorable role on bone metabolism and bone mass maintenance and to produce a significant reduction especially in vertebral fractures; still, the available literature is contradictory regarding the exact effect of vitamin K on bone metabolism [9–12]. Although supplementary distribution of all of these bone metabolism nutrients is one approach to achieving adequate intakes, consumption of fortified staple foods, such as dairy products, has also been proven to be an effective approach [13–17].
The aim of this study was to examine the effect of nutrition and lifestyle counseling combined with consumption of milk enriched with calcium, vitamin D3, and phylloquinone or menaquinone-7 on daily nutrient intakes and physical activity levels, biochemical indices of bone metabolism, and BMD of postmenopausal women. Furthermore, the current study aimed to specifying any possible different actions of phylloquinone and menaquinone-7 on bone metabolism and BMD.
Methods
Sampling
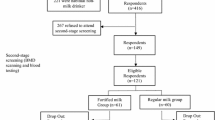
First Screening
In March 2008 volunteers were invited to participate by informational brochures and posters distributed in public buildings and community centers in municipalities from the wider district of Athens. The study was approved by the Ethical Committee of Harokopio University of Athens and conducted in accordance with the code of ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. The study protocol registration number was NTR1396. Through the initial screening of the study a sample of 720 postmenopausal women volunteered to participate. The first screening comprised a short questionnaire, primarily focusing on gathering information on women’s medical history, demographic data, diet, physical activity, and smoking habits. Furthermore, the bone status of all volunteers was assessed by calcaneal quantitative ultrasound (QUS) measurements, carried out by the SAHARA Clinical Bone Sonometer (Hologic, Waltham, MA). Through this initial screening those women diagnosed as having a T score lower than –2.5, taking medications (i.e., thiazide diuretics, glucocorticoids) and/or dietary supplements (calcium, magnesium, phosphate, or vitamin D) that affect bone metabolism; having any kind of degenerative chronic disease (i.e., diabetes, nephrolithiasis, heart disease, cancer, hyper- or hypothyroidism, hyperparathyroidism, impaired renal and liver function); smoking more than 5 cigarettes/day; having a habitual dietary calcium intake, assessed by a valid food-frequency questionnaire [18], of >800 mg/day; being at menopause (defined as no menses during the past 60 months) for <5 years; and being unwilling to participate in a 12-month intervention program were excluded from the second screening of the study.
Second Screening
After the initial screening, 219 women (age 55–65 years) satisfying the inclusion criteria were identified and invited to participate at the second screening of the study. During the second screening all volunteers underwent dual-energy X-ray absorptiometry (DXA; DPX-MD; Lunar, Madison, WI) as well as hematological and biochemical examinations, comprising erythrocyte sedimentation rate (ESR) and serum calcium, phosphorus, glutamic-oxaloacetic and glutamic pyruvic transaminases, gamma-glutamyl transferase, albumin, alkaline phosphatase, and creatinine levels. Forty-six women found to be osteoporotic, according to the data provided by the DXA examination, or having abnormal values on the aforementioned blood indices were excluded from the study. This second screening yielded 173 eligible women who prior to their entry to the study signed detailed consent forms to participate and proceeded in the intervention study. The intervention component of the study was initiated in October 2008.
Study Groups and Intervention
These 173 eligible women were randomly assigned to three intervention groups and one control group using a table of random digits. More specifically, the three intervention groups comprised a group receiving 800 mg of calcium and 10 μg of vitamin D3 (CaD, n = 38); a group receiving 800 mg of calcium, 10 μg of vitamin D3, and 100 μg phylloquinone (0.221 μmol; CaDK1, n = 38); and a group receiving 800 mg of calcium, 10 μg of vitamin D3, and 100 μg menaquinone-7 (0.154 μmol; CaDK2, n = 39) via fortified milk and yoghurt. No dietary intervention was delivered to the control group (CG), and they continued with their usual diet throughout the 12-month intervention period. Subjects in the three intervention groups were advised to consume one portion of fortified low-fat milk and one portion of fortified low-fat yoghurt on a daily basis. In order to avoid excess caloric intake, subjects in the three intervention groups were advised to substitute other dairy products in their diet with those provided. Subjects in the three intervention groups also attended nutrition and lifestyle counseling sessions that were held biweekly within the setting of the university. The theoretical framework upon which these nutrition and lifestyle counseling sessions were based was a combined application of the health belief model [19] and social cognitive theory [20]. More specifically, the aim of the sessions delivered to the three intervention groups was to increase awareness of the subjects on health issues, primarily related to osteoporosis, but also to motivate them to change certain lifestyle and dietary habits in order to improve their health status. The first sessions primarily focused on educating the subjects on the pathophysiology of osteoporosis as well as the risk factors (health-related behaviors) related to its development. Gradually, the sessions became more interactive and emphasis was given to guiding and assisting the subjects in changing their dietary habits. Finally, all study groups, including CG, were given the results of medical examinations as well as general health and nutrition guidelines right after every screening phase.
