Abstract
The purpose of the present study was to examine the effect of vitamin K2 on cancellous and cortical bone mass in rats with streptozotocin (STZ)-induced type 1 diabetes. Twenty-seven male Sprague-Dawley rats aged 12 weeks were randomized by the weight-stratified method into the following three groups: age-matched control group, STZ + vehicle group, and STZ + vitamin K2 group. STZ (40 + 50 mg/kg) was administered intravenously twice during the initial 1-week period. Vitamin K2 (menatetrenone, 30 mg/kg) was administered orally 5 days a week. After 12 weeks of treatment, the serum glucose concentration and femoral length and weight were measured and histomorphometric analysis was performed on the cancellous and cortical bone of the distal femoral metaphysis and femoral diaphysis, respectively. STZ administration induced hyperglycemia and a decrease in femoral weight. The STZ + vehicle group also showed cancellous osteopenia due to a decrease in the number of osteoblasts/bone surface (N.Ob/BS) and the osteoblast surface (ObS)/BS without any significant changes in bone-resorption parameters, but it did not have a significant decrease in cortical bone mass. Administration of vitamin K2 to STZ-treated rats prevented the development of hyperglycemia and a decrease in femoral weight. Vitamin K2 also prevented cancellous osteopenia by inhibiting the decrease in N.Ob/BS and ObS/BS without significantly affecting bone-resorption parameters, but it did not significantly increase cortical bone mass. These results suggest that vitamin K2 has beneficial effects on glucose concentration and cancellous bone mass in rats with STZ-induced type 1 diabetes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The metabolic and endocrine alterations of diabetes adversely affect bone and may increase the risk of fractures. Patients with type 1 diabetes have reduced bone mass and increased risk of fragility fracture [1]. Patients with recent onset of type 1 diabetes may have impaired bone formation because of the absence of the anabolic effects of insulin and amylin, whereas in long-standing type 1 diabetes, vascular complications may account for low bone mass and increased fracture risk [2]. A meta-analysis conducted by Janghorbani et al. [3] found that type 1 diabetes was associated with an increased risk of hip fractures (relative risk = 6.3).
Type 1 diabetes can be induced in animals by intravenous injection of streptozotocin (STZ), which is toxic to insulin-producing cells and thus causes acute insulinopenia and hyperglycemia [4]. Young rats with type 1 diabetes due to STZ administration display progressive cancellous osteopenia, cessation of cortical bone growth, and diminished whole-bone strength [5]. The skeletal changes associated with STZ-induced type 1 diabetes mainly result from decreased bone formation [6–15]. In particular, STZ decreases the serum osteocalcin level in treated rats [12–15]. A decrease in bone strength, despite normal bone mineral density (BMD), in rats with type 2 diabetes is partly attributable to abnormal cross-linking of bone collagen (a steady decrease in enzymatic cross-links and a marked increase in nonenzymatic cross-links, i.e., pentosidine) [16]. Conversely, the short-term skeletal effects of type 1 diabetes in rats involve changes in bone structure rather than alterations in collagen cross-linking [5].
So far, two preclinical studies have shown an effect of parathyroid hormone (PTH), one of the commercially available antifracture drugs, on the bones in rats with STZ-induced type 1 diabetes [6, 7]. Tsuchida et al. [6] reported that PTH(1–34) enhanced bone turnover and increased bone mass, but not trabecular connectivity, in rats with advanced diabetes. Suzuki et al. [7] showed that combined treatment with insulin and PTH(1–34) was more effective for improving bone mass, trabecular connectivity, and bone strength than treatment with insulin or PTH(1–34) alone in rats with STZ-induced type 1 diabetes. However, there is little information about the effects of available antifracture drugs other than PTH(1–34) on osteopenia in STZ-treated rats.