Assessment of the Effectiveness of the Intervention
During the intervention period the subjects from all study groups were invited to go through certain examinations primarily focusing on the assessment of behavioral and clinical indices. The data obtained from the second screening of the study were used as baseline data. Follow-up examinations took place after 12 months of intervention at October 2009. At baseline and follow-up examinations the following measurements were obtained.
Nutritional Assessment
The 24-hour recall technique was used to collect information on dietary intake for a total of 3 days—2 weekdays and 1 weekend day, preferably a Sunday. These data were obtained during scheduled interviews with the study participants at the university. All interviewers were rigorously trained to minimize interviewer effects. Respondents were asked to recall the type and amount of any foods and beverages consumed during the previous day (i.e., 24 h) in chronological order—i.e., from the time they woke up in the morning to the same time on the day of the examination. To improve the accuracy of food descriptions and portion sizes, standard household measures (e.g., cups and tablespoons) and picture food models (Western Dairy Council, Thornton, CO) were used during interviews to define amounts when appropriate. Food intake data were analyzed using NUTRITIONIST V diet analysis software (version 2.1; First Databank, San Bruno, CA), which was extensively amended to include food composition tables for Greek foods and recipes [21, 22] and chemically analyzed commercial food items widely consumed in Greece.
Physical Activity
Physical activity was assessed by recording the number of steps for 1 week with the use of waist-mounted pedometers (Yamax SW-200 Digiwalker; Yamax, Tokyo, Japan) that were provided to all subjects at baseline and follow-up. The pedometer was positioned according to the manufacturer’s instructions on the right waistband in line with the patella. This pedometer uses a spring-suspended horizontal lever arm that moves up and down in response to the hip’s vertical accelerations. This movement opens and closes an electrical circuit; the lever arm makes an electrical contact, and a step is registered. The pedometer used in the present study displayed the cumulative number of steps from the time it was worn in the morning until the time it was removed at night, i.e., before bedtime.
Anthropometry
Anthropometric measurements were carried out during the initial screening as well as at baseline and follow-up examinations. At all aforementioned time points, body weight and standing height were measured in light clothing and with no shoes using a digital scale (Seca Alpha, model 770; Seca, Hamburg, Germany) with an accuracy of ± 100 g and a commercial stadiometer (Leicester Height Measure; Invicta Plastics, Oadby, UK) to the nearest 0.5 cm. Body mass index (BMI) was calculated as weight (kg) divided by height squared (m2).
Biochemical Indices
Blood Indices
Early-morning venous blood samples were obtained from each subject for biochemical screening tests following a 12-hour overnight fast. Professional staff performed venipuncture to obtain a maximum of 25 mL blood. The blood was collected in tubes with no added anticoagulant, where it was allowed to clot for approximately 2 h for serum separation. Centrifugation for serum separation was conducted at 3,000 rpm for 15 min. A part of the collected serum was used for biochemical analyses, while aliquots of 1.5 mL from the remaining serum were pipetted into plastic Eppendorf tubes and stored at –80°C.
Biochemical analyses included radioimmunoassay for amino-terminal procollagen 1 extension peptide (P1NP) levels (Orion Diagnostica, Espoo, Finland); immunoradiometric assay for total and undercarboxylated osteocalcin (OC and UnOC, respectively) before and after extraction with hydroxyapatite (Medgenix-H-Ost, Fleurus, Belgium). For extraction with hydroxyapatite 250 mL serum were incubated with 7.5 mg hydroxyapatite (calcium phosphate tribasic type IV; Sigma, St. Louis, MO) in an Eppendorf tube, mixed end over end for 1 h at 4°C, and then centrifuged at 3,000 rpm for 15 min. For intact PTH levels (Nichols Advantage®; Nichols Institute Diagnostics, San Clemente, CA), an enzyme immunoassay for matrix-Gla protein (Biomedica, Vienna, Austria) and a chemiluminescence immunoassay for 25-hydroxyvitamin D (25[OH]D3) and insulin-like growth factor I (IGF-I, Nichols Advantage) were performed. Furthermore, the ELISA method was used for the quantitative determination of human OPG and human ampli-sRANKL in duplicate serum samples of the participants by the Biomedica immunoassay kits. Both baseline and follow-up blood samples were analyzed at the end of the trial so as to avoid interassay bias.
Urine Indices
Second-morning urine samples were collected prior to any food or drink consumption, at baseline and follow-up. Samples were prepared with 2 mL of HCl 6 N and stored at –80°C until analysis. Bone resorption indices of hydrolyzed Pyr and D-Pyr were analyzed using HPLC (Chromsystems Instruments & Chemicals, Munich, Germany). Urinary excretion of Pyr and D-Pyr was corrected for urinary creatinine.
BMD and Total-Body Composition Measurements
BMD (g/cm2) of the lumbar spine (L2–L4), hip (i.e., total proximal femur, femoral neck, trochanter, intertrochanter, Ward’s triangle), and total body (i.e., total and segmental BMD, lean and fat mass) were measured at baseline and at 12-month follow-up examination, using DXA with the analysis software version 4.6. The BMDs of regional skeletal sites (i.e., arms, legs, pelvis, and total spine) were extracted from the analyses of total-body scans. Correct positioning of each study participant at repeated measures was ensured by geometrically positioning subjects exactly as indicated in the manufacturer’s manual. Furthermore, all follow-up scans were analyzed by using the compare-scans mode of the equipment’s software in order to define exactly the same profile lines at baseline and follow-up measurements for each subject. A daily quality-assurance check was performed at each time point of follow-up examination, using a calibration standard of known composition, provided by the manufacturer. Before the beginning of the study the coefficients of variation were estimated and found to be 0.7% for total-body BMD and to range from 1% to 2% for other regional skeletal sites. Scans were performed in the morning by an experienced technician, who was blinded to the therapy.
Compliance
Compliance to the intervention scheme was assessed via information obtained at the biweekly sessions, combined with data obtained from the nutritional assessments conducted at baseline and follow-up examinations. These data showed that compliance to the intervention scheme reached 95%. Subjects with compliance <75% for any of the supplemented key nutrients were excluded, resulting in a total of 115 subjects. The sample sizes in the four study groups (i.e., 39 in CG, 26 in CaD, 26 in CaDK1, and 24 in CaDK2) were adequate since we achieved statistical power >90% for standardized differences for the main outcomes of our study (i.e., BMD indices) between groups of >2.5 (SE = 1.4) with a probability of Type I error of <0.05.
Statistical Analysis
All data are reported as means and standard deviations and as mean percentage changes (with 95% CI) over baseline. The Kolmogorov–Smirnov test was used to determine the normality of distribution of the examined variables. Differences in baseline characteristics among the three groups of women were evaluated using one-way analysis of variance (ANOVA). Repeated measures ANOVA was used to evaluate the significance of the differences between groups at baseline and 12 months of follow-up (treatment effect), the significance of the changes observed within each group (time effect), and the effect of treatment × time interaction (i.e., the significance of the differences among groups regarding the changes observed from baseline to follow-up) after controlling for certain potential covariates. The between-group factor was the study groups; the within-group factor was the time point of measurement (i.e., baseline and 12 months of intervention). The inflation in Type I error due to multiple post hoc comparisons between groups was corrected using the Bonferroni rule. In case of variables being not normally distributed, the Friedman test for repeated measures was used. All P values are two-tailed. Statistical analysis was conducted with SPSS version 13.0 (SPSS, Inc., Chicago, IL). The level of statistical significance was set at P ≤ 0.05.
Results
The mean age of study participants at baseline was 62.0 (SD = 5.8) years (range 54–73), the average elapsed time since menopause was 11.9 (SD = 6.9) years, and the mean educational level was 10.4 (SD = 4.0) years, with no significant differences among study groups. Furthermore, no significant differences were observed between study groups in baseline hematological indices of inflammation (i.e., ESR) and in a wide range of biochemical indices mainly of renal and hepatic function (data not shown), indicating homogeneity among groups at baseline.
Tables 1 and 2 summarize the differences among groups at baseline and 12 months of follow-up with respect to certain anthropometric (i.e., BMI), body composition (i.e., percent fat mass), and behavioral (i.e., dietary intake and physical activity) indices. No significant differences were observed in the changes of BMI and percent of fat mass levels among the four study groups from baseline to follow-up. On the contrary, the increases in the average daily number of steps recorded by subjects in the three dietary intervention groups were found to differentiate significantly compared to the change observed in the CG (P = 0.003). Regarding dietary intake indices, no significant differences were observed among groups with respect to energy and macronutrient intake. Furthermore, subjects in all three dietary intervention groups were found to significantly increase their intakes of calcium, vitamin D, phosphorus, and magnesium from baseline, leading to significantly higher intakes at follow-up compared to the CG (P = 0.001). Similarly, dietary phylloquinone intake significantly increased from baseline in the CaDK1 group (P = 0.001). The additional intakes from phylloquinone and menaquinone-7 supplementation in the CaDK1 and CaDK2 groups, respectively, resulted in significantly higher overall vitamin K intakes in these groups compared to the CaD and CG groups (P = 0.001). There was no significant difference in dietary intakes of phylloquinone in the CaDK2 group at baseline and after 12 months.