Vitamin K2 is widely used for the treatment of osteoporosis in Asia. This vitamin prevents vertebral fractures in postmenopausal women with osteoporosis and both nonvertebral and hip fractures in patients with neurological diseases [17, 18]. Vitamin K2 is known to have an anabolic effect on bone; regulation of bone formation by vitamin K2 may be related to its influence on the γ-carboxylation of osteocalcin and may be mediated via steroid and xenobiotic receptors (SXRs) [19–23]. Thus, vitamin K2 could potentially influence cancellous bone mass and cortical bone growth in type 1 diabetes. The purpose of the present study was to examine the effect of vitamin K2 on cancellous and cortical bone in rats with STZ-induced type 1 diabetes. The serum glucose concentration was measured to confirm STZ-induced hyperglycemia in terms of diabetes.
Materials and Methods
Treatment of Animals
Thirty male Sprague-Dawley rats (11 weeks old) were purchased from Charles River (Kanagawa, Japan). Male Sprague-Dawley rats were used according to the report by Silva et al. [5], who established a type 1 diabetes animal model induced by STZ injection using male Sprague-Dawley rats. Rats were fed a pelleted standard chow diet containing 1.25% calcium, 0.9% phosphorus, and 618 IU/100 g of vitamin D3 (CRF-1; Oriental Yeast, Tokyo, Japan). They were housed under local vivarium conditions: temperature of 24°C and humidity of 50%, with a 12-h lighting time and free access to water. After allowing 1 week for adaptation to the new environment, the 12-week-old rats were randomized by the weight-stratified method into the following three groups (n = 10/group): age-matched control group, STZ + vehicle group, and STZ + vitamin K2 group. STZ (Wako Pure Chemical Industries, Osaka, Japan) was reconstituted in 0.1 M phosphate-buffered saline (Invitrogen, Carlsbad, CA) and then administered intravenously at a dosage of 40–50 mg/kg body weight on days 1 and 7 of the experimental period. Vitamin K2 (menatetrenone; Eisai, Tokyo, Japan) was suspended in fatty acid (Miglyol 812; Mitsuba Trading, Tokyo, Japan) at a dosage of 30 mg/kg body weight and administered by gavage deep into the mouth five times a week. Administration of vitamin K2 at 30 mg/kg three to five times weekly has previously been reported to be effective in rats [24, 25]. The body weight of rats was monitored weekly, and the total duration of the experiment was 12 weeks. This study was carried out at the laboratory of Hamri (Ibaraki, Japan), which has been approved by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC) International. The experimental protocol was approved by the Institutional Animal Care and Use Committee (IACUC) of Hamri.
Preparation of Specimens
Three rats in the STZ + vitamin K2 group were excluded from the study because of unexpected death during the experimental period. Thus, 27 rats were subjected to the following analyses. Rats were labeled by injection of 10 mg/kg of calcein (Dojindo Laboratories, Kumamoto, Japan) subcutaneously at 7 and 3 days before death. At 12 weeks after the start of the experiment, the animals were killed by exsanguination after being anesthetized with 2–3% isoflurane (Mylan, Tokyo, Japan) using a Table Top Laboratory Animal Anesthesia System (V1 Type; VetEquip, Pleasanton, CA). Blood was collected from each animal after a 12-h fast, and the bilateral femora were harvested. Serum glucose concentration was measured with an Accu-Check (Roche Diagnostics, Tokyo, Japan). The length of the left femur was measured using a dial caliper, and it was weighed with an electronic balance (A&D Company, Tokyo, Japan). The right femur was processed for histomorphometric analysis of the distal metaphysis and diaphysis.
Each right femur was preserved overnight in 70% cold ethanol and then cut into three parts, the proximal femur, femoral diaphysis, and distal femur, using an Isomet saw (Buehler, Lake Bluff, IL). The distal metaphysis and mid-diaphysis were stained with Villanueva Osteochrome Bone Stain (Polyscience, Warrington, PA) for 5 days. Then, specimens were dehydrated sequentially in ascending concentrations of ethanol (70, 95, and 100%) and xylene and embedded in methyl methacrylate (EM Science, Gibbstown, NJ) at 4°C according to the method of Erben [26].