According to the data presented in Table 3, regarding changes in hormonal and osteoclast differentiation molecules, significant treatment × time interaction effects were observed for serum IGF-I and 25(OH)D (P = 0.028 and P = 0.001, respectively) levels. Specifically, serum IGF-I levels increased significantly in the CaDK2 group and serum 25(OH)D levels increased significantly in both the CaDK1 and CaDK2 groups, resulting in higher mean serum IGF-I and 25(OH)D levels at follow-up, compared to the CG (P = 0.036 and P = 0.010, respectively). No other treatment × time interaction effect was observed. In this context, although OPG levels were not found to differentiate significantly among groups, they increased significantly in the CG and CaD groups (0.38, 95% CI 0.10–0.66, and 0.40, 95% CI 0.06–0.74, respectively).
The changes in serum and urine levels of biochemical indices of bone turnover are presented in Table 4. Regarding bone formation indices, a significant treatment × time interaction effect was found for serum percent UnOC, with the CaDK1 and CaDK2 groups having significantly lower levels at follow-up compared to the CaD and CG groups (P = 0.001). Furthermore, significant decreases in P1NP levels were also observed for CaD and CaDK1. Regarding bone resorption indices, urine Pyd significantly decreased in CaDK2, while urine D-Pyd was significantly reduced in both CaDK1 and CaDK2, leading to significantly lower follow-up levels in these two groups compared to CaD and CG (P = 0.047).
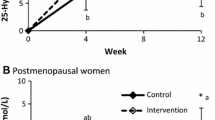
According to the data presented in Fig. 1, at the end of the 12-month intervention period all three dietary intervention groups were found to have significantly higher increases in total-body BMD compared to the decrease observed in CG (P = 0.001). Specifically, total-body BMD values observed at baseline and follow-up were, respectively, 1.112 (SD = 0.077) and 1.135 (SD = 0.083) g/cm2 in CaD, 1.117 (SD = 0.101) and 1.129 (SD = 0.099) g/cm2 in CaDK1, 1.105 (SD = 0.078) and 1.117 (SD = 0.077) g/cm2 in CaDK2, as well as 1.095 (SD = 0.079) and 1.094 (SD = 0.079) g/cm2 in CG. Furthermore, the changes for L2–L4 BMD observed in CaDK1 and CaDK2 were found to differentiate significantly (P = 0.001) compared to the decrease observed in CG. More specifically, L2–L4 BMD values observed at baseline and follow-up were, respectively, 1.256 (SD = 0.192) and 1.273 (SD = 0.0.195) g/cm2 in CaDK1, 1.165 (SD 0.169) and 1.171 (SD = 0.158) g/cm2 in CaDK2, 1.121 (SD = 0.158) and 1.113 (SD 0.160) g/cm2 in CaD, as well as 1.134 (SD = 0.176) and 1.101 (SD = 0.167) g/cm2 in CG. However, when the analysis presented in Fig. 1 was repeated controlling for serum 25(OH)D levels and dietary calcium intake, the significant differences observed for total-body BMD among the three intervention groups and the CG were lost (P = 0.073), while the differences observed for L2–L4 BMD among CADK1, CADK2, and CG remained significant (P = 0.002). No other significant differences among groups were observed regarding changes in pelvis, leg, arm, and hip BMD as described analytically elsewhere [23].
Changes in lumbar spine (L2–L4) and total-body BMD for women in the three intervention groups and the control group. In all analyses adjustments were made for physical activity levels. In case of lumbar spine BMD, adjustment was also made for baseline values. *Treatment × time interaction effect. †P < 0.05 compared to the baseline value within group, ‡P < 0.05 compared to CG
Discussion
As expected, the present study showed that the intake of calcium and vitamin D significantly increased in all three dietary intervention groups, reaching and remaining close to the recommended adequate intake (AI) levels (i.e., 1,200 mg and 10 μg per day, respectively) [24] throughout the intervention period. Consumption of the fortified dairy products also led to significant increases in magnesium intake, a nutrient which is also essential for bone mineralization [25]. Additionally, vitamin K intake significantly increased in the two intervention groups that consumed milk enriched with phylloquinone and menaquinone-7, leading to total dietary intakes that were more than twice the recommended AI level of 90 μg/day [24] but close to or still lower than recommendations based on latest scientific insights [26]. As far as physical activity levels were concerned, significant increases were observed in all three intervention groups by more than 2,000 steps per day. Contrary to the current findings, other similar studies have confronted difficulties in motivating middle-aged women who already have a sedentary lifestyle to become more active [13, 16, 27]. The use of subjective methods (i.e., questionnaires) to assess changes in physical activity levels during the intervention period could provide an explanation for the lack of significant findings in these studies. Overall, the favorable dietary and physical activity changes recorded in the present study for the intervention groups are probably indicative of the effectiveness of the “nutrition and lifestyle counseling” component of the program in increasing the self-efficacy of the intervention subjects to comply with the given health behavior instructions.
The effect of the intervention on bone metabolism and BMD should be attributed to the changes induced in both the diet and physical activity levels of the intervention subjects. Moreover, in order to examine the independent effect of the dietary intervention (i.e., nutrient intake and nutrition counseling) on the examined biochemical and bone mass indices, all relative analyses were adjusted for physical activity levels. With regard to calcium homeostasis hormones, serum 25(OH)D levels increased in all three dietary intervention groups, leading to significantly higher mean serum levels in these groups at follow-up compared to the CG. Although the major source of 25(OH)D3 endogenous synthesis is photoconversion of 7-dehydrocholesterol to previtamin D3 in the skin following exposure to UVB radiation, measurements of sunlight exposure via personal UV dosimetry in a previous similar intervention study on Greek postmenopausal women also living in municipalities from the wider district of Athens revealed no significant sunlight exposure effect on their vitamin D status [13]. These data indicate that supplementation of 10 μg of vitamin D3 via fortified milk in the three intervention groups was effective at inducing more favorable changes in serum 25(OH)D levels compared to control subjects who continued with their usual diet. Furthermore, the results of the present study regarding serum IGF-I levels showed that only the CaDK2 group had a significant increase over the CG. The reason for this finding remains unclear, and to the best of our knowledge a relationship between IGF-I and menaquinone-7 has not been described before. This anabolic hormone-like peptide has been reported by recent studies to stimulate bone formation activity in postmenopausal women, basically via osteoblast differentiation [28].
To our knowledge, the current study is the first to examine the effect of a dietary intervention on osteoclast differentiation molecules, i.e., in serum RANKL and OPG levels. The discovery of the unique role of RANK/RANKL/OPG signaling has led to the targeting of this pathway as a novel therapeutic approach in the management of osteoporosis [29, 30]. Although very little is known so far about the effect of dietary interventions on bone markers, there have been studies in postmenopausal women where 24 months of genistein administration resulted in higher OPG and lower RANKL levels [31]. However, no significant differences were observed in the present study among groups with respect to these two biochemical indices of bone metabolism, probably indicating the need for additional research.
Examination of the changes induced over the intervention period in certain biochemical indices of bone remodeling revealed significant differences among groups with respect to the percent UcOC. Specifically, the significant decrease observed in the CG and the increases observed in the two vitamin K–supplemented groups led to significantly lower percent UcOC at follow-up in these two groups compared to the CG. Vitamin K is necessary for the carboxylation of OC, a process that is vital for bone calcification. Yet, under vitamin K insufficiency, OC does not undergo complete γ-carboxylation, resulting in the release of UnOC from osteoblasts into the circulating blood [32]. The results of the present study are consistent with those reported by other similar studies conducted with postmenopausal women, showing that either supplementation with [10, 33–35] or increased dietary intake [36] of vitamin K can significantly reduce UnOC levels. Furthermore, the present study showed that increased dietary intake of phylloquinone or menaquinone-7 by subjects in the two relative intervention groups resulted in significant decreases in urine D-Pyd levels, producing significantly lower mean levels at follow-up compared to CaD and CG. This finding combined with the significant decrease observed for urine Pyd levels in CaDK2 is in line with in vitro studies [37, 38] and other similar clinical trials [34] demonstrating that treatment with vitamin K, especially menaquinone-7, is effective enough to reduce bone resorption. Summarizing the above findings, the present study showed that increased dietary intake of phylloquinone and menaquinone-7 via milk also fortified with calcium and vitamin D is likely to lead to a suppression of bone remodeling, which has been associated with lower fracture risk [39–41].
According to the results derived from DXA measurements, the findings of the present study revealed more favorable changes over the intervention period in total-body BMD in the three intervention groups compared to the CG (Table 3), with the absolute BMD values at baseline and follow-up examinations presented analytically elsewhere [23]. Similar to the findings from the present study, other intervention studies showed that total dietary calcium intake of 1,200 mg/day or higher was adequate to prevent bone loss from the total body, lumbar spine, femoral neck, and greater trochanter in susceptible populations, such as postmenopausal women [17, 42]. Furthermore, calcium intake has been proven to be even more effective with respect to total-body bone mass maintenance when combined with other essential nutrients for bone health and especially with vitamin D [13, 43].