Serial frontal sections of the distal femoral metaphysis were cut at a thickness of 5 μm on a microtome (RM2155; Leica, Nussloch, Germany). The first section of each series was transferred onto a chromium/gelatin-coated slide, dried overnight under pressure at 42°C, and coverslipped with Eukitt mounting medium (Calibrated Instruments, Hawthorne, NY) for static and dynamic histomorphometric analysis. The second section was deplasticized and rehydrated, treated according to the Goldner trichrome staining procedure, and mounted with Eukitt to evaluate osteoid, osteoblasts, and osteoclasts.
Cross sections of the mid-diaphysis of the femur were cut at a thickness of 6 μm on the same microtome (Leica RM2155), then transferred onto chromium/gelatin-coated slides and dried overnight under pressure at 42°C. Then, sections were coverslipped with Eukitt for static and dynamic histomorphometric analysis.
Histomorphometric Analysis of the Distal Femoral Metaphysis and Femoral Diaphysis
A digitizing morphometric system was used to measure bone histomorphometric parameters. The system consisted of an epifluorescence microscope (BX51TF; Olympus, Center Valley, PA), an Osteomeasure High Resolution Color Subsystem (Olympus BX51TF) coupled to an IBM computer, and a morphometry software program (OsteoMetrics, Atlanta, GA).
The parameters measured for cancellous bone included the total tissue volume (TV), bone volume (BV), bone surface (BS), eroded surface (ES), single- and double-labeled surfaces (sLS and dLS, respectively), interlabel width, osteoblast surface (ObS), number of osteoblasts (N.Ob), osteoclast surface (OcS), and number of osteoclasts (N.Oc). These data were then used to calculate percent trabecular bone volume (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), ES/BS, mineralizing surface (MS)/BS [(sLS/2 + dLS)/BS], mineral apposition rate (MAR), bone formation rate (BFR)/BS (MS/BS × MAR), ObS/BS, N.Ob/BS, OcS/BS, and N.Oc/BS in accordance with the standard nomenclature proposed by Parfitt et al. [27]. The region of cancellous bone measured was 1–4 mm proximal to the higher margin of the growth plate in the distal metaphysis, which consists of secondary spongiosa.
The parameters measured for cortical bone were the total tissue area and marrow area, as well as periosteal and endocortical BS (perimeter), sLS, dLS, interlabel width, and endocortical ES, in accordance with the method described by Chen et al. [28]. These data were then used to calculate the cortical area as well as periosteal and endocortical MS/BS [(sLS/2 + dLS)/BS], MAR, BFR/BS (MS/BS × MAR), and endocortical ES/BS.
Statistical Analysis
Data are described as the mean and standard deviation (SD). Comparisons among the groups were performed by analysis of variance (ANOVA) with Fisher’s protected least significant difference (PLSD) test. All statistical analyses were done with Stat View J-5.0 software (SAS Institute, Cary, NC) on a Windows computer, and P < 0.05 was used as the level of significance.
Results
Body Weight, Serum Glucose, Femoral Length, and Weight
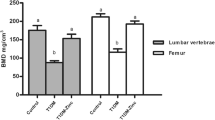
As shown in Table 1, the initial body weight did not differ significantly among the three groups. STZ administration induced weight loss and hyperglycemia and decreased femoral weight but not femoral length. Vitamin K2 administration to STZ-treated rats prevented weight loss and hyperglycemia and inhibited the decrease in femoral weight, without significantly affecting femoral length.
Histomorphometry of Cancellous Bone at the Distal Femoral Metaphysis
As shown in Table 2, in the STZ + vehicle group, there were decreases in BV/TV, Tb.Th, and Tb.N and an increase in Tb.Sp as a result of decreases in BFR/BS, ObS/BS, and N.Ob/BS and an increase in ES/BS. However, OcS/BS and N.Oc/BS were not significantly affected. The increase in ES/BS, which is an indicator of the remodeling balance, was attributable to decreased bone formation because bone-resorption parameters were not significantly affected by STZ administration.