Furthermore, there is only very little evidence available in the literature regarding the exact effect of vitamin K supplementation on bone mass in humans [9–12, 44–48], while there is no evidence of the combined effect of calcium, vitamin D, and vitamin K when these are administered as components of fortified dairy products. According to the findings of the present study, further supplementation of phylloquinone and menaquinone-7 induced more favorable changes in lumbar spine (L2–L4) BMD. These changes could probably point to additional site-specific skeletal benefits derived from increased vitamin K intake, which mainly apply for trabecular (cancellous), but not for cortical (compact), bone tissue [44]. Similar changes have been reported by another 2-year randomized controlled intervention study that combined phylloquinone and vitamin D supplementation [44]. Safer conclusions can be reached only by conducting further clinical research and by applying more sensitive bone scanning techniques to examine the changes in BMD distribution, while the question of which isoprenologue of vitamin K (i.e., phylloquinone or menaquinone-7) is more effective on bone metabolism cannot be addressed by the outcomes of the present study. With respect to the relative efficacies of phylloquinone versus menaquinone-7, other considerations are that on a molecular basis the daily intake of menaquinone-7 (0.154 μmol) in this study was about 30% less than that of phylloquinone (0.221 μmol). On the other hand, there is evidence that on a molar basis menaquinone-7 is much more effective than phylloquinone in increasing the degree of γ-carboxylation of OC [49]. The reason for this greater efficacy of menaquinone-7 seems to be its longer half-life of clearance from the circulation than phylloquinone [49].
In summary, the present study showed more favorable changes on bone metabolism and bone mass indices, mainly for the vitamin K–supplemented groups. The beneficial intervention effect in these two study groups was mainly reflected in the suppression of the bone remodeling process and the positive changes observed in L2–L4 BMD. The holistic nature of the current intervention and probably the synergistic effect of supplementing nutrients that are essential for bone health (i.e., milk protein, vitamin D, vitamin K, calcium, magnesium, and phosphorus) together with increasing physical activity levels and improving dietary patterns as a result of counseling sessions could probably provide an explanation for these favorable changes. However, further clinical trials, probably of longer duration and higher vitamin K doses, could help clarify the exact role of each one of these two vitamin K isoprenologues on bone metabolism and BMD.
References
Heaney RP, McCarron DA, Dawson-Hughes B, Oparil S, Berga SL, Stern JS, Barr SI, Rosen CJ (1999) Dietary changes favorably affect bone remodeling in older adults. J Am Diet Assoc 99:1228–1233
Bonde M, Qvist P, Fledelius C, Riis BJ, Christiansen C (1994) Immunoassay for quantifying type I collagen degradation products in urine evaluated. Clin Chem 40:2022–2025
Jensen C, Holloway L, Block G, Spiller G, Gildengorin G, Gunderson E, Butterfield G, Marcus R (2002) Long-term effects of nutrient intervention on markers of bone remodeling and calciotropic hormones in late-postmenopausal women. Am J Clin Nutr 75:1114–1120
Wittrant Y, Theoleyre S, Chipoy C, Padrines M, Blanchard F, Heymann D, Redini F (2004) RANKL/RANK/OPG: new therapeutic targets in bone tumours and associated osteolysis. Biochim Biophys Acta 1704:49–57
Martin RB (2002) Is all cortical bone remodeling initiated by microdamage? Bone 30:8–13
Nieves JW (2005) Osteoporosis: the role of micronutrients. Am J Clin Nutr 81:1232S–1239S
Ahlborg HG, Johnell O, Turner CH, Rannevik G, Karlsson MK (2003) Bone loss and bone size after menopause. N Engl J Med 349:327–334
Zaidi M, Turner CH, Canalis E, Pacifici R, Sun L, Iqbal J, Guo XE, Silverman S, Epstein S, Rosen CJ (2009) Bone loss or lost bone: rationale and recommendations for the diagnosis and treatment of early postmenopausal bone loss. Curr Osteoporos Rep 7:118–126
Iwamoto J, Takeda T, Ichimura S (2001) Effect of menatetrenone on bone mineral density and incidence of vertebral fractures in postmenopausal women with osteoporosis: a comparison with the effect of etidronate. J Orthop Sci 6:487–492
Cheung AM, Tile L, Lee Y, Tomlinson G, Hawker G, Scher J, Hu H, Vieth R, Thompson L, Jamal S, Josse R (2008) Vitamin K supplementation in postmenopausal women with osteopenia (ECKO trial): a randomized controlled trial. PLoS Med 5:e196
Shiraki M, Shiraki Y, Aoki C, Miura M (2000) Vitamin K2 (menatetrenone) effectively prevents fractures and sustains lumbar bone mineral density in osteoporosis. J Bone Miner Res 15:515–521
Ishida Y, Kawai S (2004) Comparative efficacy of hormone replacement therapy, etidronate, calcitonin, alfacalcidol, and vitamin K in postmenopausal women with osteoporosis: the Yamaguchi Osteoporosis Prevention Study. Am J Med 117:549–555
Manios Y, Moschonis G, Trovas G, Lyritis GP (2007) Changes in biochemical indexes of bone metabolism and bone mineral density after a 12-mo dietary intervention program: the Postmenopausal Health Study. Am J Clin Nutr 86:781–789
Chee WS, Suriah AR, Chan SP, Zaitun Y, Chan YM (2003) The effect of milk supplementation on bone mineral density in postmenopausal Chinese women in Malaysia. Osteoporos Int 14:828–834
McKenna MJ, Freaney R, Byrne P, McBrinn Y, Murray B, Kelly M, Donne B, O’Brien M (1995) Safety and efficacy of increasing wintertime vitamin D and calcium intake by milk fortification. QJM 88:895–898
Prince R, Devine A, Dick I, Criddle A, Kerr D, Kent N, Price R, Randell A (1995) The effects of calcium supplementation (milk powder or tablets) and exercise on bone density in postmenopausal women. J Bone Miner Res 10:1068–1075
Storm D, Eslin R, Porter ES, Musgrave K, Vereault D, Patton C, Kessenich C, Mohan S, Chen T, Holick MF, Rosen CJ (1998) Calcium supplementation prevents seasonal bone loss and changes in biochemical markers of bone turnover in elderly New England women: a randomized placebo-controlled trial. J Clin Endocrinol Metab 83:3817–3825
Magkos F, Manios Y, Babaroutsi E, Sidossis LS (2006) Development and validation of a food frequency questionnaire for assessing dietary calcium intake in the general population. Osteoporos Int 17:304–312
Janz NK, Becker MH (1984) The health belief model: a decade later. Health Educ Q 11:1–47
Bandura A, Adams NE, Beyer J (1977) Cognitive processes mediating behavioral change. J Pers Soc Psychol 35:125–139
Trichopoulou A (2004) Composition tables of foods and Greek dishes. Department of Epidimiology, School of Medicine, University of Athens, Athens
University of Crete (1991) Food composition tables (website in Greek). http://nutrition.med.uoc.gr/GreekTables
Moschonis G, Kanellakis S, Papaioannou N, Schaafsma A, Manios Y (2011) Possible site-specific effect of an intervention combining nutrition and lifestyle counselling with consumption of fortified dairy products on bone mass: the Postmenopausal Health Study II. J Bone Miner Metab 29:501–506
Institute of Medicine Food and Nutrition Board (2001) Dietary reference intakes: applications in dietary assessment. National Academy Press, Washington, DC
Kitchin B, Morgan SL (2007) Not just calcium and vitamin D: other nutritional considerations in osteoporosis. Curr Rheumatol Rep 9:85–92
Bugel S (2008) Vitamin K and bone health in adult humans. Vitam Horm 78:393–416
Wolff I, van Croonenborg JJ, Kemper HC, Kostense PJ, Twisk JW (1999) The effect of exercise training programs on bone mass: a meta-analysis of published controlled trials in pre- and postmenopausal women. Osteoporos Int 9:1–12
Ghiron LJ, Thompson JL, Holloway L, Hintz RL, Butterfield GE, Hoffman AR, Marcus R (1995) Effects of recombinant insulin-like growth factor-I and growth hormone on bone turnover in elderly women. J Bone Miner Res 10:1844–1852
Hamdy NA (2007) Targeting the RANK/RANKL/OPG signaling pathway: a novel approach in the management of osteoporosis. Curr Opin Investig Drugs 8:299–303
Hofbauer LC, Schoppet M (2004) Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. Jama 292:490–495
Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Atteritano M, Gaudio A, Mazzaferro S, Frisina A, Frisina N, Lubrano C, Bonaiuto M, D’Anna R, Cannata ML, Corrado F, Cancellieri F, Faraci M, Marini R, Adamo EB, Wilson S, Squadrito F (2008) OPG and sRANKL serum concentrations in osteopenic, postmenopausal women after 2-year genistein administration. J Bone Miner Res 23:715–720
Hauschka PV, Lian JB, Cole DE, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K–dependent proteins in bone. Physiol Rev 69:990–1047
Iwamoto J, Sato Y, Takeda T, Matsumoto H (2009) High-dose vitamin K supplementation reduces fracture incidence in postmenopausal women: a review of the literature. Nutr Res 29:221–228
Ozuru R, Sugimoto T, Yamaguchi T, Chihara K (2002) Time-dependent effects of vitamin K2 (menatetrenone) on bone metabolism in postmenopausal women. Endocr J 49:363–370