Vitamin K2 administration to STZ-treated rats prevented both the decreases in BV/TV, Tb.Th, and Tb.N and the increase in Tb.Sp by inhibiting both the decreases in ObS/BS and N.Ob/BS and the increase in ES/BS. However, OcS/BS and N.Oc/BS were not significantly affected. The inhibition of the increase in ES/BS was attributable to inhibition of the decrease in bone formation because bone-resorption parameters were not significantly affected by vitamin K2 administration.
Histomorphometric Analysis of Cortical Bone at the Femoral Diaphysis
As shown in Table 3, in the STZ + vehicle group, there was a decrease in periosteal BFR/BS and an increase in endocortical ES/BS. However, total tissue area, cortical area, and marrow area were not significantly affected. The increase in endocortical ES/BS, which is an indicator of remodeling balance, was attributable to increased bone resorption because endocortical BFR/BS was not significantly affected by STZ administration.
Vitamin K2 administration to STZ-treated rats prevented both the decrease in BFR/BS and the increase in endocortical ES/BS. However, total tissue area, cortical area, and marrow area were not significantly affected. The prevention of the increase in endocortical ES/BS was attributable to the inhibition of the increase in bone resorption because endocortical BFR/BS was not significantly affected by vitamin K2 administration.
Discussion
The short-term skeletal consequences of STZ-induced type 1 diabetes in young rats are attributable to decreased bone formation and deterioration of bone structure [5]. Thus, histomorphometric analysis may provide important information on the bone of rats with type 1 diabetes. Administration of vitamin K2 to STZ-treated rats prevented hyperglycemia and a decrease in femoral weight, without significantly affecting femoral length, as well as cancellous osteopenia by inhibiting a decrease in bone formation; but it did not significantly increase cortical bone mass. These results suggest that vitamin K2 has beneficial effects on glucose concentration and cancellous bone mass in rats with STZ-induced type 1 diabetes.
STZ administration induced hyperglycemia and a decrease in body weight and femoral weight, suggesting that this rat model of type 1 diabetes is associated with osteopenia. The rat model of type 1 diabetes induced by STZ administration is well established. The cause of osteopenia in young male Sprague-Dawley rats (12 weeks old) at 12 weeks after a single STZ injection (50 mg/kg) differs between cancellous and cortical bone [5]. Cancellous osteopenia is caused by loss of bone, whereas cortical osteopenia is attributable to premature cessation of bone growth [5]. STZ administration decreases the serum osteocalcin level in rats [12–15], and cancellous osteopenia is largely due to diminished bone formation [6–15].
In the present study, treatment of 12-week-old male Sprague-Dawley rats with STZ (40–50 mg/kg, twice) induced cancellous osteopenia in the distal femoral metaphysis as a result of decreased bone formation after 12 weeks but did not significantly reduce cortical bone growth as indicated by the lack of any significant change in the total tissue area of femoral cortical bone. The reason for the lack of any significant alteration in total tissue area of cortical bone by STZ administration remains uncertain. Because STZ administration decreased periosteal bone formation and increased endocortical bone resorption, a longer observation period might have revealed a significant effect on cortical bone growth. To our empirical knowledge, however, STZ-treated rats may not be able to survive for a longer term due to complications caused by type 1 diabetes.
Administration of vitamin K2 to STZ-treated rats prevented the development of hyperglycemia. A survey of the Framingham Offspring Cohort showed that higher phylloquinone (vitamin K1) intake was associated with increased insulin sensitivity and an improved glycemic status [29]. This evidence suggests a potential beneficial role of vitamin K in glucose homeostasis. The present study was designed to examine the skeletal effect of vitamin K2 in rats with STZ-induced type 1 diabetes, and the serum glucose concentration was measured to confirm STZ-induced hyperglycemia in terms of diabetes. Thus, the mechanistic data, regarding the effect of vitamin K2 on serum glucose concentration, such as osteocalcin and insulin gene expression in the β cells, are lacking. Lee et al. [30] showed that mice lacking the osteoblast-secreted molecule osteocalcin displayed decreased β-cell proliferation, glucose intolerance, and insulin resistance and that osteocalcin stimulated insulin expression in β cells and adiponectin (an insulin-sensitizing adipokine) in adipocytes and improved glucose tolerance. Ferron et al. [31] also suggested that osteocalcin had the ability to regulate glucose metabolism and affected insulin sensitivity. They showed that long-term treatment of wild-type mice with osteocalcin significantly weakened the deleterious effect on body mass and glucose metabolism of gold thioglucose-induced hyperphagia and a high-fat diet. Thus, osteocalcin can regulate glucose metabolism and affect insulin sensitivity. Further studies are needed to clarify the mechanism for the inhibition of hyperglycemia by vitamin K2 in STZ-treated rats.
However, there is a criticism for this result. Because vitamin K2 administration was started at the time of STZ injection in the present study, it cannot be neglected that vitamin K2 might have prevented the influence of STZ on the pancreas in the beginning of the study. In this regard, vitamin K2 administration should have been started after STZ injections. However, if this suggestion is true, the difference in cancellous BFR/BS between the age-matched control group and the STZ + vitamin K2 group remains to be explained. Serum glucose concentration as well as pancreatic function should have been monitored throughout the experimental period. Well-controlled time-course studies including the baseline control group and the vehicle injection + vitamin K2 group are needed to resolve these issues.
Administration of vitamin K2 to STZ-treated rats prevented cancellous osteopenia by inhibiting a decrease in bone formation (N.Ob/BS and ObS/BS) and did not induce a decrease of femoral weight. Vitamin K2 is known to have an anabolic action through SXRs [23], and it increases mRNA levels for osteoblast markers such as bone alkaline phosphatase, osteoprotegerin, osteopontin, and matrix Gla protein. In the present study vitamin K2 was suggested to be useful to prevent cancellous osteopenia induced by decreased bone formation in rats with type 1 diabetes. However, there was a discrepancy among the changes in N.Ob/BS, ObS/BS, and BFR/BS with vitamin K2 administration. A time course study may be needed to confirm longitudinal changes in terms of the transient increase of BFR/BS during the current 12-week experiment.
Administration of vitamin K2 to STZ-treated rats did not significantly increase cortical bone mass, suggesting a lesser effect of vitamin K2 on cortical bone growth. However, vitamin K2 administration prevented a decrease in BFR/BS and an increase in endocortical ES/BS. The influence of vitamin K2 on endocortical ES/BS was attributable to its antiresorptive effect because endocortical bone formation was not significantly increased by vitamin K2 administration. In addition to the anabolic action of vitamin K2, an antiresorptive effect has been confirmed [32, 33]. Hara et al. [34, 35] showed that the inhibition of bone resorption by vitamin K2 is partly mediated by inhibition of prostaglandin E2 synthesis and that the side chain of vitamin K2 may play an important role in preventing bone resorption. Cortical bone mass was less responsive to vitamin K2 administration than cancellous bone mass, and the changes in the total tissue area of cortical bone caused by STZ or vitamin K2 administration were very small.
A study in northern Italy suggests that the incidence of type 1 diabetes is higher in male young adults than in female young adults (incidence rate ratio = 1.41) [36]. Silva et al. [5] established a type 1 diabetes animal model induced by STZ injection using male Sprague-Dawley and Fisher 344 rats. Because of this report, male Sprague-Dawley rats were used in the present study. However, it is certain that osteoporosis commonly affects postmenopausal women. Therefore, it would be of interest to test the effect of vitamin K2 on bone in female rats with or without ovariectomy. The gender difference in the response of bone to STZ injection in rats and vitamin K2 administration in STZ-treated rats remains uncertain. Further studies are needed to clarify these issues.
In conclusion, the present study showed that vitamin K2 administration to rats with STZ-induced diabetes prevented the onset of hyperglycemia and cancellous osteopenia by inhibiting a decrease in bone formation but did not significantly increase cortical bone mass. These results suggest that vitamin K2 has beneficial effects on glucose concentration and cancellous bone mass in rats with STZ-induced type 1 diabetes.