Shiraki M, Itabashi A (2009) Short-term menatetrenone therapy increases gamma-carboxylation of osteocalcin with a moderate increase of bone turnover in postmenopausal osteoporosis: a randomized prospective study. J Bone Miner Metab 27:333–340
Yamauchi M, Yamaguchi T, Nawata K, Takaoka S, Sugimoto T (2010) Relationships between undercarboxylated osteocalcin and vitamin K intakes, bone turnover, and bone mineral density in healthy women. Clin Nutr 29:761–765
Hara K, Akiyama Y, Tajima T, Shiraki M (1993) Menatetrenone inhibits bone resorption partly through inhibition of PGE2 synthesis in vitro. J Bone Miner Res 8:535–542
Kameda T, Miyazawa K, Mori Y, Yuasa T, Shiokawa M, Nakamaru Y, Mano H, Hakeda Y, Kameda A, Kumegawa M (1996) Vitamin K2 inhibits osteoclastic bone resorption by inducing osteoclast apoptosis. Biochem Biophys Res Commun 220:515–519
Weisman SM, Matkovic V (2005) Potential use of biochemical markers of bone turnover for assessing the effect of calcium supplementation and predicting fracture risk. Clin Ther 27:299–308
Stepan JJ (2000) Prediction of bone loss in postmenopausal women. Osteoporos Int 11(suppl 6):S45–S54
Delmas PD, Eastell R, Garnero P, Seibel MJ, Stepan J (2000) The use of biochemical markers of bone turnover in osteoporosis. Committee of Scientific Advisors of the International Osteoporosis Foundation. Osteoporos Int 11(suppl 6):S2–S17
Riggs BL, O’Fallon WM, Muhs J, O’Connor MK, Kumar R, Melton LJ 3rd (1998) Long-term effects of calcium supplementation on serum parathyroid hormone level, bone turnover, and bone loss in elderly women. J Bone Miner Res 13:168–174
Moschonis G, Katsaroli I, Lyritis GP, Manios Y (2010) The effects of a 30-month dietary intervention on bone mineral density: the Postmenopausal Health Study. Br J Nutr 104:100–107
Bolton-Smith C, McMurdo ME, Paterson CR, Mole PA, Harvey JM, Fenton ST, Prynne CJ, Mishra GD, Shearer MJ (2007) Two-year randomized controlled trial of vitamin K1 (phylloquinone) and vitamin D3 plus calcium on the bone health of older women. J Bone Miner Res 22:509–519
Cockayne S, Adamson J, Lanham-New S, Shearer MJ, Gilbody S, Torgerson DJ (2006) Vitamin K and the prevention of fractures: systematic review and meta-analysis of randomized controlled trials. Arch Intern Med 166:1256–1261
Schaafsma A, Muskiet FA, Storm H, Hofstede GJ, Pakan I, Van der Veer E (2000) Vitamin D3 and vitamin K1 supplementation of Dutch postmenopausal women with normal and low bone mineral densities: effects on serum 25-hydroxyvitamin D and carboxylated osteocalcin. Eur J Clin Nutr 54:626–631
Vermeer C, Shearer MJ, Zittermann A, Bolton-Smith C, Szulc P, Hodges S, Walter P, Rambeck W, Stocklin E, Weber P (2004) Beyond deficiency: potential benefits of increased intakes of vitamin K for bone and vascular health. Eur J Nutr 43:325–335
Eisai (2005) Eisai announces the intermediate analysis of anti-osteoporosis treatment post-marketing research to investigate the benefits of menatetrenone as part of the Ministry of Health, Labour and Welfare’s. Pharmacoepidemiological Drug Review Program
Schurgers LJ, Teunissen KJ, Hamulyak K, Knapen MH, Vik H, Vermeer C (2007) Vitamin K–containing dietary supplements: comparison of synthetic vitamin K1 and natto-derived menaquinone-7. Blood 109:3279–3283
Acknowledgement
This work was supported by a research grant from FrieslandCampina. The study sponsor had no role in the collection and analysis of the data, or the submission of the report. The authors thank Anastasia Vandorou, Katerina Kyriakou, Vasiliki Dede, Odysseas Androutsos, Konstantina Maragkopoulou, Efstathoula Argyri, Angeliki Giannopoulou, Vassiliki Iatridi, Christina Maurogianni, Sotiria Pitsikou, Sofia Chondrou, George Saltaouras, and Dimitra Meladaki (dietitians) for their valuable contribution in data collection and processing.
Author information
Authors and Affiliations
Corresponding author
Additional information
A. Schaafsma and E. G. H. M. van den Heuvel have consultant/advisory role to FrieslandCampina BV. Y. Manios has consultant/advisory role to FrieslandCampina Hellas. All other authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Kanellakis, S., Moschonis, G., Tenta, R. et al. Changes in Parameters of Bone Metabolism in Postmenopausal Women Following a 12-Month Intervention Period Using Dairy Products Enriched with Calcium, Vitamin D, and Phylloquinone (Vitamin K1) or Menaquinone-7 (Vitamin K2): The Postmenopausal Health Study II. Calcif Tissue Int 90, 251–262 (2012). https://doi.org/10.1007/s00223-012-9571-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-012-9571-z