References
Adami S (2009) Health in diabetes: considerations for clinical management. Curr Med Res Opin 25:1057–1072
Hofbauer LC, Brueck CC, Singh SK, Dobnig H (2007) Osteoporosis in patients with diabetes mellitus. J Bone Miner Res 22:1317–1328
Janghorbani M, Van Dam RM, Willett WC, Hu FB (2007) Systematic review of type 1 and type 2 diabetes mellitus and risk of fracture. Am J Epidemiol 166:495–505
Shires R, Teitelbaum SL, Bergfeld MA, Fallon MD, Slatopolsky E, Avioli LV (1981) The effect of streptozotocin-induced chronic diabetes mellitus on bone and mineral homeostasis in the rat. J Lab Clin Med 97:231–240
Silva MJ, Brodt MD, Lynch MA, McKenzie JA, Tanouye KM, Nyman JS, Wang X (2009) Type 1 diabetes in young rats leads to progressive trabecular bone loss, cessation of cortical bone growth, and diminished whole bone strength and fatigue life. J Bone Miner Res 24:1618–1627
Tsuchida T, Sato K, Miyakoshi N, Abe T, Kudo T, Tamura Y, Kasukawa Y, Suzuki K (2000) Histomorphometric evaluation of the recovering effect of human parathyroid hormone (1–34) on bone structure and turnover in streptozotocin-induced diabetic rats. Calcif Tissue Int 66:229–233
Suzuki K, Miyakoshi N, Tsuchida T, Kasukawa Y, Sato K, Itoi E (2003) Effects of combined treatment of insulin and human parathyroid hormone (1–34) on cancellous bone mass and structure in streptozotocin-induced diabetic rats. Bone 33:108–114
Yamaguchi M, Uchiyama S, Lai YL (2007) Oral administration of phytocomponent p-hydroxycinnamic acid has a preventive effect on bone loss in streptozotocin-induced diabetic rats. Int J Mol Med 19:803–807
Facchini DM, Yuen VG, Battell ML, McNeill JH, Grynpas MD (2006) The effects of vanadium treatment on bone in diabetic and non-diabetic rats. Bone 38:368–377
Epstein S, Takizawa M, Stein B, Katz IA, Joffe II, Romero DF et al (1994) Effect of cyclosporin A on bone mineral metabolism in experimental diabetes mellitus in the rat. J Bone Miner Res 9:557–566
Goodman WG, Hori MT (1984) Diminished bone formation in experimental diabetes. Relationship to osteoid maturation and mineralization. Diabetes 33:825–831
Pennisi P, Clementi G, Prato A, Luca T, Martinez G, Mangiafico RA, Pulvirenti I, Muratore F, Fiore CE (2009) l-Arginine supplementation normalizes bone turnover and preserves bone mass in streptozotocin-induced diabetic rats. J Endocrinol Invest 32:546–551
Horcajada-Molteni MN, Chanteranne B, Lebecque P, Davicco MJ, Coxam V, Young A, Barlet JP (2001) Amylin and bone metabolism in streptozotocin-induced diabetic rats. J Bone Miner Res 16:958–965
Ward DT, Yau SK, Mee AP, Mawer EB, Miller CA, Garland HO, Riccardi D (2001) Functional, molecular, and biochemical characterization of streptozotocin-induced diabetes. J Am Soc Nephrol 12:779–790
Herrero S, Calvo OM, García-Moreno C, Martín E, San Román JI, Martín M, García-Talavera JR, Calvo JJ, del Pino-Montes J (1998) Low bone density with normal bone turnover in ovariectomized and streptozotocin-induced diabetic rats. Calcif Tissue Int 62:260–265
Saito M, Marumo K (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214
WHO Scientific Group (2003) Prevention and management of osteoporosis. World Health Organ Tech Rep Ser 921:86–109
Iwamoto J, Matsumoto H, Takeda T (2009) Efficacy of menatetrenone (vitamin K2) against non-vertebral and hip fractures in patients with neurological diseases: meta-analysis of three randomized, controlled trials. Clin Drug Investig 29:471–479
Hauschka PV, Lian JB, Cole DEC, Gundberg CM (1989) Osteocalcin and matrix Gla protein: vitamin K-dependent proteins in bone. Physiol Rev 69:990–1047
Koshihara Y, Hoshi K (1995) Vitamin K2 enhances osteocalcin accumulation in the extracellular matrix of human osteoblasts in vitro. J Bone Miner Res 12:431–438
Shearer MJ (1995) Vitamin K. Lancet 345:229–234
Vermeer C, Jie KSG, Knapen MHJ (1995) Role of vitamin K in bone metabolism. Annu Rev Nutr 15:1–22
Tabb MM, Sun A, Zhou C, Grun F, Errandi J, Romero K, Pham H, Inoue S, Mallick S, Lin M, Forman BM, Blumberg B (2003) Vitamin K2 regulation of bone homeostasis is mediated by the steroid and xenobiotic receptor SXR. J Biol Chem 278:43919–43927
Iwamoto J, Yeh JK, Takeda T (2003) Effect of vitamin K2 on cortical and cancellous bones in orchidectomized and/or sciatic neurectomized rats. J Bone Miner Res 18:776–783
Iwamoto J, Yeh JK, Schmidt A, Rowley E, Stanfield L, Takeda T, Sato M (2005) Raloxifene and vitamin K2 combine to improve the femoral neck strength of ovariectomized rats. Calcif Tissue Int 77:119–126
Erben RG (1997) Embedding of bone samples in methylmethacrylate: an improved method suitable for bone histomorphometry, histochemistry, and immunohistochemistry. J Histochem Cytochem 45:307–313
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units. Report of the ASMBR Histomorphometry Nomenclature Committee. J Bone Miner Res 2:595–610
Chen MM, Yeh JK, Aloia JF, Tierney JM, Sprintz S (1994) Effect of treadmill exercise on tibial cortical bone in aged female rats: a histomorphometry and dual energy X-ray absorptiometry study. Bone 15:313–319
Yoshida M, Booth SL, Meigs JB, Saltzman E, Jacques PF (2008) Phylloquinone intake, insulin sensitivity, and glycemic status in men and women. Am J Clin Nutr 88:210–215
Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, Dacquin R, Mee PJ, McKee MD, Jung DY, Zhang Z, Kim JK, Mauvais-Jarvis F, Ducy P, Karsenty G (2007) Endocrine regulation of energy metabolism by the skeleton. Cell 130:456–469
Ferron M, Hinoi E, Karsenty G, Ducy P (2008) Osteocalcin differentially regulates beta cell and adipocyte gene expression and affects the development of metabolic diseases in wild-type mice. Proc Natl Acad Sci USA 105:5266–5270
Iwamoto J, Takeda T, Sato Y (2004) Effects of vitamin K2 on osteoporosis. Curr Pharm Des 10:2557–2576
Iwamoto J, Takeda T, Sato Y (2006) Effects of vitamin K2 on the development of osteopenia in rats as the models of osteoporosis. Yonsei Med J 47:157–166
Hara K, Akiyama Y, Nakamura T, Murota S, Morita I (1995) The inhibitory effect of vitamin K2 (menatetrenone) on bone resorption may be related to its side chain. Bone 16:179–184
Hara K, Akiyama Y, Tajima T, Shiraki M (1993) Menatetrenone inhibits bone resorption partly through inhibition of PGE2 synthesis in vitro. J Bone Miner Res 8:535–542
Bruno G, Novelli G, Panero F, Perotto M, Monasterolo F, Bona G, Perino A, Rabbone I, Cavallo-Perin P, Cerutti F (2009) The incidence of type 1 diabetes is increasing in both children and young adults in northern Italy: 1984–2004 temporal trends. Diabetologia 52:2531–2535
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Iwamoto, J., Seki, A., Sato, Y. et al. Vitamin K2 Prevents Hyperglycemia and Cancellous Osteopenia in Rats with Streptozotocin-Induced Type 1 Diabetes. Calcif Tissue Int 88, 162–168 (2011). https://doi.org/10.1007/s00223-010-9441-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-010-9441-5